Fig. 6.1
Preterm delivery risk factor in a specific country
Aetiology of PTB is multifactorial, and numerous exposures including social, psychological, biological and genetic factors are known to be associated with.
There is consistent evidence that having a history of PTB or second trimester miscarriages is the most strong predicting factor. In literature it is shown that previous abortion, independently from the type (spontaneous or induced), increases the possibility of PTB; moreover, there is an increasing risk of very preterm birth associated with increasing numbers of abortions.
Singleton pregnancies resulting from assisted reproductive treatment (IVF, ICSI) have an increased risk of PTB. Similarly even an invasive diagnostic tool, as amniocentesis or villocentesis, is demonstrated to be a potential risk factor. Other factors that can increase the risk of PTB are history of cervical surgery for CIN disease, late termination of pregnancy, collagen diseases, lupus anticoagulant positivity, current multifoetal gestation, use of recreational drugs and urinary tract infections.
It has been observed that results of studies coming from ‘western world’ not always are applicable from one situation to another. In a recent multicentre, observational and retrospective, cross-sectional study, the Italian Preterm Network Study Group wanted to identify maternal risk factors for spontaneous preterm birth compared to women delivering at term, in order to recognise high-risk women and to provide a global overview of the Italian situation. The study demonstrated that there are peculiar risk factors for spontaneous preterm birth in the Italian population examined. It showed, in fact, an association between preterm delivery and certain maternal factors as BMI, employment, previous abortions, previous preterm births and previous caesarean section (Table 6.1). The increased risk of spontaneous PTB in a woman with a previous caesarean delivery has been substantiated by other studies subsequently, and it has been hypothesised that it can depend by the altered uterine milieu resulting from uterine scar and the more frequent altered placentation.
Table 6.1
Multivariate logistic analysis of various maternal risk factors for spontaneous preterm birth in Italian population (Italian Preterm Network Study Group)
Covariate | Contrast | Odds ratio estimate | Lower 95 % confidence limit for odds ratio | Upper 95 % confidence limit for odds ratio | P-value |
|---|---|---|---|---|---|
Age (cat.) | 2. Age ≥35 vs 1. age <35 | 1.234 | 0.699 | 2.177 | 0.4686 |
BMI | 2. BMI >25 vs 1. BMI ≤25 | 1.662 | 1.033 | 2.676 | 0.0365 |
Employment | 1. Physical work vs 2. intellectual work | 1.947 | 1.182 | 3.207 | 0.0089 |
Diabetes mellitus | 1. Yes vs 2. no | 2.286 | 0.942 | 5.544 | 0.0675 |
Chronic arterial hypertension | 1. Yes vs 2. no | 2.621 | 0.746 | 9.206 | 0.1327 |
Asthma | 1. Yes vs 2. no | 1.555 | 0.367 | 6.580 | 0.5489 |
Endocrinological diseases | 1. Yes vs 2. no | 1.420 | 0.594 | 3.396 | 0.4307 |
Congenital/acquired uterine malformations | 1. Yes vs 2. no | 2.660 | 0.602 | 11.745 | 0.1967 |
Previous abortion | 1. Yes vs 2. no | 1.954 | 1.162 | 3.285 | 0.0116 |
Previous PTLs | 1. Yes vs 2. no | 3.412 | 1.342 | 8.676 | 0.0099 |
Previous caesarean section | 1. Yes vs 2. no | 2.904 | 1.066 | 7.910 | 0.0371 |
Previous pregnancies <1 year before current delivery | 1. Yes vs 2. no | 0.919 | 0.398 | 2.124 | 0.8440 |
IVF | 1. Yes vs 2. no | 2.065 | 0.263 | 16.223 | 0.4906 |
Cigarette smoking | 1. Yes vs 2. no | 1.340 | 0.702 | 2.557 | 0.3746 |
Amniocentesis/villocentesis | 1. Yes vs 2. no | 1.006 | 0.540 | 1.875 | 0.9845 |
6.3 Pathophysiology
Human parturition is the expression of anatomic, biochemical, physiologic and clinical events that occur in the mother and in the foetus in both term and preterm labour (Fig. 6.2). This pathway consists in:
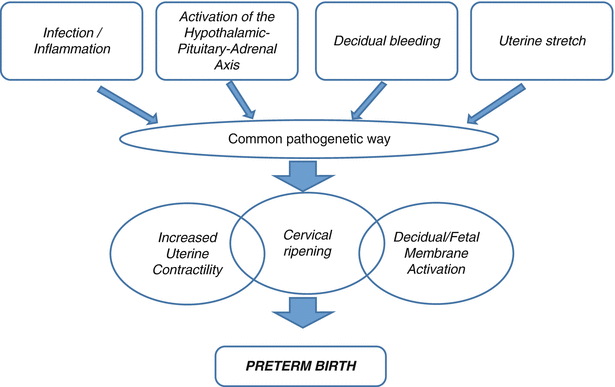
Decidual/foetal membrane activation
Increased uterine contractility
Cervical ripening (dilatation and effacement)

Fig. 6.2
Mechanism of activation of preterm versus term labour
Preterm labour is defined as the presence of uterine contractions between 22 and 36 weeks of gestation at a frequency of at least 6 per hour associated with at least one of the following elements: progressive changes in the cervix or cervical dilation >= 2 cm and/or premature rupture of membranes.
Preterm labour is the consequence of the pathologic premature activation of one or more of these elements (Fig. 6.3). A key hormone regulating the progress of pregnancy and the uterine quiescence is progesterone. In many species progesterone withdrawal is a prerequisite for the activation of labour. However, in the human, apparently no decrease of progesterone concentration in blood is demonstrated. However, the apparent loss of progesterone sensitivity and activity at term could be a consequence of several different mechanisms including alterations in progesterone receptors (PR) isoform ratios, the loss of anti-inflammatory function of progesterone, the catabolism of progesterone in the uterus into inactive compounds, changes in cofactor protein levels affecting PR transactivation and the inflammation-induced trans-repression of PR.


Fig. 6.3
Labour, term and preterm, is characterised by increased myometrial contractility, cervical dilatation and rupture of the chorioamniotic membranes (Adapted from Romero R et al. Science. 2014)
The activation of the mechanisms of labour at term and preterm can be divided into two groups:
- 1.
Infection/inflammation: the route of infection is generally ascending from the vagina through the cervix to membrane’s interface, and from there it can move to the amniotic fluid or, in more serious cases, to the foetus (foetal inflammatory syndrome).
Evidence has now shown that at least half of ‘idiopathic spontaneous preterm labour’ is attributable to subclinical genital tract infections, mostly due to micro-organisms such as Mycoplasma hominis, Ureaplasma urealyticum, anaerobic species and a host of other sub-pathogenic organisms associated with bacterial vaginosis.

- 2.
Not related to infection/inflammation (Fig. 6.4). Such cases comprise:
Uterine over distension, as in the case of multiple pregnancy or polyhydramnios
Cervical diseases, due to congenital anomalies or surgical trauma, defined as cervix-isthmic incontinence, a condition that leads to premature ripening and/or dilatation of the cervix
Uteroplacental ischaemia, due to abnormal angiogenesis during pregnancy at the level of the spiral arteries, resulting in increased incidence of thrombosis
Autoimmunity, as abnormal maternal mechanism of adaptation to the foetal-placental unit
Allergies
Unknown causes

Fig. 6.4
Pathways of preterm delivery
6.4 Identification
Before undertaking any therapeutic strategy, careful identification is needed, so as to detect manageable conditions and foetal and/or maternal contraindications.
Symptoms reported by patients with suspected preterm labour are pelvic pain, vaginal discharge, back pain and menstrual-like cramps. To improve the accuracy of diagnosis of the nosological entity we still name ‘threatened preterm labour’, the combination of two methods has been proposed:
Transvaginal ultrasound cervical length
Research of foetal fibronectin (fFN) or placental alpha-1 microglobulin (PAMG-1) in cervical-vaginal secretions
6.4.1 Biophysical Marker
Cervical length >2.5 cm has a high negative predictive value in symptomatic women. Cervicometry can be used as a diagnostic tool in two different clinical conditions: (1) Symptomatic patients reporting uterine contractile activity, in order to make differential diagnosis with other conditions that can mimic contractions. Cervicometry <2.5 cm, independently from the presence of funnelling, is able to identify a population at risk of preterm delivery with a sensitivity between 60 and 80 %. (2) Asymptomatic woman, in this case, the method is applied as screening. It is performed at the time of anatomic scan in the second trimester (19–23 weeks of gestation). The finding of a cervicometry <2.5 cm is associated with a subsequent preterm birth with a sensitivity between 30 % and 60 %.
Cervical screening can be undertaken in asymptomatic or symptomatic women, and studies have been performed in both high- and low-risk populations. The role of scanning in low-risk women is less clear, as the interventions to improve outcome are not established. However, more than half of all preterm births come from this group, and prediction remains good. A CL of <15 mm on transvaginal ultrasonography between 14 and 24 weeks’ gestation is associated with approximately a 50 % chance of sPTB prior to 32 weeks’ gestation (Hassan SS). In 2915 low-risk women at 24 weeks, the CL at 24 weeks averaged 34 ± 7.8 mm and was normally distributed. Only 5 % of women had a CL <20 mm, but the preterm birth rate <35 weeks was 23 % (PPV 25.7, NPV 96.5 %). The authors noted the concept that risk was a continuum across the CL range, rather than a threshold where risks begin. The risk of preterm delivery is greater in high-risk women with a cervical length (CL) less than 25 mm (10th centile) between 14 and 24 weeks’ gestation, and the risk is greater as the CL shortens. In a prospective study (183 women), a measurement of CL <25 mm at 16–19 weeks’ gestation had a relative risk of 3.3 (CI 2.1–5.0) for preterm delivery <35 weeks’ gestation, which increased to 4.5 (2.7–7.6) with serial measures.
6.4.2 Biochemical Markers
Foetal fibronectin (fFn) has proved to be one of the most promising markers among potential new indicators of impending preterm delivery. The test is available in two primary formats (Hologic, Marlborough, MA, USA). fFN is a glycoprotein produced by the chorion, and it functions as a ‘glue’ between placenta, amnion-chorion membranes and decidua. It is found in cervical-vaginal fluid from 16 to 19 weeks of gestation, it disappears and it is again detectable around term (after 36 weeks) or a week or so before preterm labour. It is believed that fFN is a marker of chorio-decidual interface alteration due to infection or inflammation, placental abruption or mechanical causes. This test is mainly used to exclude a preterm delivery rather than to identify it as its negative predictive power (97 %) has been shown to be significantly greater than its positive predictive value (<50 %) for delivery within 7–14 days. In a recent systematic review of the accuracy of the fFN test to predict preterm delivery in women with symptoms of preterm labour, Deshpande et al. reported pooled sensitivity and specificity from 27 studies for predicting PTB 7–10 days after testing of 76.7 % (70.4–82.0 %) and 82.7 % (79.4–85.5 %), respectively, presented with 95 % confidence intervals. In line with several previous systematic reviews, the authors suggested that the sensitivity of fFN testing may be highest for predicting PTB within 7–10 days of testing.
Most recently, a quantitative bedside foetal fibronectin test has been developed. The value of the test relies on the use of alternative thresholds of fFN detection (10, 50, 200 and 500 ng/mL, respectively) that may allow optimal selection of higher PPV for sPTB (an improved ‘rule in’ test) within 14 days and before <34 weeks, whilst the NPV remains reasonable as a rule out throughout all thresholds.
The identification of women at high risk of preterm delivery carried out with the fFN test or with transvaginal ultrasound cervicometry is clinically valid, but it has been demonstrated that the contextual use of biochemical and biophysical tests reaches a high negative predictive value (100 %), making it a very useful combined method to identify patients truly at risk and to further reduce the incidence of inappropriate treatment (Fig. 6.5).
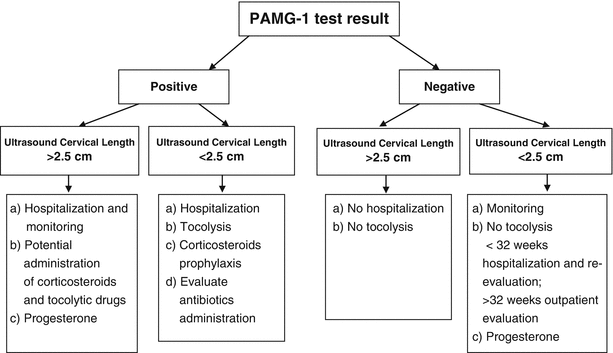

Fig. 6.5
Combined use of PAMG-1 test and ultrasound cervical length for predicting preterm birth in symptomatic patients
Another marker is based on identification of phosphorylated insulin-like growth factor binding protein (phIGFBP-1) in vaginal fluid. phIGFBP-1 is produced by the placental decidual cells and thought to be released into the CVF after tissue damage to the choriodecidual interface. A qualitative test, either positive or negative, is measured from a vaginal swab taken with a speculum between 22 and 36 weeks of gestation. An immunochromatography-based dipstick test (Actim Partus, Medix Biochemica, Kauniainen, Finland) is used to obtain the result within 5 min.
Conde-Agudelo and Romero reported pooled sensitivity and specificity from 18 studies for predicting PTB 7 days after testing symptomatic women of 67 % (62–72 %) and 77 % (75–79 %), respectively, presented with 95 % confidence intervals.
A new test, recently introduced, is based on the research of placental alpha-1 microglobulin (PAMG-1) in cervical-vaginal secretions. PAMG-1 is another glycoprotein synthesised by the decidua. It is found in the amniotic fluid in high concentrations. Little is found in the cervicovaginal fluid. A vaginal swab can be inserted directly into the vagina, removing the need for a speculum in patients between 20 and 37 weeks of gestation. An immunoassay bedside ‘dipstick test’ (PartoSure Test, Parsagen Diagnostics, Inc., USA) is used to obtain the result within 5 min. It has been shown that the positive test in patients symptomatic with intact membranes and cervical dilatation <=3 cm indicates the possibility of a spontaneous preterm delivery within 7 days with a high degree of accuracy (>80 %). A negative result indicated that spontaneous preterm delivery within 14 days is highly unlikely (negative predictive value >97 %) (Table 6.2).
Table 6.2
Biochemical marker tests to predict spontaneous preterm delivery within 7 days of testing in women with symptoms of preterm labour
Biomarker | Name of test | Test cutoff (ng/mL) | N | SN (%) | SP (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
fFN (qualitative) | Rapid fFN /QuikCheck | 50 | 4285 | 76 | 83 | 25 | 98 |
fFN (quantitative) | Rapid fFN 10Q Analyser | 10 | 350 | 96 | 42 | 29 | 98 |
50 | 350 | 91 | 65 | 39 | 97 | ||
200 | 350 | 71 | 84 | 52 | 92 | ||
500 | 350 | 42 | 96 | 71 | 87 | ||
phIGFBP-1 | Actim Partus | 10 | 2159 | 67 | 79 | 35 | 93 |
PAMG-1 | PartoSure | 1 | 353 | 84 | 95 | 77 | 97 |
Comparing fFN test, PAMG1 test and measuration of cervical length, it has been demonstrated that PAMG1 is the single most accurate test for prediction of imminent spontaneous delivery in patients presenting with signs, symptoms or complaints suggestive of PTL. Therefore, nowadays the identification of the patient at risk of impending preterm delivery is based on the two assumptions:
- 1.
Bearing in mind the excellent NEGATIVE predictive value of such tests, in patients with fFn or PAMG-1 negative and cervical length by ultrasound >2.5 cm, tocolytic therapy and steroid prophylaxis should be withheld, and it is recommended not to admit to hospital.
- 2.
Bearing in mind the excellent POSITIVE predictive value, patients with a positive PAMG-1 test should be admitted to hospital and tocolytic therapy, and steroid prophylaxis should be administered.
Other markers are under evaluation; particularly proinflammatory cytokines have been demonstrated in the second trimester amniotic fluid of pregnancies hesitating in PTB; and maternal salivary oestriol can be detected as early as 3 weeks prior to the onset of spontaneous preterm labour.
The challenge is to develop sensitive and specific tests that reliably detect these pregnancy’s changes before they became irreversible and to find effective intervention of arresting the process of preterm labour to enhance the effectiveness of current available interventions (Table 6.3).
Table 6.3
Benefits of identifying patients at real risk of preterm birth
Benefits to the hospital | Benefits to the patient |
|---|---|
Reduces unnecessary admissions and transfer to NICU | Unnecessary medical intervention |
Cost savings to the hospital | Peace of mind |
Reduction in administering medical management | Uninterrupted travel plans |
Availability of beds | Employment |
Less burden on spouse and family |
6.5 Management
Women who present with signs and symptoms of labour frequently will not deliver in the short term, and many will continue to full term, even in the absence of interventions. Women with risk factors will usually not deliver preterm. Conversely, even women who receive prophylactic interventions may still deliver early. Improved prediction in all these groups would be clinically beneficial, to target preventative interventions, admit to hospital with optimal neonatal facilities and therefore triage the need for in utero transfers as well as instigate in utero therapies to improve outcomes (e.g. steroids and magnesium sulphate).
6.5.1 Pharmacological Aspects
Tocolysis and administration of corticosteroids to induce lung maturation is the first therapeutic tool for the management of threatened preterm labour, in selected patients; also bed rest and hydration are usually recommended in the management of these patients (without evidence of real benefits).
Antenatal corticosteroids given to mothers with anticipated preterm delivery (betamethasone or dexamethasone 12 mg intramuscularly in two doses, 24 h apart) will improve survival and reduce the risk of respiratory distress syndrome (RDS), necrotising enterocolitis and intraventricular haemorrhage, and a single course does not appear to be associated with any significant maternal or short-term foetal adverse effects. The beneficial effects of antenatal steroids were similar in studies conducted in the 1970s as in those conducted more recently implying that they remain beneficial in the presence of modern neonatal care.
Betamethasone is likely to be more effective than dexamethasone, but has also more side effects. It reduces foetal body and breathing movements and foetal heart rate variation for about 1–3 days, without evidence for an impaired foetal condition. Due account for this phenomenon has to be given when monitoring the foetal condition. Betamethasone does not induce heart rate decelerations nor does it affect foetal Doppler.
Although with relative contraindications, accompanying tocolysis is preferable to guarantee the full course of corticosteroids. The current accepted indications for the use of tocolysis, in fact, are delaying delivery for 24–48 h to initiate and/or complete corticosteroid administration or for controlling uterine activity during in utero transfer to maternity units provided with neonatal intensive care especially for gestations <32 weeks.
6.5.1.1 Tocolytic Agents
Therapeutic indications are:
Gestational age (GA) at 22–34 weeks (advanced GA in exceptional cases)
Presence of four or more contractions for 30 min, lasting at least 30 s possibly evaluated by cardiotography
Cervical dilatation of 1–3 cm (0–3 for nulliparous) and cervical shortening >50 % or less than 1.5 cm by ultrasound
Normal foetal cardiac activity
Inhibitors of Prostaglandin Synthesis (Indomethacin, Naproxen, Ketoprofen, Diclofenac)
This group of drugs interfere with the synthesis of prostaglandins, inhibiting both cyclooxygenases (COX), which catalyse the conversion of arachidonic acid into prostaglandins, precursors of prostaglandins E and F.
Indomethacin is a generic inhibitor of COX, after absorption by oral or rectal administration, which achieves the plasmatic peak in 1–2 h. Several studies have shown tocolytic effects of this drug comparable to β-sympathomimetic and fewer maternal side effects. Prolonged administration may be associated with headache, dizziness and depression, besides it could double the maternal bleeding time.
The major side effects linked to the tocolysis with inhibitors of prostaglandin synthesis are possible closure of the ductus arteriosus (which can resolve within 24 h after drug interruption), neonatal pulmonary hypertension (possibly due to the prolonged deviation of blood from the ductus arteriosus to the pulmonary vascular bed), ventricular haemorrhage (when it is used for prolonged time after failure of the other therapies or in combination with magnesium sulphate) and oligohydramnios (due to foetal urinary production) especially if the drug is administered after 32 weeks of pregnancy. Indomethacin can also increase the risk of necrotising enterocolitis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


