Fig. 12.1
Complete placenta previa (major previa) at transabdominal ultrasound. Placenta lies over the internal cervical os

Fig. 12.2
Minor or partial placenta previa at transvaginal ultrasound
Currently, the definition of placenta previa is based on the distance between the placenta and internal os of the cervix. Definitely, there is an agreement that complete placenta previa is one which completely covers the internal cervical os [4–6]. However, when the placental edge just reaches the margin of a closed cervix, not crossing the os, but not a measurable distance away, terminology becomes confusing [2].
Different terms have been used, such as incomplete, partial, or marginal placenta previa, referring to different conditions in each case. When the cervix is visibly dilated and partially covered by the placenta, the term partial previa would seem appropriate, as this parallels the clinical definition of partial previa [4, 7], but this is a rare incidental finding and this term is no longer mentioned in placental terminology [8]. Conversely, the term marginal previa has been commonly used referring to a placenta that either reaches the margin of the internal os or is located a short distance away from the os in the lower uterine segment, typically within 20–25 mm [4, 5, 8]. However, some other authors have made a distinction, defining “low-lying” a placenta that is a short distance from the internal os (≤20 mm) (Fig. 12.3) [6, 9].


Fig. 12.3
Lower placental edge – internal cervical os distance measuring <20 mm at transvaginal ultrasound
Controversies in terminology reflect different recommended managements in a placenta that covers any part of the internal os compared to one that is a short distance from the os. In order to limit confusion, a placenta that reaches the internal os should be simply termed placenta previa, while the term “low-lying placenta” should be used if placenta implants in the lower uterine segment, but does not reach the cervix. In the latter case, vaginal delivery may be achieved, depending on the distance between the lower placental edge and the cervical os [2].
Conversely, if the distance between the internal cervical os and the placental edge is >20 mm, the patients should be managed as per routine, because the majority of studies have not demonstrated an increased risk for caesarean delivery for hemorrhage [10].
12.3 Incidence and Prevalence
The occurrence of placenta previa has increased in the last decades. A systematic review on published studies from 1966 to 2000 showed that the overall prevalence rate of placenta previa was 4.0 per 1000 births [11]. A more recent meta-analysis on placenta previa prevalence on the last 30 years reported a higher prevalence (5.2 per 1000 pregnancies), with a pooled prevalence of major placenta previa of 4.3 per 1000 pregnancies. [12]. According to regional differences, the prevalence of placenta previa was reported to be highest among Asian population (12.2 per 1000) and lower among studies from Europe (3.6 per 1000), North America (2.9 per 1000), and sub-Saharan Africa (2.7 per 1000).
The increasing rate of caesarean deliveries in the past three decades may have contributed to this increase in the prevalence of placenta previa [13, 14]. If primary and secondary caesarean rates continue to rise as they have in recent years, in 2020 the caesarean delivery rate will be 56.2 %, and there will be an additional 6236 placenta previas, 4504 placenta accretas, and 130 maternal deaths annually [15].
12.4 Risk Factors
The etiology of placenta previa remains unclear, but several epidemiological studies reported a panel of predisposing factors [16]. Previous caesarean section and uterine surgery (i.e., previous curettage, previous myomectomy, Asherman’s syndrome) represent major risk factors for placenta previa, as uterine scars predispose to a low placental implantation. The occurrence of placenta previa seems to be correlated also with the number of caesarean sections.
The incidence of placenta previa increased from 10 per 1000 deliveries with one previous caesarean section to 28 per 1000 with more than three caesarean deliveries [17]. Damage and scarring to the endometrial and myometrial lining during caesarean delivery and spontaneous and induced abortion are known to predispose to the low implantation of the placenta in the uterus [18–20].
In addition, advanced maternal age (over 35 years), low socioeconomic status, grand multiparity, smoking, cocaine abuse, recurrent miscarriages, history of induced abortions, submucous myomas, a short caesarean- or curettage-to-conception interval, male fetal gender, and multiple pregnancy are other risk factors associated with the occurrence of placenta previa [16, 21–24] (Table 12.1).
Table 12.1.
Risk factors for placenta previa
Risk factors | OR (95 % CI) |
|---|---|
Prior caesarean section | 2.7 (2.3–3.2) |
Advanced maternal age (≥35 years) | 1.8 (1.2–2.5) |
Spontaneous or induced abortion | 1.9 (1.7–2.2) |
Cigarette smoking | 1.6 (1.4–1.8) |
Cocaine abuse | 2.9 (1.9–4.3) |
Male fetal gender | 1.2 (1.1–1.3) |
Finally, women with history of placenta previa in a prior pregnancy are at higher risk of developing this condition in a subsequent pregnancy [11]. The increased risk of PP in women aged more than 35 years may be explained by atherosclerotic changes in the uterine blood vessels causing compromised uteroplacental blood flow. In order to maintain optimal blood flow, an increased surface area may be required for placental attachment, and this may result in placental encroachment on the lower uterine segment [25].
The high prevalence of PP in multiparous women may be due to endometrial scarring at the site of prior placental attachments resulting in lower placental implantation; alternatively, changes of blood vessels at the sites of prior placental attachments may lead to decreased uteroplacental blood, resulting in a larger placenta encroaching on the cervical os with repeated pregnancies. The relationship between cigarette smoking during pregnancy and PP risk may be attributed to the vasoactive properties of nicotine and to chronic hypoxia associated with carbon monoxide: chronic hypoxic changes in the uterine vasculature of smokers, resulting in a larger placenta with increased likelihood of placental encroachment on the cervical os [21].
Similarly, maternal cocaine use is known to cause catecholamine-mediated vasoconstriction and vasospasm in blood vessels innervated by the sympathetic nervous system, resulting in underperfusion of the uteroplacental vessels and a larger placenta encroaching on the cervical os [23].
There is an increased frequency of placenta previa among women with preexisting or chronic hypertension. The exact mechanism that leads to lower implantation of the placenta among women with chronic hypertension is not clear. However, it has been suggested that a better blood supply and oxygenation of the placenta in the lower uterine segment prevents the release of vasoactive substances into the bloodstream and thus reduces the risk of pregnancy-induced hypertension and preeclampsia in cases of placenta previa [26].
Furthermore, recent studies indicate that assisted reproductive technology (ART) also constitutes a risk factor for PP [27–29]. Indeed, in a group of 318 patient who consecutively conceived by ART, it was found that endometriosis (OR 15.1) and tubal disease (OR 4.4) increased the risk of PP. Thus, even factors causing infertility may contribute to the pathogenesis of PP because most women who undergo ART have some underlying infertile factors [30]. Besides, a recent study on ART cycles showed that endometrial thickness is directly proportional to the risk of placenta previa, independently of significant risk factors, such as smoking and endometriosis, but related to the endometrial preparation and hormonal stimulating therapy [31].
12.5 Pathophysiology
The pathophysiology of placenta previa is not completely understood [32]. As gestation advances, the relationship between the placental edge and the internal cervical os changes. In fact, a low implanted placenta in the early second trimester of pregnancy would move away from the internal os in the third trimester in most of the cases. This placental “migration” from the lower uterine segment toward the fundus may be explained by a greater vascularization of fundus compared to the rest of the uterus, allowing a better development of the trophoblastic tissue. This phenomenon would occur because of a process of a degeneration of the trophoblast close to the internal orifice secondary to decreased vascularization rather than a true migration of the placental tissue [33]. Thus, distortion of the normal anatomy of the lower uterine segment induced by a previous uterine scar would prevent this “migration” [20]. Alternatively, defective decidual vascularization and subsequent endometrial hypoxemia may increase the surface area of the placental tissue, predisposing to a lower implantation close to the cervix [34].
12.6 Diagnosis
Placenta previa characteristically presents with painless vaginal bleeding, in late second trimester or in the third trimester. The initial bleed in more than 50 % of cases occurs prior to 36 weeks’ gestation. Bleeding episodes can be recurrent in most cases, with a worsening of bleeding. The absence of abdominal pain is regarded as a significant differentiating feature between placenta previa and abruption, although 10 % of women will have a coexisting abruption or the presence of uterine contractions. Alternatively, the condition would remain unknown until labor onset [35]. Even though clinical signs are very important in the initial management of placenta previa suspect, definitive diagnosis is made only by ultrasound [10].
Placental location is usually reported during the routine anomaly scan in the second trimester. A follow-up scan in the third trimester should be scheduled when the placental edge is found to be reaching or overlapping the internal cervical orifice, to confirm this finding and to plan the management of delivery [3, 36] (Fig. 12.4).


Fig. 12.4
Transvaginal ultrasound at 20 weeks: the placental edge is found to be overlapping the internal cervical orifice
The ultrasound can either be done by transabdominal, transvaginal, or translabial methods (Fig.12.5). Transvaginal ultrasound is considered to be the most accurate, with less risk of false-positive results [37, 38]. This approach allows the sonographer to visualize the relationship between the lower placental edge and the internal cervical orifice with greater clarity because there is no obstruction of visualization caused by the fetus. Transvaginal ultrasound is safe, even in suspected cases of placenta previa, if the sonographer visualizes the placement of the intravaginal probe and avoids close cervical contact [38–43] (Fig. 12.6).



Fig. 12.5
Major placenta previa at transabdominal ultrasound

Fig. 12.6
Transvaginal ultrasound: complete placenta previa (major previa)
Translabial sonography is an acceptable technique to visualize placental location if there are concerns about the insertion of a transvaginal probe, but it is not as precise as transvaginal ultrasound [8].
The scanner head is placed between the labia majora anterior to the vaginal introitus and oriented along the axis of the vagina [44]. If placenta previa or low-lying placenta is diagnosed early (15–19 weeks’ gestation) in pregnancy, repeated sonographic screening performed throughout the pregnancy is critical [45].
The suspected diagnosis of placenta previa at 20 weeks of gestation by abdominal scan should be confirmed by transvaginal scan. In the second trimester, transvaginal sonography will reclassify 26–60 % of cases where the abdominal scan diagnosed a low-lying placenta, meaning fewer women will need follow-up [3, 40, 41] (Fig. 12.7).


Fig. 12.7
Transvaginal ultrasound: placenta previa in the third trimester
Anyhow, a conclusive diagnosis of placenta previa is possible only in the third trimester of pregnancies, because almost 90 % of the placentas defined as low in the second trimester move away out of the lower uterine segment later on in gestation [46, 47]. This occurs as a result of atrophy of the placental tissue in the lower uterine segment, secondary to a poor blood supply combined with the subsequent growth of placental tissue in the areas of increased vascularity in the fundal area of the uterus. This developmental event is called trophotropism [1].
The study by Heller [48] on second-trimester low-lying placentas demonstrates a very high (>98 %) likelihood of migration of the placenta away from the cervix (>2 cm from the internal os) by the time of delivery. These results can be used to counsel patients and reduce their level of anxiety regarding peripartum complications or the need for caesarean delivery because of the second-trimester finding. Since only 66 % of low-lying placentas resolve by the end of 27 weeks’ gestation, whereas almost 90 % of cases will be clear of the cervix by 32 weeks, it would be cost-effective to delay reassessment of the placental location until after 28–30 weeks in those pregnancies uncomplicated by bleeding or preterm labor (Fig. 12.8).


Fig. 12.8
Transvaginal ultrasound: placental edge reaching the internal cervical os at 28 weeks
Some investigators have attempted to correlate the position of the placenta in the second trimester, as detected at the scan, with the likelihood of migration in the third trimester of pregnancy. In fact, in some cases of midtrimester low-lying placenta, the placental edge is more likely to “migrate” than others are. Ultrasound may be useful in predicting both the likelihood and extent of placental migration as a function of time. In women with placental edge within 3 cm of the internal cervical orifice at 26 weeks of pregnancy or later, the mean rate of migration was 5.4 mm per week in women in whom placental edge migration did occur. If the placental edge overlapped the internal cervical orifice by more than 2 cm, migration was not observed in any woman, while migration always occurred when the placental edge was more than 2 cm from the internal cervical orifice (Fig. 12.9).


Fig. 12.9
The placental edge overlaps the internal cervical orifice by more than 2 cm at transvaginal ultrasound
On the contrary, if the distance of the placental edge was less than 2 cm from the internal cervical orifice, placental migration occurred in most cases [49]. The extent to which the placenta overlaps the internal cervical os in the second trimester can be used to determine if the placenta previa will persist until term. With complete previas that cover the os at 20 weeks’ gestation, 40 % will continue as a complete previa until birth. Women with a history of a prior caesarean are at greater risk for a placenta previa or a low-lying placenta to persist until birth [38]. Scarring from the caesarean birth impairs the ability of a low-lying placenta to migrate as the uterus expands with the advancing pregnancy [38, 50, 51].
The shape of the placental edge detected at 28–32 weeks is another factor in predicting placental migration. Placental migration was seen in 29.6 % where the placental edge was thin, but only in 5.8 % where it was thick (if the thickness was 1 cm or less, within 1 cm from the edge, the angle between the basal and the chorionic plate exceeded 45°, or both). Indeed, a significantly higher rate of antepartum hemorrhage, abdominal delivery, adherent placenta, and low birth weight was found in cases where the placental edge was thick [52] (Fig. 12.10).


Fig. 12.10
Placental edge shape at transvaginal ultrasound
In summary, when a low-lying placenta is diagnosed in the second trimester from 16 to 24 weeks, more than 98 % of placentas will no longer approach the cervix by the time of delivery. In almost 90 % of cases, the placenta will be clear of the cervix by 32 weeks’ gestation and almost 96 % by 36 weeks. A very small percentage of second-trimester low-lying placentas persist or progress to placenta previa requiring caesarean delivery, and a few will develop into vasa previa. Careful scanning at follow-up is essential for determining whether part of the placenta or a fetal vessel crosses the internal surface of the cervix to plan appropriately for delivery.
A low-lying placenta is a known risk factor for the development of vasa previa [53], and this factor must be kept in mind when following up on an earlier diagnosis of a low-lying placenta. Color Doppler imaging is recommended to assess the internal surface of the cervix to diagnose or exclude vasa previa as well as to identify the umbilical cord insertion to the placenta. [54] (Fig. 12.11).
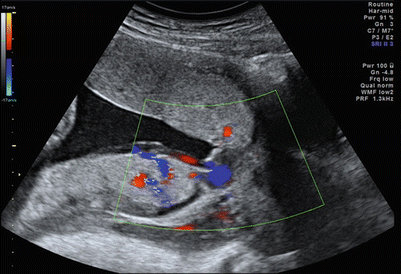

Fig. 12.11
Posterior placenta previa and anterior accessory placental lobe, with vasa previa
12.6.1 Vasa Previa
Vasa previa describes fetal blood vessels coursing through the membranes over the internal cervical os and below the fetal presenting part, unprotected by placental tissue or the umbilical cord. [55]. This can be secondary to a velamentous cord insertion in a single or bilobed placenta (vasa previa type 1) or from fetal vessels running between lobes of a placenta with one or more accessory lobes (vasa previa type 2) [56]. The reported incidence varies between 1 in 2000 and 1 in 6000 pregnancies [57]. Unlike placenta previa, vasa previa carries no major maternal risk but is associated with significant risk to the fetus. When the fetal membranes are ruptured, either spontaneously or artificially, the unprotected fetal vessels are at risk of disruption with consequent fetal hemorrhage. Vasa previa therefore often presents with fresh vaginal bleeding at the time of membrane rupture and fetal heart rate abnormalities such as decelerations, bradycardia, a sinusoidal trace, or fetal demise. The mortality rate in this situation is around 60 %, although significantly improved survival rates of up to 97 % have been reported where the diagnosis has been made antenatally. More rarely, bleeding can occur in the absence of membrane rupture. Because the fetal blood volume is around 80–100 ml/kg, the loss of relatively small amounts of blood can have major implications for the fetus. Very rarely, fetal heart rate abnormalities in the absence of bleeding may be present secondary to compression of the fetal vessels by the fetal presenting part.
Risk factors for vasa previa include placental anomalies, such as a bilobed placenta or succenturiate lobes, where the fetal vessels run through the membranes joining the separate lobes together, a history of low-lying placenta in the second trimester, multiple pregnancy, and in vitro fertilization, where the incidence of vasa previa has been reported to be as high as one in 300 [58].
Vasa previa can be accurately diagnosed with color Doppler ultrasound, by using transvaginal probe and assessing the lower pole of the uterus in the region of the internal cervical os to identify any fetal vessels. The accuracy of ultrasound in the prenatal diagnosis of vasa previa is good with a sensitivity of 100 % and specificity of 99.0–99.8 %, when performed transvaginally with color Doppler [59] (Figs. 12.12, 12.13, 12.14, and 12.15).





Fig. 12.12
Vasa previa at transvaginal ultrasound

Fig. 12.13
Vasa previa at color Doppler

Fig. 12.14
Placenta previa and vasa previa at 30 weeks

Fig. 12.15
Placenta previa and vasa previa at 32 weeks
A surgical birth is indicated for women diagnosed with vasa previa. Because the exposed blood vessels are not protected by Wharton’s jelly or placental tissue, a planned caesarean birth is recommended before the likelihood of spontaneous labor, at approximately 35–36 weeks’ gestation.
12.7 Management
Clinicians should determine if a woman has risk factors for placenta previa during the initial prenatal visit. Any episode of painless vaginal bleeding during pregnancy should be evaluated for placenta previa with ultrasound [51]. Placenta previa is responsible for potentially life-threatening conditions for the mother and the fetus, and women with confirmed diagnosis in the third trimester should be counseled about potential risks and their care should be tailored to their individual needs.
Maternal risks [32, 60]:
- 1.
Maternal mortality: from 5 % lo less than 0.1 %
- 2.
Antepartum and postpartum hemorrhage, with need for hysterectomy and/or blood transfusions
- 3.
Placenta accreta (occurring in approximately 15 % of PP)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


