Mechanisms of chemotherapy-induced gonadal damage
Although the relationship between chemotherapy and decreased ovarian reserve has been well-established, the underlying mechanism is not well understood. There are multiple proposed mechanisms of how chemotherapy may exert an effect on ovarian follicles, which includes both direct and indirect pathways ( Figure 1 ). The mechanisms may also vary depending on the specific chemotherapeutic agent used.
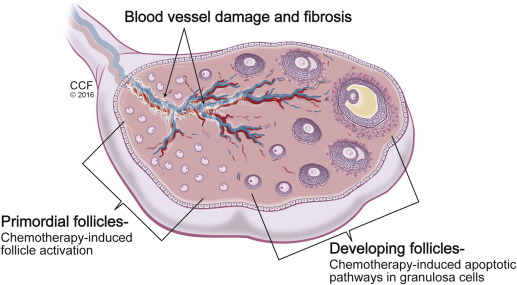
Amenorrhea that occurs during treatment is secondary to the loss of growing follicles. Chemotherapeutic agents can cause apoptosis of follicles directly, with the dividing granulosa cells being particularly susceptible to damage. The direct loss of growing follicles causes an accelerated recruitment of primordial follicles and a decrease in the total ovarian follicular reserve. This results in a faster depletion of the primordial follicle pool and, in turn, decreased fertility. The diminished reserve may manifest immediately after treatment with persistent amenorrhea or have a delayed clinical presentation with infertility or POF long term. Last, changes to ovarian stromal tissue are evident after exposure to chemotherapy. Studies of ovarian tissue after chemotherapy treatment demonstrate stromal fibrosis and damage to blood vessels. The resultant local ischemia further contributes to overall follicular depletion.
Theories that support the concomitant use of gonadotropin-releasing hormone agonists (GnRHa) during chemotherapy treatment describe ovarian protection from both the direct and indirect influences. GnRHa act at the level of the anterior pituitary, where it binds GnRH receptors. Initially, GnRHa have an agonistic effect, stimulating secretion of luteinizing hormone and follicle-stimulating hormone. This “flare” effect is the most significant in the early follicular phase, when a large reserve of gonadotropins is present in the anterior pituitary. Prolonged activation of the receptors leads to a desensitization and disruption of GnRH pulsatility. As such, gonadotropin secretion becomes down-regulated, which causes a hypogonadal state. Overtime, a loss of GnRH receptors also occurs. At the ovary, GnRHa are thought to decrease vascularity, thereby reducing the concentration of chemotherapy acting directly on the ovary. GnRHa have also been shown to inhibit primordial follicle recruitment, thereby attenuating the accelerated depletion of ovarian reserve and providing protection to fertility.
Management
The impact of a cancer diagnosis on a woman of reproductive age is significant and has a potential impact on fertility, quality of life, bone health, cardiovascular disease, sexual health, and overall mortality rates. The oncologist serves a crucial role in patient education. After cancer diagnosis, the oncologist must explain the implications of cancer treatment, including the planned chemotherapeutic regimen, the potential side-effects of treatment, and the benefit of the regimen proposed, which includes the anticipated disease-free survival ( Figure 2 ). The oncologist should also initiate a discussion on the risk of POF if a gonadotoxic regimen is planned and ensure that a referral to a reproductive endocrinologist is provided so that fertility and other concerns related to hypogonadism can be explained further to the patient. The development of a multidisciplinary treatment team is necessary for the treatment of reproductive-aged women with a malignancy.
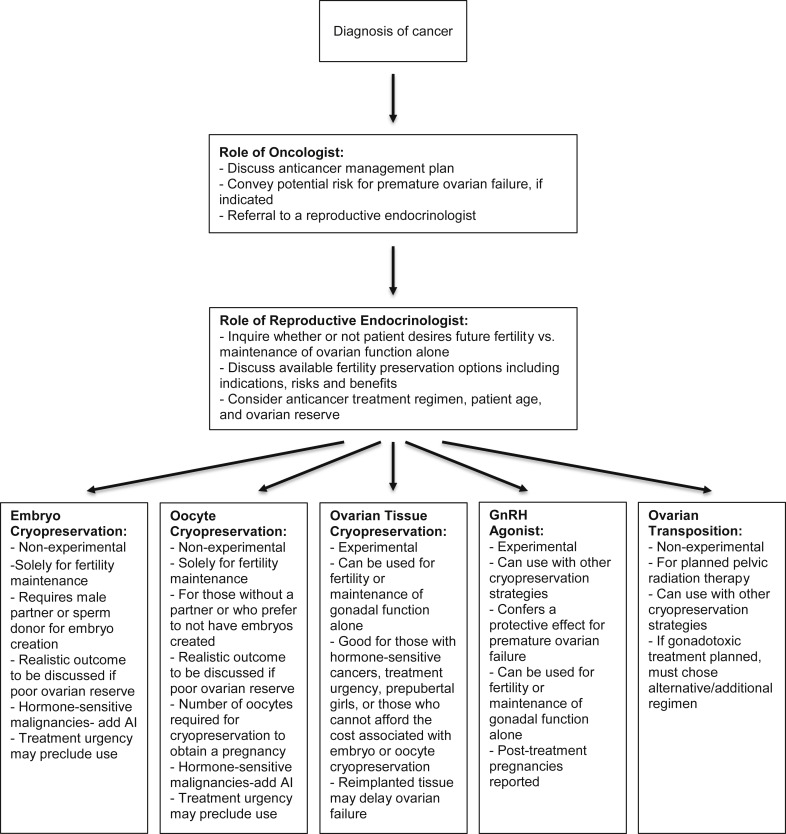
The reproductive endocrinologist also plays an important role in the counseling of a woman with a cancer diagnosis ( Figure 2 ). A comprehensive discussion with the patient regarding whether future fertility is desired is essential. If future fertility is desired, consideration of the cancer diagnosis and timeline for treatment may impact the options that are available to the patient. Other patient-specific features that can impact treatment options include the patient’s age and ovarian reserve work up. It is important that consideration of the potential gonadotoxicity, patient’s wishes, and risks/benefits of fertility preservation treatment be weighed carefully. Ovarian reserve and future fertility can be impacted even by regimens with low gonadotoxicity, and patients whose condition relapses may progress to subsequent, more gonadotoxic treatments. Patients, when appropriate, should also be counseled that they may not require assistance with conception in the future.
There are various proposed strategies for preservation of fertility and gonadal function in women who undergo cancer treatment. For fertility preservation, these include embryo, oocyte, or ovarian tissue cryopreservation (OTC) and pharmacologic ovarian suppression with GnRHa. Options are further limited for gonadal function preservation, because GnRHa and OTC are currently the main options available to address ovarian preservation and thereby endocrine function.
Embryo and oocyte cryopreservation before the initiation of chemotherapy has proved to be very successful and to date has been considered the first-line options for fertility preservation. Embryo preservation requires the use of ovarian stimulation and fertilization of embryos with either a partner or donor’s sperm, which are then stored for later use. Oocyte cryopreservation is an alternative option for postpubertal girls, single women who prefer not to use donor sperm, and individuals with moral or ethical concerns regarding embryo cryopreservation. It is important to counsel patients that the pregnancy rates after oocyte cryopreservation are related to the number oocytes cryopreserved. In general, with a slow-freeze technique, a live birth rate per embryo transfer of 22% was found in a meta-analysis by Oktay et al. Similarly, when a vitrification technique was used, a live birth rate per transfer of 37% was found. Unfortunately, there is a significant financial cost associated with embryo and oocyte cryopreservation, and this may be prohibitive for some patients. Patients must also have access to an appropriate assisted reproductive center, which may further preclude the use of these methods. Embryo or oocyte cryopreservation may not be an option for some patients, such as prepubertal girls and women with hormone-sensitive malignancies who cannot or choose not to be exposed to the high levels of estrogen needed for ovarian stimulation. Letrozole use during ovarian stimulation, however, has had a significant impact on reducing serum estrogen levels during ovarian stimulation, making this a more accessible option for women with breast and endometrial cancer. Last, cryopreservation of embryos and oocytes also focuses solely on preservation of fertility, without providing any affect on long-term ovarian function and the systemic sequelae that are associated with POF.
For some patients, OTC may be an important option to consider. OTC can be performed when time constraints are a factor. For some patients, the urgency of their treatment may preclude in vitro fertilization as an option, even with random start protocols; the controlled ovarian stimulation process generally takes at least 2 weeks. OTC is not a suitable option for women with ovarian or hematologic malignancies and those with tumors prone to metastasize to the ovaries. OTC techniques have continued to evolve since first developed >20 years ago in animal models. A retrospective cohort study of Danish women has demonstrated encouraging results, with a positive pregnancy test in 63% (21/32 patients) and 13 live births. Additionally, for some of the cohort, the graft longevity has lasted upwards of 7–10 years, with a large number of individuals experiencing graft longevity of 2–4 years. Other studies have also reported an average graft survival of 4–5 years, with some reports of grafts that lasted >7 years. Ovarian activity with premenopausal hormonal profiles, and in many cases menstrual cycles, has been reported for upwards of 10 years after transplantation. Although the primary utility of OTC has been for fertility preservation, it has also been proposed that ovarian tissue grafts may be a novel method to postpone menopause by restoring the hormonal milieu and thereby alleviating some of the menopause-related medical conditions in the aging population. This interesting proposal would require a considerable amount of further investigation; however, it does pose a unique solution that could have major impact on the physiologic process of reproductive aging.
It is important to note that OTC currently is considered experimental, and its use is limited to research centers with institutional review board approval. The American Society for Reproductive Medicine committee opinion recommends the use of OTC when the benefits outweigh the risks that are associated with more traditional fertility preservation methods, such as patients with treatment time constraints or hormone-sensitive malignancies. Although OTC has the potential to affect both fertility and endocrine outcomes, clinical data are still limited with conflicting results. To date, optimal techniques for cryopreservation, ideal location for graft transplantation, and duration of graft survival remain not entirely clear. Additionally, the percentage of patients who regain ovarian function and become pregnant after autotransplantation is difficult to discern, likely because of publication bias. Last, OTC requires a laparoscopic procedure, which has inherent surgical and anesthetic risks and associated costs.
The use of GnRHa during chemotherapy is still a controversial, albeit appealing, method for many patients and providers alike. GnRHa are associated with a relatively low risk, time, and cost. Typical side-effects during treatment are related to hypoestrogenic state and include hot flashes, vaginal dryness, mood swings, depression, amenorrhea or spotting, and headaches. A localized allergic reaction at the injection site may occur. Anaphylaxis is very rare. The treatment-associated amenorrhea may be especially helpful for patients with anemia secondary to abnormal uterine bleeding.
GnRHa may also protect reproductive potential. GnRHa can be useful for the preservation of gonadal function to alleviate hypogonadal consequences. Most evidence to this effect has been derived from cotreatment with the standard chemotherapeutic regimens for patients with breast cancer or lymphoma. GnRHa often are used in conjunction with standard chemotherapy for breast cancer in estrogen receptor–positive cancers. Studies have shown that GnRHa are safe, with no impact on grade 3 or 4 side-effect incidence during chemotherapy. Additionally, GnRHa are often beneficial as adjuvant treatment in combination with chemotherapy for a subset of patients. Patients with breast cancer, including those with estrogen receptor positive tumors, who received GnRHa cotreatment had increased or no impact on disease-free survival and overall survival compared with chemotherapy alone. In the Prevention of Early Menopause Study (POEMS), a trend towards a higher rate of disease-free survival in those individuals who were treated with GnRHa was observed as was a statistically significant higher rate of overall survival in this group compared with those who were treated with chemotherapy alone ( P =.04). Similarly, in the study by Lambertini et al, a trend towards improved 5-year disease-free survival was observed in the GnRHa group vs control subjects ( P =.52).
Regarding the impact of GnRHa on improving fertility potential, a positive impact has been noted in several studies to date. In the POEMS trial, women in the GnRHa group had a higher pregnancy rate than those in the chemotherapy alone group (odds ratio, 2.45; 95% confidence interval, 1.09–5.51; P =.03). Other randomized controlled trials (RCTs) have also looked at posttreatment pregnancy outcomes but report very small numbers in both the treatment and control groups. These data must be interpreted with caution, because a variety of confounders such as patient age, psychologic impact of cancer diagnosis, and precancer parity may all affect one’s desire to pursue pregnancy after treatment. Two cohort studies also investigated the impact of GnRHa cotreatment on pregnancy outcomes in women with breast cancer and found spontaneous pregnancy rates of nearly 70% in those who attempted to conceive. Taken together with the POEMS data, these findings are encouraging for a protective impact of GnRHa on future fertility.
To date the American Society for Reproductive Medicine recommends use of GnRHa in concert with other fertility preservation methods for patients who desire future pregnancies. A recent expert meeting on cancer and fertility preservation also recommended that GnRHa treatment be considered a “reliable strategy to preserve ovarian function and fertility” in light of data that support its efficacy, while still underscoring the experimental nature of the procedure. Additionally, the National Comprehensive Cancer Network and the St. Gallen International Expert Consensus panel guidelines support the use of GnRHa for the prevention of ovarian failure secondary to gonadotoxic chemotherapy. For individuals who have completed childbearing, GnRHa with the goal of preserving of ovarian function is also important to consider.
Management
The impact of a cancer diagnosis on a woman of reproductive age is significant and has a potential impact on fertility, quality of life, bone health, cardiovascular disease, sexual health, and overall mortality rates. The oncologist serves a crucial role in patient education. After cancer diagnosis, the oncologist must explain the implications of cancer treatment, including the planned chemotherapeutic regimen, the potential side-effects of treatment, and the benefit of the regimen proposed, which includes the anticipated disease-free survival ( Figure 2 ). The oncologist should also initiate a discussion on the risk of POF if a gonadotoxic regimen is planned and ensure that a referral to a reproductive endocrinologist is provided so that fertility and other concerns related to hypogonadism can be explained further to the patient. The development of a multidisciplinary treatment team is necessary for the treatment of reproductive-aged women with a malignancy.
The reproductive endocrinologist also plays an important role in the counseling of a woman with a cancer diagnosis ( Figure 2 ). A comprehensive discussion with the patient regarding whether future fertility is desired is essential. If future fertility is desired, consideration of the cancer diagnosis and timeline for treatment may impact the options that are available to the patient. Other patient-specific features that can impact treatment options include the patient’s age and ovarian reserve work up. It is important that consideration of the potential gonadotoxicity, patient’s wishes, and risks/benefits of fertility preservation treatment be weighed carefully. Ovarian reserve and future fertility can be impacted even by regimens with low gonadotoxicity, and patients whose condition relapses may progress to subsequent, more gonadotoxic treatments. Patients, when appropriate, should also be counseled that they may not require assistance with conception in the future.
There are various proposed strategies for preservation of fertility and gonadal function in women who undergo cancer treatment. For fertility preservation, these include embryo, oocyte, or ovarian tissue cryopreservation (OTC) and pharmacologic ovarian suppression with GnRHa. Options are further limited for gonadal function preservation, because GnRHa and OTC are currently the main options available to address ovarian preservation and thereby endocrine function.
Embryo and oocyte cryopreservation before the initiation of chemotherapy has proved to be very successful and to date has been considered the first-line options for fertility preservation. Embryo preservation requires the use of ovarian stimulation and fertilization of embryos with either a partner or donor’s sperm, which are then stored for later use. Oocyte cryopreservation is an alternative option for postpubertal girls, single women who prefer not to use donor sperm, and individuals with moral or ethical concerns regarding embryo cryopreservation. It is important to counsel patients that the pregnancy rates after oocyte cryopreservation are related to the number oocytes cryopreserved. In general, with a slow-freeze technique, a live birth rate per embryo transfer of 22% was found in a meta-analysis by Oktay et al. Similarly, when a vitrification technique was used, a live birth rate per transfer of 37% was found. Unfortunately, there is a significant financial cost associated with embryo and oocyte cryopreservation, and this may be prohibitive for some patients. Patients must also have access to an appropriate assisted reproductive center, which may further preclude the use of these methods. Embryo or oocyte cryopreservation may not be an option for some patients, such as prepubertal girls and women with hormone-sensitive malignancies who cannot or choose not to be exposed to the high levels of estrogen needed for ovarian stimulation. Letrozole use during ovarian stimulation, however, has had a significant impact on reducing serum estrogen levels during ovarian stimulation, making this a more accessible option for women with breast and endometrial cancer. Last, cryopreservation of embryos and oocytes also focuses solely on preservation of fertility, without providing any affect on long-term ovarian function and the systemic sequelae that are associated with POF.
For some patients, OTC may be an important option to consider. OTC can be performed when time constraints are a factor. For some patients, the urgency of their treatment may preclude in vitro fertilization as an option, even with random start protocols; the controlled ovarian stimulation process generally takes at least 2 weeks. OTC is not a suitable option for women with ovarian or hematologic malignancies and those with tumors prone to metastasize to the ovaries. OTC techniques have continued to evolve since first developed >20 years ago in animal models. A retrospective cohort study of Danish women has demonstrated encouraging results, with a positive pregnancy test in 63% (21/32 patients) and 13 live births. Additionally, for some of the cohort, the graft longevity has lasted upwards of 7–10 years, with a large number of individuals experiencing graft longevity of 2–4 years. Other studies have also reported an average graft survival of 4–5 years, with some reports of grafts that lasted >7 years. Ovarian activity with premenopausal hormonal profiles, and in many cases menstrual cycles, has been reported for upwards of 10 years after transplantation. Although the primary utility of OTC has been for fertility preservation, it has also been proposed that ovarian tissue grafts may be a novel method to postpone menopause by restoring the hormonal milieu and thereby alleviating some of the menopause-related medical conditions in the aging population. This interesting proposal would require a considerable amount of further investigation; however, it does pose a unique solution that could have major impact on the physiologic process of reproductive aging.
It is important to note that OTC currently is considered experimental, and its use is limited to research centers with institutional review board approval. The American Society for Reproductive Medicine committee opinion recommends the use of OTC when the benefits outweigh the risks that are associated with more traditional fertility preservation methods, such as patients with treatment time constraints or hormone-sensitive malignancies. Although OTC has the potential to affect both fertility and endocrine outcomes, clinical data are still limited with conflicting results. To date, optimal techniques for cryopreservation, ideal location for graft transplantation, and duration of graft survival remain not entirely clear. Additionally, the percentage of patients who regain ovarian function and become pregnant after autotransplantation is difficult to discern, likely because of publication bias. Last, OTC requires a laparoscopic procedure, which has inherent surgical and anesthetic risks and associated costs.
The use of GnRHa during chemotherapy is still a controversial, albeit appealing, method for many patients and providers alike. GnRHa are associated with a relatively low risk, time, and cost. Typical side-effects during treatment are related to hypoestrogenic state and include hot flashes, vaginal dryness, mood swings, depression, amenorrhea or spotting, and headaches. A localized allergic reaction at the injection site may occur. Anaphylaxis is very rare. The treatment-associated amenorrhea may be especially helpful for patients with anemia secondary to abnormal uterine bleeding.
GnRHa may also protect reproductive potential. GnRHa can be useful for the preservation of gonadal function to alleviate hypogonadal consequences. Most evidence to this effect has been derived from cotreatment with the standard chemotherapeutic regimens for patients with breast cancer or lymphoma. GnRHa often are used in conjunction with standard chemotherapy for breast cancer in estrogen receptor–positive cancers. Studies have shown that GnRHa are safe, with no impact on grade 3 or 4 side-effect incidence during chemotherapy. Additionally, GnRHa are often beneficial as adjuvant treatment in combination with chemotherapy for a subset of patients. Patients with breast cancer, including those with estrogen receptor positive tumors, who received GnRHa cotreatment had increased or no impact on disease-free survival and overall survival compared with chemotherapy alone. In the Prevention of Early Menopause Study (POEMS), a trend towards a higher rate of disease-free survival in those individuals who were treated with GnRHa was observed as was a statistically significant higher rate of overall survival in this group compared with those who were treated with chemotherapy alone ( P =.04). Similarly, in the study by Lambertini et al, a trend towards improved 5-year disease-free survival was observed in the GnRHa group vs control subjects ( P =.52).
Regarding the impact of GnRHa on improving fertility potential, a positive impact has been noted in several studies to date. In the POEMS trial, women in the GnRHa group had a higher pregnancy rate than those in the chemotherapy alone group (odds ratio, 2.45; 95% confidence interval, 1.09–5.51; P =.03). Other randomized controlled trials (RCTs) have also looked at posttreatment pregnancy outcomes but report very small numbers in both the treatment and control groups. These data must be interpreted with caution, because a variety of confounders such as patient age, psychologic impact of cancer diagnosis, and precancer parity may all affect one’s desire to pursue pregnancy after treatment. Two cohort studies also investigated the impact of GnRHa cotreatment on pregnancy outcomes in women with breast cancer and found spontaneous pregnancy rates of nearly 70% in those who attempted to conceive. Taken together with the POEMS data, these findings are encouraging for a protective impact of GnRHa on future fertility.
To date the American Society for Reproductive Medicine recommends use of GnRHa in concert with other fertility preservation methods for patients who desire future pregnancies. A recent expert meeting on cancer and fertility preservation also recommended that GnRHa treatment be considered a “reliable strategy to preserve ovarian function and fertility” in light of data that support its efficacy, while still underscoring the experimental nature of the procedure. Additionally, the National Comprehensive Cancer Network and the St. Gallen International Expert Consensus panel guidelines support the use of GnRHa for the prevention of ovarian failure secondary to gonadotoxic chemotherapy. For individuals who have completed childbearing, GnRHa with the goal of preserving of ovarian function is also important to consider.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


