FIGURE 9-1 Percentage of women in the United States with prenatal care beginning in the first trimester by ethnicity in 1989, 2001, and 2006. (Adapted from Martin, 2002b, 2009.)
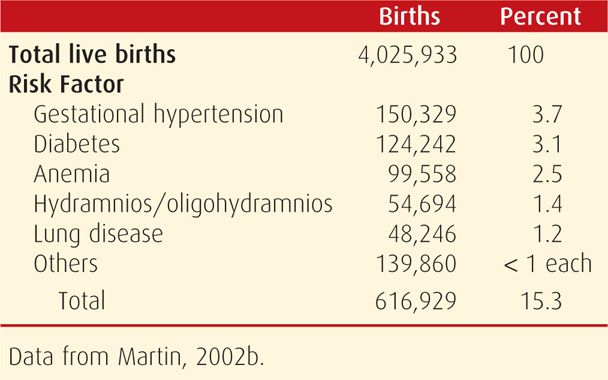
TABLE 9-1. Obstetrical and Medical Risk Factors Detected During Prenatal Care in the United States in 2001
 Assessing Prenatal Care Adequacy
Assessing Prenatal Care Adequacy
A commonly employed system for measuring prenatal care adequacy is the index of Kessner and colleagues (1973). This Kessner Index incorporates three items from the birth certificate: length of gestation, timing of the first prenatal visit, and number of visits. Although it does not measure the quality of care, the index remains a useful measure of prenatal care adequacy. Using this index, the National Center for Health Statistics concluded that 12 percent of American women who were delivered in 2000 received inadequate prenatal care (Martin, 2002a).
The Centers for Disease Control and Prevention (2000) analyzed birth certificate data for the years 1989 to 1997 and found that half of women with delayed or no prenatal care wanted to begin care earlier. Barriers to care varied by social and ethnic group, age, and payment method. The most common reason cited was late identification of pregnancy by the patient. The second most commonly cited barrier was lack of money or insurance. The third was inability to obtain an appointment.
 Prenatal Care Effectiveness
Prenatal Care Effectiveness
Care designed during the early 1900s focused on lowering the extremely high maternal mortality rate. Such care undoubtedly contributed to the dramatic decline in this rate from 690 per 100,000 births in 1920 to 50 per 100,000 by 1955 (Loudon, 1992). As discussed in Chapter 1 (p. 5), the relatively low current maternal mortality rate of approximately 10 to 15 per 100,000 is likely associated with the high utilization of prenatal care (Xu, 2010). Indeed, in their analysis of data from 1998 to 2005 from the Pregnancy Mortality Surveillance System (PRAMS), Berg and associates (2010) identified a fivefold increased risk for maternal death in women who received no prenatal care.
There are other studies that attest to the efficacy of prenatal care. Herbst and colleagues (2003) found that lack of prenatal care was associated with more than a twofold increased risk of preterm birth. National Center for Health Statistics data showed that women with prenatal care had an overall stillbirth rate of 2.7 per 1000 compared with 14.1 per 1000 for women without prenatal care (Vintzileos, 2002a). These same investigators later reported that prenatal care was associated with lower rates of preterm birth as well as neonatal death associated with placenta previa, fetal-growth restriction, and postterm pregnancy (Vintzileos, 2002b, 2003). Evaluating the format of care, Ickovics and associates (2007) compared individual prenatal care and group prenatal care. The latter provided traditional pregnancy surveillance in a group setting with special focus on support, education, and active health-care participation. Women enrolled in group prenatal care had significantly reduced preterm birth rates compared with those receiving individual care.
DIAGNOSIS OF PREGNANCY
Pregnancy is usually identified when a woman presents with symptoms and possibly a positive home urine pregnancy test result. Typically, such women receive confirmatory testing of urine or blood for human chorionic gonadotropin (hCG). Further, there may be presumptive or diagnostic findings of pregnancy during examination. Sonography is often used, particularly if miscarriage or ectopic pregnancy is a concern.
 Signs and Symptoms
Signs and Symptoms
Amenorrhea
The abrupt cessation of menstruation in a healthy reproductive-aged woman who previously has experienced spontaneous, cyclical, predictable menses is highly suggestive of pregnancy. As discussed in Chapter 5 (p. 80), menstrual cycles vary appreciably in length among women and even in the same woman. Thus, amenorrhea is not a reliable pregnancy indicator until 10 days or more after expected menses. Occasionally, uterine bleeding occurs after conception and can be somewhat suggestive of menstruation. During the first month of pregnancy, such episodes are likely the consequence of blastocyst implantation. Still, first-trimester bleeding should generally prompt evaluation for an abnormal pregnancy.
Lower-Reproductive-Tract Changes
During pregnancy, the vaginal mucosa usually appears dark-bluish red and congested—Chadwick sign, popularized by him in 1886. Although presumptive evidence of pregnancy, it is not conclusive. Also, there is increased cervical softening as pregnancy advances. Other conditions, however, such as estrogen–progestin contraceptives, may cause similar softening. As pregnancy progresses, the external cervical os and cervical canal may become sufficiently patulous to admit a fingertip, but the internal os should remain closed.
The substantial increase in progesterone secretion associated with pregnancy affects the consistency and microscopic appearance of cervical mucus. Specifically, microscopic observation of a fernlike pattern of mucus, which is typically seen in the midportion of the menstrual cycle, makes pregnancy unlikely (Fig. 4-2, p. 49).
Uterine Changes
During the first few weeks of pregnancy, uterine size grows principally in the anteroposterior diameter. During bimanual examination, it feels doughy or elastic. At 6 to 8 weeks’ menstrual age, the firm cervix contrasts with the now softer fundus and the compressible interposed softened isthmus—Hegar sign. Isthmic softening may be so marked that the cervix and uterine body seem to be separate organs. By 12 weeks’ gestation, the uterine body is almost globular, with an average diameter of 8 cm.
In later pregnancy, using a stethoscope for auscultation, one may hear the uterine souffle. This is a soft, blowing sound that is synchronous with the maternal pulse. It is produced by the passage of blood through the dilated uterine vessels and is heard most distinctly near the lower portion of the uterus. In contrast, the funic souffle is a sharp, whistling sound that is synchronous with the fetal pulse. It is caused by the rush of blood through the umbilical arteries and may not be heard consistently. Fetal heart tones can also be heard and are described on page 176.
Breast and Skin Changes
Anatomical changes in the breasts that accompany pregnancy are characteristic during a first pregnancy (Chap. 4, p. 50). These are less obvious in multiparas, whose breasts may contain a small amount of milky material or colostrum for months or even years after the birth of their last child, especially if the child was breast fed.
Increased pigmentation and visual changes in abdominal striae are common to, but not diagnostic of, pregnancy. They may be absent during pregnancy and may also be seen in women taking estrogen-containing contraceptives.
Fetal Movement
Maternal perception of fetal movement depends on factors such as parity and habitus. In general, after a first successful pregnancy, a woman may first perceive fetal movements between 16 and 18 weeks’ gestation. A primigravida may not appreciate fetal movements until approximately 2 weeks later. At about 20 weeks, depending on maternal habitus, an examiner can begin to detect fetal movements.
 Pregnancy Tests
Pregnancy Tests
Detection of hCG in maternal blood and urine is the basis for endocrine assays of pregnancy. This hormone is a glycoprotein with high carbohydrate content. There are subtle hCG variants, and these differ by their carbohydrate moieties. The general structure of hCG is a heterodimer composed of two dissimilar subunits, designated α and β, which are noncovalently linked. As described in Chapter 5 (p. 101), the α-subunit is identical to those of luteinizing hormone (LH), follicle-stimulating hormone (FSH), and thyroid-stimulating hormone (TSH). HCG prevents involution of the corpus luteum, which is the principal site of progesterone formation during the first 6 weeks of pregnancy.
Syncytiotrophoblast produce hCG in amounts that increase exponentially during the first trimester following implantation. With a sensitive test, the hormone can be detected in maternal serum or urine by 8 to 9 days after ovulation. The doubling time of serum hCG concentration is 1.4 to 2.0 days. As shown in Figure 9-2, serum hCG levels increase from the day of implantation and reach peak levels at 60 to 70 days. Thereafter, the concentration declines slowly until a plateau is reached at approximately 16 weeks.
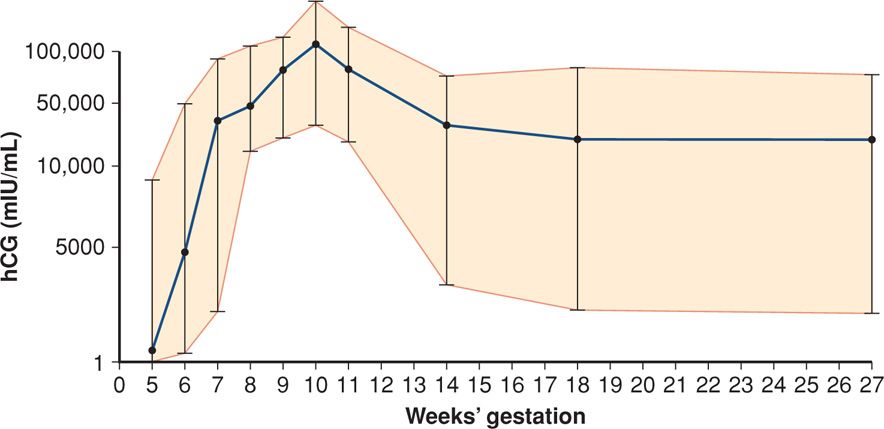
FIGURE 9-2 Mean concentration (95% CI) of human chorionic gonadotropin (hCG) in serum of women throughout normal pregnancy.
Measurement of hCG
As noted, hCG is composed of both an α- and a β-subunit, but the β-subunit is structurally distinct from that of LH, FSH, and TSH. With this recognition, antibodies were developed with high specificity for the hCG β-subunit. This specificity allows its detection, and numerous commercial immunoassays are available for measuring serum and urine hCG levels. Although each immunoassay detects a slightly different mixture of hCG variants, its free subunits, or its metabolites, all are appropriate for pregnancy testing (Cole, 1998).
One commonly employed technique is the sandwich-type immunoassay. With this test, a monoclonal antibody against the β-subunit is bound to a solid-phase support. The attached antibody is then exposed to and binds hCG in the serum or urine specimen. A second antibody is then added, binds to another site on the hCG molecule, and “sandwiches” the bound hCG between the two antibodies. In some assays, the second antibody is linked to an enzyme, such as alkaline phosphatase. When substrate for the enzyme is added, a color develops. The color intensity is proportional to the amount of enzyme and thus to the amount of the second antibody bound. This, in turn, is a function of the hCG concentration in the test sample. The sensitivity for the laboratory detection of hCG in serum is as low as 1.0 mIU/mL using this technique. With extremely sensitive immunoradiometric assays, the detection limit is even lower (Wilcox, 2001).
False-positive hCG test results are rare (Braunstein, 2002). A few women have circulating serum factors that may bind erroneously with the test antibody directed to hCG in a given assay. The most common factors are heterophilic antibodies. These are produced by an individual and bind to the animal-derived test antibodies used in a given immunoassay. Thus, women who have worked closely with animals are more likely to develop such antibodies, and alternative laboratory techniques are available (American College of Obstetricians and Gynecologists, 2013a). Elevated hCG levels may also reflect molar pregnancy and its associated cancers (Chap. 20, p. 396). Other rare causes of positive assays without pregnancy are: (1) exogenous hCG injection used for weight loss, (2) renal failure with impaired hCG clearance, (3) physiological pituitary hCG, and (4) hCG-producing tumors that most commonly originate from gastrointestinal sites, ovary, bladder, or lung (Montagnana, 2011).
Home Pregnancy Tests
Millions of over-the-counter pregnancy test kits are sold annually in the United States. In one study, Cole and associates (2011) found that a detection limit of 12.5 mIU/mL would be required to diagnose 95 percent of pregnancies at the time of missed menses. They noted that only one brand had this degree of sensitivity. Two other brands gave false-positive or invalid results. In fact, with an hCG concentration of 100 mIU/mL, clearly positive results were displayed by only 44 percent of brands. As such, only about 15 percent of pregnancies could be diagnosed at the time of the missed menses. Some manufacturers of even newer home urine assays claim > 99-percent accuracy on the day of—and some up to 4 days before—the expected day of menses. But, careful analysis suggests that these assays are often not as sensitive as advertised (Cole, 2011).
 Sonographic Recognition of Pregnancy
Sonographic Recognition of Pregnancy
Transvaginal sonography has revolutionized early pregnancy imaging and is commonly used to accurately establish gestational age and confirm pregnancy location. A gestational sac—a small anechoic fluid collection within the endometrial cavity—is the first sonographic evidence of pregnancy. It may be seen with transvaginal sonography by 4 to 5 weeks’ gestation. A fluid collection, however, can also be seen within the endometrial cavity with an ectopic pregnancy and is termed a pseudogestational sac or pseudosac (Fig. 19-5, p. 382). Thus, further evaluation may be warranted if this is the only sonographic finding, particularly in a patient with pain or bleeding. A normal gestational sac implants eccentrically in the endometrium, whereas a pseudosac is seen in the midline of the endometrial cavity. Other potential indicators of early intrauterine pregnancy are an anechoic center surrounded by a single echogenic rim—the intradecidual sign—or two concentric echogenic rings surrounding the gestational sac—the double decidual sign (Fig. 9-3) (Chiang, 2004). If sonography yields equivocal findings—the so-called pregnancy of unknown location, then serial serum hCG levels can also help differentiate a normal intrauterine pregnancy from an extrauterine pregnancy or an early miscarriage (Chap. 19, p. 381).
Visualization of the yolk sac—a brightly echogenic ring with an anechoic center—confirms with certainty an intrauterine location for the pregnancy and can normally be seen by the middle of the fifth week. As shown in Figure 9-3, after 6 weeks, an embryo is seen as a linear structure immediately adjacent to the yolk sac, and cardiac motion is typically noted at this point. Up to 12 weeks’ gestation, the crown-rump length is predictive of gestational age within 4 days (Chap. 10, p. 195).
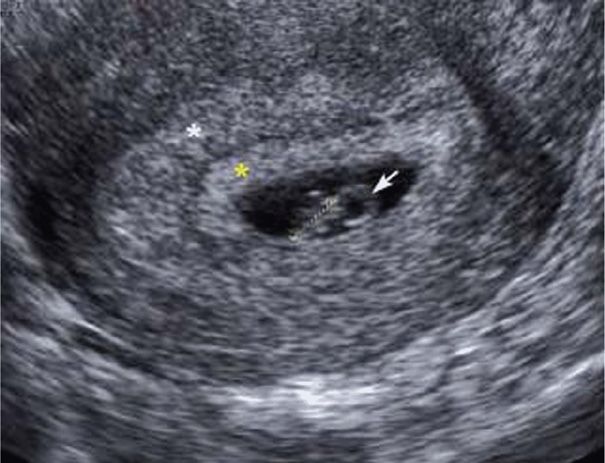
FIGURE 9-3 Transvaginal sonogram of a first-trimester intrauterine pregnancy. The double decidual sign is noted surrounding the gestational sac and is defined by the decidua parietalis (white asterisk) and the decidua capsularis (yellow asterisk). The arrow notes the yolk sac, and the crown-rump length of the embryo is marked with measuring calipers. (Image contributed by Dr. Elysia Moschos.)
INITIAL PRENATAL EVALUATION
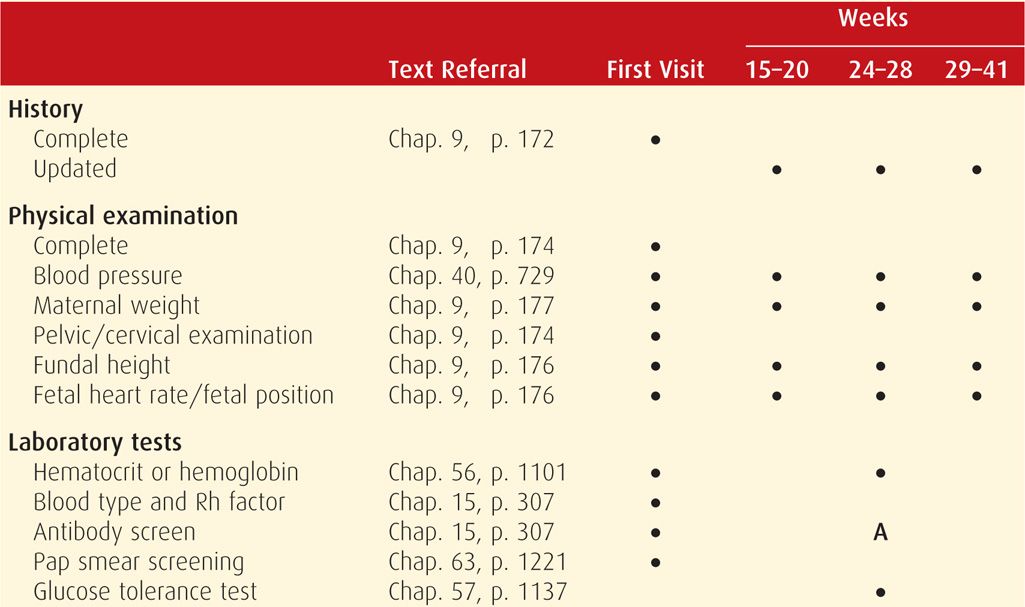
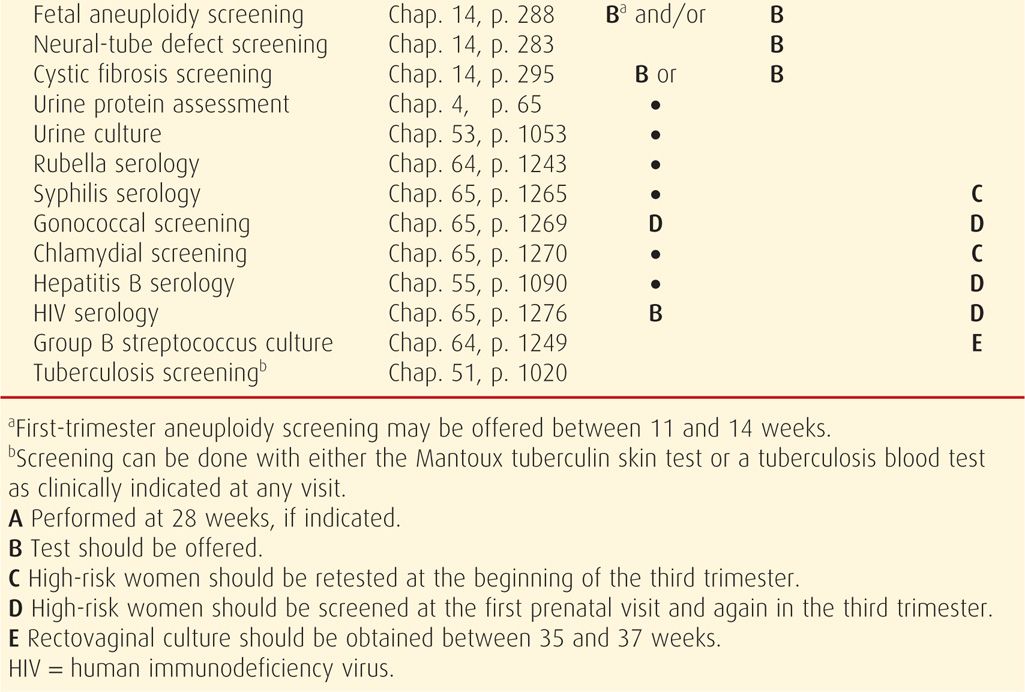
Prenatal care should be initiated as soon as there is a reasonable likelihood of pregnancy. Major goals are to: (1) define the health status of the mother and fetus, (2) estimate the gestational age, and (3) initiate a plan for continuing obstetrical care. Typical components of the initial visit are summarized in Table 9-2. The initial plan for subsequent care may range from relatively infrequent routine visits to prompt hospitalization because of serious maternal or fetal disease.
TABLE 9-2. Typical Components of Routine Prenatal Care
 Prenatal Record
Prenatal Record
Use of a standardized record within a perinatal health-care system greatly aids antepartum and intrapartum management. Standardizing documentation may allow communication and care continuity between providers and enable objective measures of care quality to be evaluated over time and across different clinical settings (Gregory, 2006). A prototype is provided by the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (2012) in their Guidelines for Perinatal Care, 7th edition.
Definitions
There are several definitions pertinent to establishment of an accurate prenatal record.
1. Nulligravida—a woman who currently is not pregnant nor has ever been pregnant.
2. Gravida—a woman who currently is pregnant or has been in the past, irrespective of the pregnancy outcome. With the establishment of the first pregnancy, she becomes a primigravida, and with successive pregnancies, a multigravida.
3. Nullipara—a woman who has never completed a pregnancy beyond 20 weeks’ gestation. She may not have been pregnant or may have had a spontaneous or elective abortion(s) or an ectopic pregnancy.
4. Primipara—a woman who has been delivered only once of a fetus or fetuses born alive or dead with an estimated length of gestation of 20 or more weeks. In the past, a 500-g birthweight threshold was used to define parity. As discussed in Chapter 1 (p. 2), this threshold is now controversial because many states still use this weight to differentiate a stillborn fetus from an abortus. However, the survival of neonates with birthweights < 500 g is no longer uncommon.
5. Multipara—a woman who has completed two or more pregnancies to 20 weeks’ gestation or more. Parity is determined by the number of pregnancies reaching 20 weeks. It is not increased to a higher number if multiples are delivered in a given pregnancy. Moreover, stillbirth does not lower this number. In some locales, the obstetrical history is summarized by a series of digits connected by dashes. These refer to the number of term infants, preterm infants, abortuses younger than 20 weeks, and children currently alive. For example, a woman who is para 2–1–0–3 has had two term deliveries, one preterm delivery, no abortuses, and has three living children. Because these are nonconventional, it is helpful to specify the outcome of any pregnancy that did not end normally.
Normal Pregnancy Duration
The mean duration of pregnancy calculated from the first day of the last normal menstrual period is very close to 280 days or 40 weeks. In a study of 427,581 singleton pregnancies from the Swedish Birth Registry, Bergsjø and coworkers (1990) found that the mean pregnancy duration was 281 days with a standard deviation of 13 days.
It is customary to estimate the expected delivery date by adding 7 days to the date of the first day of the last normal menstrual period and counting back 3 months—Naegele rule. For example, if the last menstrual period began September 10, the expected date of delivery is June 17. However, a gestational age or menstrual age calculated in this way assumes pregnancy to have begun approximately 2 weeks before ovulation, which is not always the case.
Clinicians use this gestational age to mark temporal events during pregnancy. In contrast, embryologists and other reproductive biologists more often employ ovulatory age or fertilization age, both of which are typically 2 weeks earlier. Somewhat related, Bracken and Belanger (1989) tested the accuracy of various “pregnancy wheels” provided by three pharmaceutical companies and found that such devices predicted incorrect delivery dates in 40 to 60 percent of estimates, with a 5-day error being typical. As physicians and hospitals increasingly transition to electronic medical records, however, such errors should be largely obviated by more precise estimates of gestational age produced by calculator software applications.
Trimesters
It has become customary to divide pregnancy into three equal epochs of approximately 3 calendar months. Historically, the first trimester extends through completion of 14 weeks, the second through 28 weeks, and the third includes the 29th through 42nd weeks of pregnancy. Thus, there are three periods of 14 weeks each. Certain major obstetrical problems tend to cluster in each of these time periods. For example, most spontaneous abortions take place during the first trimester, whereas most women with hypertensive disorders due to pregnancy are diagnosed during the third trimester.
In modern obstetrics, the clinical use of trimesters to describe a specific pregnancy is imprecise. For example, it is inappropriate in cases of uterine hemorrhage to categorize the problem temporally as “third-trimester bleeding.” Appropriate management for the mother and her fetus will vary remarkably depending on whether bleeding begins early or late in the third trimester (Chap. 41, p. 782). Because precise knowledge of fetal age is imperative for ideal obstetrical management, the clinically appropriate unit is weeks of gestation completed. And more recently, clinicians designate gestational age using completed weeks and days, for example, 334/7 weeks or 33 + 4, for 33 completed weeks and 4 days.
 Previous and Current Health Status
Previous and Current Health Status
For the most part, the same essentials go into appropriate history taking from the pregnant woman as elsewhere in medicine. In addition to queries concerning medical or surgical disorders, detailed information regarding previous pregnancies is essential as many obstetrical complications tend to recur in subsequent pregnancies.
The menstrual history is also important. The woman who spontaneously menstruates approximately every 28 days is most likely to ovulate at midcycle. Thus, gestational or menstrual age is the number of weeks since the onset of the last menstrual period. If her menstrual cycles were significantly longer than 28 to 30 days, ovulation more likely occurred well beyond 14 days. If the intervals were much longer and irregular, chronic anovulation is likely to have preceded some of the episodes identified as menses. Thus, without a history of regular, predictable, cyclic, spontaneous menses that suggest ovulatory cycles, accurate dating of pregnancy by history and physical examination is difficult.
It is also important to ascertain whether or not steroidal contraceptives were used before the pregnancy. Because ovulation may not have resumed 2 weeks after the onset of the last withdrawal bleeding and instead may have occurred at an appreciably later and highly variable date, using the time of ovulation for predicting the time of conception in this circumstance may be erroneous. Use of sonography in early pregnancy will clarify gestational age in these situations.
Psychosocial Screening
The American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (2012) define psychosocial issues as nonbiomedical factors that affect mental and physical well-being. Women should be screened regardless of social status, education level, race, or ethnicity. Such screening should seek barriers to care, communication obstacles, nutritional status, unstable housing, desire for pregnancy, safety concerns that include intimate partner violence, depression, stress, and use of substances such as tobacco, alcohol, and illicit drugs. This screening should be performed on a regular basis, at least once per trimester, to identify important issues and reduce adverse pregnancy outcomes. Coker and colleagues (2012) compared pregnancy outcomes in women before and after implementation of a universal psychosocial screening program and found that screened women were less likely to have preterm or low-birthweight newborns. Although this study was observational, these investigators also reported decreased rates of gestational diabetes, premature rupture of membranes, and vaginal bleeding in women who underwent universal screening.
Cigarette Smoking
There are unequivocal adverse perinatal sequelae for smoking (United States Department of Health and Human Services, 2000). Such data have been included on the birth certificate since 1989. The number of pregnant women who smoke continues to decline. From 2000 to 2010, the prevalences were 12 to 13 percent (Tong, 2013). Based on the Pregnancy Risk Assessment Monitoring System (PRAMS), 13 percent of women admitted to smoking. These women were more likely younger, had less education, and were either Alaska Natives or American Indians (Centers for Disease Control and Prevention, 2012b).
Numerous adverse outcomes have been linked to smoking during pregnancy. Potential teratogenic effects are reviewed in Chapter 12 (p. 255). There is a twofold risk of placenta previa, placental abruption, and premature membrane rupture compared with nonsmokers. Further, neonates born to women who smoke are more likely to be preterm, have lower birth-weights, and are more likely to die of sudden infant death syndrome (SIDS) than infants born to nonsmokers (Tong, 2009). In 2005, the incidence of low-birthweight infants born to American women who smoked during pregnancy was 11.9 percent compared with 7.5 percent born to nonsmokers (Martin, 2007). Risks for spontaneous abortion, fetal death, and fetal digital anomalies are also increased (Man, 2006). Finally, children who were exposed to smoking in utero are at increased risk for asthma, infantile colic, and childhood obesity (American College of Obstetricians and Gynecologists, 2013i).
Several pathophysiological mechanisms have been proposed to explain these adverse outcomes. They include fetal hypoxia from increased carboxyhemoglobin, reduced uteroplacental blood flow, and direct toxic effects of nicotine and other compounds in smoke (Jazayeri, 1998). Nicotine transfer is so efficient that fetal nicotine exposure is greater than that of the mother (Luck, 1985). Exposed fetuses have decreased heart rate variability due to impaired autonomic regulation (Zeskind, 2006).
Smoking Cessation. The United States Department of Health and Human Services recommends that clinicians offer counseling and effective intervention options to pregnant smokers at the first and subsequent prenatal visits. Although benefits are greatest if smoking ceases early in pregnancy or preferably preconceptionally, quitting at any stage of pregnancy can improve perinatal outcomes (England, 2001; Fiore, 2008).
Person-to-person psychosocial interventions are significantly more successful in achieving smoking abstinence in pregnancy than are simple advisements to quit (Fiore, 2008). One example is a brief counseling session covering the “5As” of smoking cessation (Table 9-3). This approach to counseling can be accomplished in 15 minutes or less and has been proven to be effective when initiated by health-care providers (American College of Obstetricians and Gynecologists, 2013i).
TABLE 9-3. Five A’s of Smoking Cessation
ASK about smoking at the first and subsequent prenatal visits. To improve assessment accuracy, a patient should choose the following statement that best describes her smoking status:
I smoke regularly now; the same as before pregnancy.
I smoke regularly now, but I’ve cut down with pregnancy. I smoke every once in a while.
I have quit smoking since pregnancy.
I wasn’t smoking before pregnancy, and I do not currently smoke.
If smoking abstinence has already begun, then reinforce her decision to quit, congratulate her on success, and encourage her continued abstinence. For persistent smokers, proceed to the following steps:
ADVISE with clear, strong statements that explain the risks of continued smoking to the woman, fetus, and newborn.
ASSESS the patient’s willingness to attempt cessation.
ASSIST with pregnancy-specific, self-help smoking cessation materials. Offer a direct referral to the smoker’s quit line (1–800-QUIT NOW) to provide ongoing counseling and support.
ARRANGE to track smoking abstinence progress at subsequent visits.
Nicotine replacement products have not been sufficiently evaluated to determine their effectiveness and safety in pregnancy. Trials evaluating such therapy have yielded conflicting evidence. Wisborg and colleagues (2000) randomly assigned 250 women who smoked at least 10 cigarettes per day to receive a nicotine or placebo patch beginning after the first trimester. There were no significant differences in birthweights or in smoking cessation or preterm delivery rates between the two groups. Pollak and associates (2007) randomized 181 pregnant smokers to cognitive-behavioral therapy alone versus this therapy plus nicotine replacement. They identified significantly improved smoking cessation rates in the women with nicotine replacement at 7 weeks after randomization and at 38 weeks’ gestation. The trial was terminated early due to an increased rate of negative birth outcomes in the nicotine replacement arm. Some of these included neonatal intensive care unit admission, small-for-gestational age, and placental abruption. Because of limited available evidence to support pharmacotherapy for smoking cessation in pregnancy, the American College of Obstetricians and Gynecologists (2013i) has recommended that if nicotine replacement therapy is used, it should be done with close supervision and after careful consideration of the risks of smoking versus nicotine replacement.
Alcohol
Ethyl alcohol or ethanol is a potent teratogen that causes a fetal syndrome characterized by growth restriction, facial abnormalities, and central nervous system dysfunction (Chap. 12, p. 245). Women who are pregnant or considering pregnancy should abstain from using any alcoholic beverages. The Centers for Disease Control and Prevention (2012a) analyzed data from the Behavioral Risk Factor Surveillance System from 2006 to 2010 and estimated that 7.6 percent of pregnant women used alcohol and 1.4 percent reported binge drinking. By comparison, in 1999, rates of alcohol use and binge drinking were estimated to be 12.8 and 2.7 percent, respectively (Centers for Disease Control and Prevention, 2002). Among pregnant women, those most likely to use alcohol were aged 35 to 44 years, white, college graduates, or employed. The American College of Obstetricians and Gynecologists (2008), in their committee opinion on this topic, has reviewed methods for screening women during pregnancy for alcohol abuse and for illicit drug use.
Illicit Drugs
It is estimated that 10 percent of fetuses are exposed to one or more illicit drugs (American Academy of Pediatrics and the American College of Obstetricians and Gynecologists, 2012). Agents may include heroin and other opiates, cocaine, amphetamines, barbiturates, and marijuana. Chronic use of large quantities is harmful to the fetus (Chap. 12, p. 253). Well-documented sequelae include fetal-growth restriction, low birthweight, and drug withdrawal soon after birth. Women who use such drugs frequently do not seek prenatal care, or if they do, they may not admit to substance abuse. El-Mohandes and associates (2003) reported that when women who use illicit drugs receive prenatal care, the risks for preterm birth and low birthweight are reduced.
For women who abuse heroin, methadone maintenance can be initiated within a registered methadone treatment program to reduce complications of illicit opioid use and narcotic withdrawal, to encourage prenatal care, and to avoid drug culture risks (American College of Obstetricians and Gynecologists, 2012c). Available programs can be found through the treatment locator of the Substance Abuse and Mental Health Services Administration at www.samhsa.gov. Methadone dosages usually are initiated at 10 to 30 mg daily and titrated as needed. Although less commonly used, buprenorphine alone or in combination with naloxone may also be offered and managed by physicians with specific credentialing.
Intimate Partner Violence
This term refers to a pattern of assaultive and coercive behaviors that may include physical injury, psychological abuse, sexual assault, progressive isolation, stalking, deprivation, intimidation, and reproductive coercion (American College of Obstetricians and Gynecologists, 2012a). Such violence has been recognized as a major public health problem. Unfortunately, most abused women continue to be victimized during pregnancy. With the possible exception of preeclampsia, domestic violence is more prevalent than any major medical condition detectable through routine prenatal screening (American Academy of Pediatrics and the American College of Obstetricians and Gynecologists, 2012). The prevalence during pregnancy is estimated to be between 4 and 8 percent. As discussed in Chapter 47 (p. 951), intimate partner violence is associated with an increased risk of several adverse perinatal outcomes including preterm delivery, fetal-growth restriction, and perinatal death.
The American College of Obstetricians and Gynecologists (2012a) has provided methods for domestic violence screening and recommends their use at the first prenatal visit, then again at least once per trimester, and again at the postpartum visit. Such screening should be done privately and away from family members and friends. Patient self-administered or computerized screenings appear to be as effective for disclosure as clinician-directed interviews (Ahmad, 2009; Chen, 2007). Physicians should be familiar with state laws that may require reporting of intimate partner violence. Coordination with social services can be invaluable in such cases. The National Domestic Violence Hotline (1–800–799-SAFE [7233]) is a nonprofit telephone referral service that provides individualized information regarding city-specific women’s shelter locations, counseling resources, and legal advocacy.
 Clinical Evaluation
Clinical Evaluation
A thorough, general physical examination should be completed at the initial prenatal encounter. Many of the expected changes that result from normal pregnancy are addressed throughout Chapter 4 (p. 46).
Pelvic examination is performed as part of the evaluation. The cervix is visualized employing a speculum lubricated with warm water or water-based lubricant gel. Bluish-red passive hyperemia of the cervix is characteristic, but not of itself diagnostic, of pregnancy. Dilated, occluded cervical glands bulging beneath the ectocervical mucosa—nabothian cysts—may be prominent. The cervix is not normally dilated except at the external os. To identify cytological abnormalities, a Pap smear is performed according to current guidelines noted in Chapter 63 (p. 1221). Specimens for identification of Chlamydia trachomatis and Neisseria gonorrhoeae are also obtained when indicated (p. 175).
Bimanual examination is completed by palpation, with special attention given to the consistency, length, and dilatation of the cervix; to uterine and adnexal size; to the bony pelvic architecture; and to any vaginal or perineal anomalies. Later in pregnancy, fetal presentation often can also be determined. Lesions of the cervix, vagina, or vulva should be further evaluated as needed by colposcopy, biopsy, culture, or dark-field examination. The perianal region should be visualized, and digital rectal examination performed as required for complaints of rectal pain, bleeding, or mass.
Gestational Age Assessment
Precise knowledge of gestational age is one of the most important aspects of prenatal care because several pregnancy complications may develop for which optimal treatment will depend on fetal age. Gestational age can be estimated with considerable precision by appropriately timed and carefully performed clinical uterine size examination that is coupled with knowledge of the last menses. Uterine size similar to a small orange roughly correlates with a 6-week gestation; a large orange, with an 8-week pregnancy; and a grapefruit, with one at 12 weeks (Margulies, 2001). That said, a first-trimester crown-rump length is the most accurate tool for gestational age assignment and is performed as clinically indicated. As described in Chapter 10 (p. 198), later sonographic interrogation can also provide an estimated gestational age, but with declining accuracy.
 Laboratory Tests
Laboratory Tests
Recommended routine tests at the first prenatal encounter are listed in Table 9-2. Initial blood tests include a complete blood count, a determination of blood type with Rh status, and an antibody screen. The Institute of Medicine recommends universal human immunodeficiency virus (HIV) testing, with patient notification and right of refusal, as a routine part of prenatal care. The Centers for Disease Control and Prevention (2006) as well as the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (2012) continue to support this practice. If a woman declines testing, this should be recorded in the prenatal record. All pregnant women should also be screened for hepatitis B virus, syphilis, and immunity to rubella at the initial visit. Based on their prospective investigation of 1000 women, Murray and coworkers (2002) concluded that in the absence of hypertension, routine urinalysis beyond the first prenatal visit was not necessary. A urine culture is performed because treating asymptomatic bacteruria significantly reduces the likelihood of developing symptomatic urinary tract infections in pregnancy (Chap. 53, p. 1053).
Cervical Infections
Chlamydia trachomatis is isolated from the cervix in 2 to 13 percent of pregnant women. The American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (2012) recommend that all women be screened for chlamydia during the first prenatal visit, with additional third-trimester testing for those at increased risk. Risk factors include unmarried status, recent change in sexual partner or multiple concurrent partners, age younger than 25 years, inner-city residence, history or presence of other sexually transmitted diseases, and little or no prenatal care. Following treatment, a second testing—a so-called test of cure—is recommended in pregnancy 3 to 4 weeks after treatment completion (Chap. 65, p. 1270).
Neisseria gonorrhoeae is the gram-negative diplococcal bacteria responsible for causing gonorrhea. Risk factors for gonorrhea are similar for those for chlamydial infection. The American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (2012) recommend that pregnant women with risk factors or those living in an area of high N gonorrhoeae prevalence be screened at the initial prenatal visit and again in the third trimester. Treatment is given for gonorrhea as well as possible coexisting chlamydial infection, as outlined in Chapter 65 (p. 1269). Test of cure is also recommended following treatment.
 Pregnancy Risk Assessment
Pregnancy Risk Assessment
Many factors exist that can adversely affect maternal and/or fetal well-being. Some are evident at conception, but many become apparent during the course of pregnancy. The designation of “high-risk pregnancy” is overly vague for an individual patient and probably should be avoided if a more specific diagnosis has been assigned. Some common risk factors for which consultation is recommended by the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (2012) are shown in Table 9-4. Some conditions may require the involvement of a maternal-fetal medicine subspecialist, geneticist, pediatrician, anesthesiologist, or other medical specialist in the evaluation, counseling, and care of the woman and her fetus.
TABLE 9-4. Conditions for Which Maternal-Fetal Medicine Consultation May Be Beneficial
Medical History and Conditions
Cardiac disease—including cyanotic, prior myocardial infarction, moderate to severe valvular stenosis or regurgitation, Marfan syndrome, prosthetic valve, American Heart Association class II or greater
Diabetes mellitus with evidence of end-organ damage or uncontrolled hyperglycemia
Family or personal history of genetic abnormalities
Hemoglobinopathy
Chronic hypertension if uncontrolled or associated with renal or cardiac disease
Renal insufficiency if associated with significant proteinuria (≥ 500 mg/24 hour), serum creatinine ≥ 1.5 mg/dL, or hypertension
Pulmonary disease if severe restrictive or obstructive, including severe asthma
Human immunodeficiency virus infection
Prior pulmonary embolus or deep-vein thrombosis
Severe systemic disease, including autoimmune conditions
Bariatric surgery
Epilepsy if poorly controlled or requires more than one anticonvulsant
Cancer, especially if treatment is indicated in pregnancy
Obstetrical History and Conditions
CDE (Rh) or other blood group alloimmunization (excluding ABO, Lewis)
Prior or current fetal structural or chromosomal abnormality
Desire or need for prenatal diagnosis or fetal therapy
Periconceptional exposure to known teratogens
Infection with or exposure to organisms that cause congenital infection
Higher-order multifetal gestation
Severe disorders of amnionic fluid volume
SUBSEQUENT PRENATAL VISITS
Subsequent prenatal visits have been traditionally scheduled at 4-week intervals until 28 weeks, then every 2 weeks until 36 weeks, and weekly thereafter. Women with complicated pregnancies often require return visits at 1- to 2-week intervals. For example, in twin pregnancies, Luke and colleagues (2003) found that a specialized prenatal care program emphasizing nutrition and education and requiring return visits every 2 weeks resulted in improved outcomes.
In 1986, the Department of Health and Human Services convened an expert panel to review the content of prenatal care. This report was subsequently reevaluated and revised in 2005 (Gregory, 2006). The panel recommended, among other things, early and continuing risk assessment that is patient specific. It also endorsed flexibility in clinical visit spacing; health promotion and education, including preconceptional care; medical and psychosocial interventions; standardized documentation; and expanded prenatal care objectives—to include family health up to 1 year after birth.
The World Health Organization (WHO) conducted a multicenter randomized trial with almost 25,000 women comparing routine prenatal care with an experimental model designed to minimize visits (Villar, 2001). In the new model, women were seen once in the first trimester and screened for certain risks. Those without anticipated complications—80 percent of those screened—were seen again at 26, 32, and 38 weeks. Compared with routine prenatal care, which required a median of eight visits, the new model required a median of only five. No disadvantages were attributed to the regimen with fewer visits, and these findings were consistent with other randomized trials (Clement, 1999; McDuffie, 1996).
 Prenatal Surveillance
Prenatal Surveillance
At each return visit, the well-being of mother and fetus are assessed (see Table 9-2). Fetal heart rate, growth, amnionic fluid volume, and activity are evaluated. Maternal blood pressure and weight and their extent of change are assessed. Symptoms such as headache, altered vision, abdominal pain, nausea and vomiting, bleeding, vaginal fluid leakage, and dysuria are sought. Uterine examination measures size from the symphysis to the fundus. In late pregnancy, vaginal examination often provides valuable information that includes confirmation of the presenting part and its station, clinical estimation of pelvic capacity and its general configuration, amnionic fluid volume adequacy, and cervical consistency, effacement, and dilatation (Chap. 22, p. 438).
Fundal Height
Between 20 and 34 weeks, the height of the uterine fundus measured in centimeters correlates closely with gestational age in weeks (Calvert, 1982; Jimenez, 1983; Quaranta, 1981). This measurement is used to monitor fetal growth and amnionic fluid volume. It is measured as the distance along the abdominal wall from the top of the symphysis pubis to the top of the fundus. Importantly, the bladder must be emptied before fundal measurement. Worthen and Bustillo (1980) demonstrated that at 17 to 20 weeks, fundal height was 3 cm higher with a full bladder. Obesity or the presence of uterine masses such as leiomyomata may also limit fundal height accuracy. In such cases, sonography may be necessary for assessment. Moreover, using fundal height alone, fetal-growth restriction may be undiagnosed in up to a third of cases (American College of Obstetricians and Gynecologists, 2013b).
Fetal Heart Sounds
Instruments incorporating Doppler ultrasound are often used to easily detect fetal heart action, and in the absence of maternal obesity, heart sounds are almost always detectable by 10 weeks with such instruments (Chap. 24, p. 474). The fetal heart rate ranges from 110 to 160 beats per minute and is typically heard as a double sound.
Using a standard nonamplified stethoscope, the fetal heart may be audible as early as 16 weeks in some women. Herbert and coworkers (1987) reported that the fetal heart was audible by 20 weeks in 80 percent of women, and by 22 weeks, heart sounds were heard in all. Because the fetus moves freely in amnionic fluid, the site on the maternal abdomen where fetal heart sounds can be heard best will vary.
Sonography
As described in detail in Chapter 10 (p. 199), sonography provides invaluable information regarding fetal anatomy, growth, and well-being, and most women in the United States have at least one prenatal sonographic examination during pregnancy (American College of Obstetricians and Gynecologists, 2011b). Recent trends suggest that the number of these examinations performed per pregnancy is increasing. Siddique and associates (2009) reported that the average number of sonographic evaluations per pregnancy increased from 1.5 in 1995 through 1997 to 2.7 almost 10 years later. This trend was noted in both high- and low-risk pregnancies. The actual clinical utility of increasing sonography use in pregnancy has not been demonstrated, and it is unclear that the cost-benefit ratio is justified (Washington State Health Care Authority, 2010). The American College of Obstetricians and Gynecologists (2011b) has concluded that sonography should be performed only when there is a valid medical indication under the lowest possible ultrasound exposure setting. The College further indicates that a physician is not obligated to perform sonography without a specific indication in a low-risk patient, but that if she requests sonographic screening, it is reasonable to honor her request.
 Subsequent Laboratory Tests
Subsequent Laboratory Tests
If initial results were normal, most tests need not be repeated. Fetal aneuploidy screening may be performed at 11 to 14 weeks and/or at 15 to 20 weeks, depending on the protocol selected as described in Chapter 14. Serum screening for neural-tube defects is offered at 15 to 20 weeks (Chap. 14, p. 284). Hematocrit or hemoglobin determination, along with syphilis serology if it is prevalent in the population, should be repeated at 28 to 32 weeks (Hollier, 2003; Kiss, 2004). For women at increased risk for HIV acquisition during pregnancy, repeat testing is recommended in the third trimester, preferably before 36 weeks’ gestation (American College of Obstetricians and Gynecologists, 2011a). Similarly, women who engage in behaviors that place them at high risk for hepatitis B infection should be retested at the time of hospitalization for delivery (American Academy of Pediatrics and the American College of Obstetricians and Gynecologists, 2012). Women who are D (Rh) negative and are unsensitized should have an antibody screening test repeated at 28 to 29 weeks, with administration of anti-D immune globulin if they remain unsensitized (Chap. 15, p. 311).
Group B Streptococcal Infection
The Centers for Disease Control and Prevention (2010b) recommend that vaginal and rectal group B streptococcal (GBS) cultures be obtained in all women between 35 and 37 weeks’ gestation, and the American College of Obstetricians and Gynecologists (2013g) has endorsed this recommendation. Intrapartum antimicrobial prophylaxis is given for those whose cultures are positive. Women with GBS bacteriuria or a previous infant with invasive disease are given empirical intrapartum prophylaxis. These infections are discussed in detail in Chapter 64 (p. 1249).
Gestational Diabetes
All pregnant women should be screened for gestational diabetes mellitus, whether by history, clinical factors, or routine laboratory testing. Although laboratory testing between 24 and 28 weeks’ gestation is the most sensitive approach, there may be pregnant women at low risk who are less likely to benefit from testing (American Academy of Pediatrics and the American College of Obstetricians and Gynecologists, 2012). Gestational diabetes is discussed in Chapter 57 (p. 1136).
Selected Genetic Screening
Selected screening for certain genetic abnormalities should be offered to those at increased risk based on family history, ethnic or racial background, or age (American College of Obstetricians and Gynecologists, 2009c, 2011c, 2013h). These are discussed in greater detail in Chapters 13 (p. 275) and 14 (p. 294). Some examples include testing for Tay-Sachs disease for persons of Eastern European Jewish or French Canadian ancestry; β-thalassemia for those of Mediterranean, Southeast Asian, Indian, Pakistani, or African ancestry; α-thalassemia for individuals of Southeast Asian or African ancestry; sickle-cell anemia for people of African, Mediterranean, Middle Eastern, Caribbean, Latin American, or Indian descent; and trisomy 21 for those with advanced maternal age.
NUTRITIONAL COUNSELING
 Weight Gain Recommendations
Weight Gain Recommendations
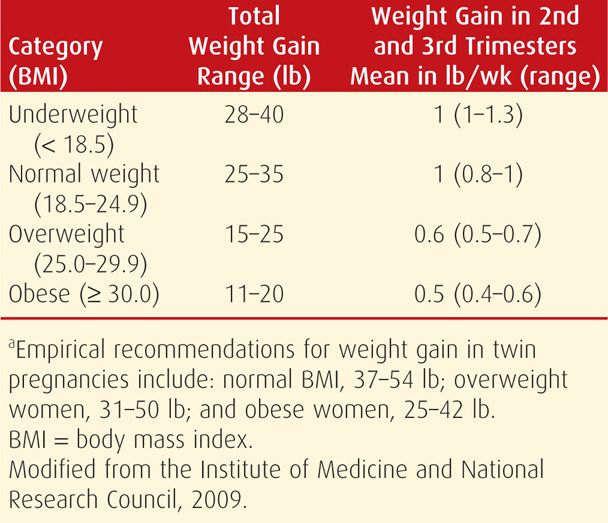
For the first half of the 20th century, it was recommended that weight gain during pregnancy be limited to less than 20 lb or about 9 kg. It was believed that such restriction would prevent gestational hypertension and fetal macrosomia. By the 1970s, however, women were encouraged to gain at least 25 lb or 11 to 12 kg to prevent preterm birth and fetal-growth restriction, a recommendation supported by subsequent research (Ehrenberg, 2003). The Institute of Medicine and National Research Council (2009) revised its guidelines for weight gain in pregnancy and continues to stratify suggested weight gain ranges based on prepregnancy body mass index (BMI) (Table 9-5). BMI can easily be calculated with commonly available graphs (Fig. 48-1, p. 962). Of note, the new guidelines include a specific, relatively narrow range of recommended weight gains for obese women. Also, the same recommendations apply to adolescents, short women, and women of all racial and ethnic groups. The American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (2012) have endorsed these guidelines.
TABLE 9-5. Recommendations for Total and Rate of Weight Gain During Pregnancy, by Prepregnancy BMIa
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


