FIGURE 49-1 Prevalence of risk factors for cardiovascular disease among reproductive-aged women. DM = diabetes mellitus. (From Centers for Disease Control and Prevention, 2011.)
PHYSIOLOGICAL CONSIDERATIONS IN PREGNANCY
 Cardiovascular Physiology
Cardiovascular Physiology
The marked pregnancy-induced anatomical and functional changes in cardiac physiology can have a profound effect on underlying heart disease (Chap. 4, p. 58). Some of these changes are listed in Table 49-1. Importantly, cardiac output increases approximately 40 percent during pregnancy. Almost half of this total increase takes place by 8 weeks and is maximal by midpregnancy (Capeless, 1989). The early increase stems from augmented stroke volume that results from decreased vascular resistance. Later in pregnancy, resting pulse and stroke volume increase even more because of increased end-diastolic ventricular volume that results from pregnancy hypervolemia. This along with an increase in heart rate translates to increased cardiac output that continues to rise across pregnancy to average 40 percent higher at term. These changes are even more profound in multifetal pregnancy (Kametas, 2003; Kuleva, 2011).
TABLE 49-1. Hemodynamic Changes in 10 Normal Pregnant Women at Term Compared with Repeat Values Obtained 12 Weeks Postpartum
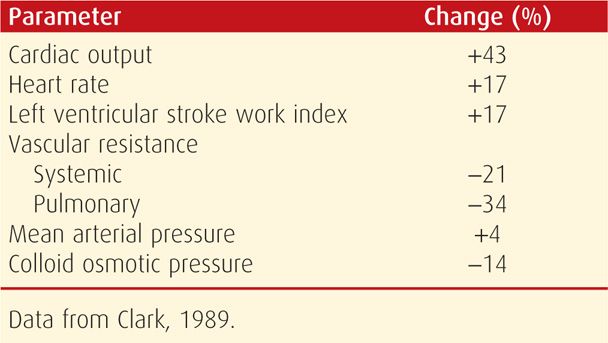
An important study by Clark and colleagues (1989) contributed greatly to the understanding of cardiovascular physiology during pregnancy. Using right-sided heart catheterization, hemodynamic function was measured in 10 healthy primigravid volunteers, and pregnancy values were compared with those measured again at 12 weeks postpartum. As shown in Table 49-1, the cardiac output near term in the lateral recumbent position increased 43 percent. Systemic and pulmonary vascular resistances were concomitantly decreased. Importantly, intrinsic left ventricular contractility did not change. Thus, normal left ventricular function is maintained during pregnancy, that is, pregnancy is not characterized by hyperdynamic function or a high cardiac-output state.
Women with underlying cardiac disease may not always accommodate these changes, and ventricular dysfunction leads to cardiogenic heart failure. A few women with severe cardiac dysfunction may experience evidence of heart failure before midpregnancy. In others, heart failure may develop after 28 weeks when pregnancy-induced hypervolemia and cardiac output reach their maximum. In most, however, heart failure develops peripartum when labor, delivery, and a number of common obstetrical conditions add undue cardiac burdens. Some of these include preeclampsia, hemorrhage and anemia, and sepsis syndrome. In a report of 542 women with heart disease, eight of 10 maternal deaths were during the puerperium (Etheridge, 1977).
 Ventricular Function in Pregnancy
Ventricular Function in Pregnancy
Ventricular volumes increase to accommodate pregnancy-induced hypervolemia. This is reflected by increasing end-systolic as well as end-diastolic dimensions. At the same time, however, there is no change in septal thickness or in ejection fraction. This is because these changes are accompanied by substantive ventricular remodeling—plasticity—which is characterized by eccentric expansion of left-ventricular mass that averages 30 to 35 percent near term. All of these adaptations return to prepregnancy values within a few months postpartum.
Certainly for clinical purposes, ventricular function during pregnancy is normal as estimated by the Braunwald ventricular function graph depicted in Figure 4-9 (p. 59). For given filling pressures, there is appropriate cardiac output so that cardiac function during pregnancy is eudynamic. Despite these findings, it remains controversial whether myocardial function per se is normal, enhanced, or depressed. Myocardial performance is measured by preload, afterload, contractility, and heart rate. Because these depend on ventricular geometry, they can only be measured indirectly (Savu, 2012). In nonpregnant subjects with a normal heart who sustain a high-output state, the left ventricle undergoes longitudinal remodeling, and echocardiographic functional indices of its deformation provide normal values. In pregnancy, there instead appears to be spherical remodeling, and these calculated indices that measure longitudinal deformation are depressed. Thus, these normal indices are likely inaccurate when used to assess function in pregnant women because they do not take into account the spherical eccentric hypertrophy characteristic of normal pregnancy.
DIAGNOSIS OF HEART DISEASE
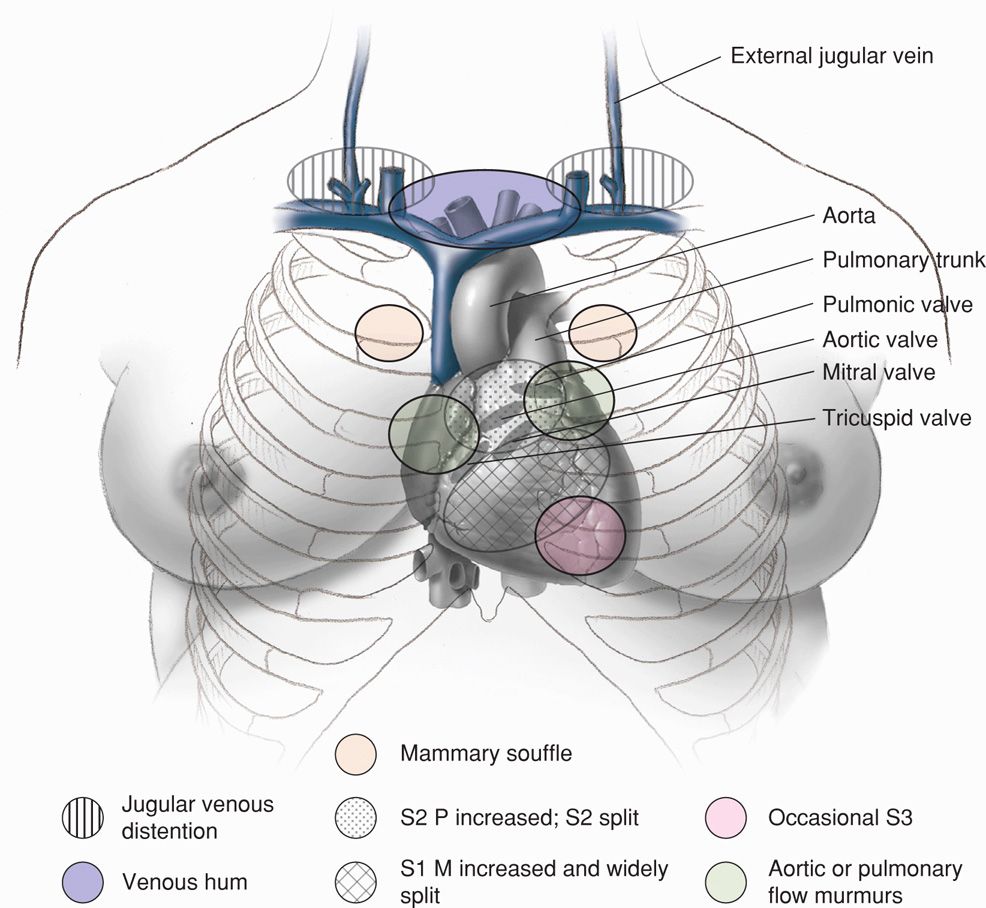
The physiological adaptations of normal pregnancy can induce symptoms and alter clinical findings that may confound the diagnosis of heart disease. For example, in normal pregnancy, functional systolic heart murmurs are common; respiratory effort is accentuated and at times suggests dyspnea; edema in the lower extremities after midpregnancy is common; and fatigue and exercise intolerance develop in most women. Some systolic flow murmurs may be loud, and normal changes in the various heart sounds depicted in Figure 49-2 may suggest cardiac disease.
FIGURE 49-2 Normal cardiac examination findings in the pregnant woman. S1 = first sound; M1 = mitral first sound; S2 = second sound; P2 = pulmonary second sound. (From Gei, 2001; Hytten, 1991.)
Clinical findings that may suggest heart disease are listed in Table 49-2. Pregnant women with none of these rarely have serious heart disease. As an interesting aside, Melchiorre and associates (2011) found that the prevalence of previously undiagnosed maternal cardiac structural abnormalities is significantly increased in women with high midtrimester uterine artery Doppler resistance indices (Chap. 17, p. 345).
TABLE 49-2. Clinical Indicators of Heart Disease During Pregnancy
Symptoms
Progressive dyspnea or orthopnea
Nocturnal cough
Hemoptysis
Syncope
Chest pain
Clinical Findings
Cyanosis
Clubbing of fingers
Persistent neck vein distention
Systolic murmur grade 3/6 or greater
Diastolic murmur
Cardiomegaly
Persistent arrhythmia
Persistent split second sound
Criteria for pulmonary hypertension
 Diagnostic Studies
Diagnostic Studies
In most women, noninvasive cardiovascular studies such as electrocardiography, chest radiography, and echocardiography will provide data necessary for evaluation. In some situations, such as complex congenital heart disease, transesophageal echocardiography may be useful. Albumin or red cells tagged with technicium-99m are rarely needed during pregnancy to evaluate ventricular function. That said, the estimated fetal radiation exposure from nuclear medicine studies of myocardial perfusion are calculated to range between 5 and 17 mGy depending on the technique employed (Colletti, 2013). If indicated, cardiac catheterization can be performed with limited fluoroscopy time. During coronary angiography, the mean radiation exposure to the unshielded abdomen is 1.5 mGy, and less than 20 percent of this reaches the fetus because of tissue attenuation (European Society of Cardiology, 2011). Shielding the fetus from direct radiation and shortening the fluoroscopic time help to minimize radiation exposure. In women with clear indications, any minimal theoretical fetal risk is outweighed by maternal benefits (Chap. 46, p. 932).
Electrocardiography
As the diaphragm is elevated in advancing pregnancy, there is an average 15-degree left-axis deviation in the electrocardiogram (ECG), and mild ST changes may be seen in the inferior leads. Atrial and ventricular premature contractions are relatively frequent (Carruth, 1981). Pregnancy does not alter voltage findings.
Chest Radiography
Anteroposterior (AP) and lateral chest radiographs are useful, and when a lead apron shield is used, fetal radiation exposure is minimal (Chap. 46, p. 931). Gross cardiomegaly can usually be excluded, but slight heart enlargement cannot be detected accurately because the heart silhouette normally is larger in pregnancy. This is accentuated further with a portable AP chest radiograph.
Echocardiography
Widespread use of echocardiography has allowed accurate diagnosis of most heart diseases during pregnancy. It allows noninvasive evaluation of structural and functional cardiac factors. Some normal pregnancy-induced changes include slightly but significantly increased tricuspid regurgitation, left atrial end-diastolic dimension, and left ventricular mass. Savu (2012) and Vitarelli (2011) and their coworkers have provided normal morphological and functional echocardiographic parameters for pregnancy, which are listed in the Appendix (p. 1293).
 Classification of Functional Heart Disease
Classification of Functional Heart Disease
There is no clinically applicable test for accurately measuring functional cardiac capacity. The clinical classification of the New York Heart Association (NYHA) was first published in 1928, and it was revised for the eighth time in 1979. This classification is based on past and present disability and is uninfluenced by physical signs:
• Class I. Uncompromised—no limitation of physical activity: These women do not have symptoms of cardiac insufficiency or experience anginal pain.
• Class II. Slight limitation of physical activity: These women are comfortable at rest, but if ordinary physical activity is undertaken, discomfort in the form of excessive fatigue, palpitation, dyspnea, or anginal pain results.
• Class III. Marked limitation of physical activity: These women are comfortable at rest, but less than ordinary activity causes excessive fatigue, palpitation, dyspnea, or anginal pain.
• Class IV. Severely compromised—inability to perform any physical activity without discomfort: Symptoms of cardiac insufficiency or angina may develop even at rest. If any physical activity is undertaken, discomfort is increased.
Siu and associates (2001b) expanded the NYHA classification and developed a scoring system for predicting cardiac complications during pregnancy. The system is based on a prospective analysis of 562 consecutive pregnant women with heart disease during 617 pregnancies in 13 Canadian teaching hospitals. Predictors of cardiac complications included the following: (1) prior heart failure, transient ischemic attack, arrhythmia, or stroke; (2) baseline NYHA class III or IV or cyanosis; (3) left-sided obstruction defined as mitral valve area < 2 cm2, aortic valve area < 1.5 cm2, or peak left ventricular outflow tract gradient > 30 mm Hg by echocardiography; (4) ejection fraction less than 40 percent. The risk of pulmonary edema, sustained arrhythmia, stroke, cardiac arrest, or cardiac death was substantially increased with one of these factors and even more so with two or more.
At least two studies have been conducted using these latter risk criteria. Khairy and colleagues (2006) reviewed 90 pregnancies in 53 women with congenital heart disease at Brigham and Women’s Hospital. There were no maternal deaths, and heart failure and symptomatic arrhythmias developed in 16.7 and 2.8 percent of the women, respectively. Similar to the Canadian study cited above, the most important predictors of complications were prior congestive heart failure, depressed ejection fraction, and smoking. In a German study that also used these predefined risk predictors, they were found to be accurate in assessing most cardiac outcomes (Stangl, 2008).
 Preconceptional Counseling
Preconceptional Counseling
Women with severe heart disease will benefit immensely from counseling before undertaking pregnancy, and they usually are referred for maternal-fetal medicine or cardiology consultation (Clark, 2012; Seshadri, 2012).
Maternal mortality rates generally vary directly with functional classification, and this relationship may change as pregnancy progresses. In the Canadian study, Siu and colleagues (2001b) observed significant worsening of NYHA class in 4.4 percent of 579 pregnancies in which the baseline class was I or II. Their experiences, however, as well as those of McFaul and coworkers (1988), were that there were no maternal deaths in 1041 women with class I or II disease. As described later, some women have life-threatening cardiac abnormalities that can be reversed by corrective surgery, and subsequent pregnancy becomes less dangerous. In other cases, such as women with mechanical valves taking warfarin, fetal considerations predominate.
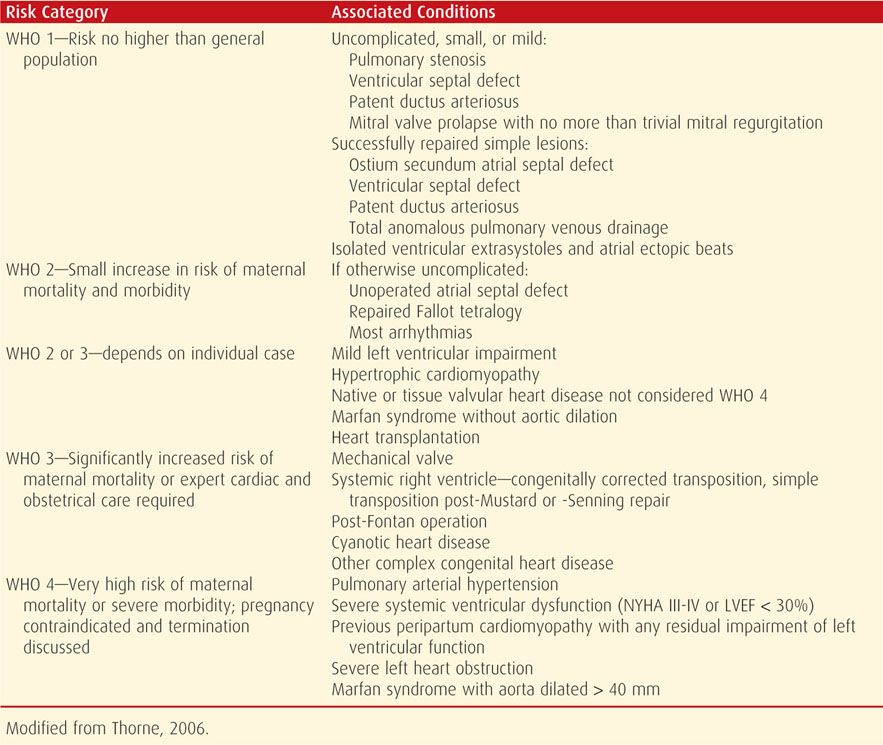
The World Health Organization Risk Classification of Cardiovascular Disease and Pregnancy is useful for assessing maternal risk associated with various cardiovascular conditions and for preconceptional counseling and planning (Thorne, 2006). Maternal risk is divided among four progressively worsening classes as shown in Table 49-3. Its use has been recommended by the European Society of Cardiology (2011).
TABLE 49-3. World Health Organization (WHO) Risk Classification of Cardiovascular Disease and Pregnancy
 Congenital Heart Disease in Offspring
Congenital Heart Disease in Offspring
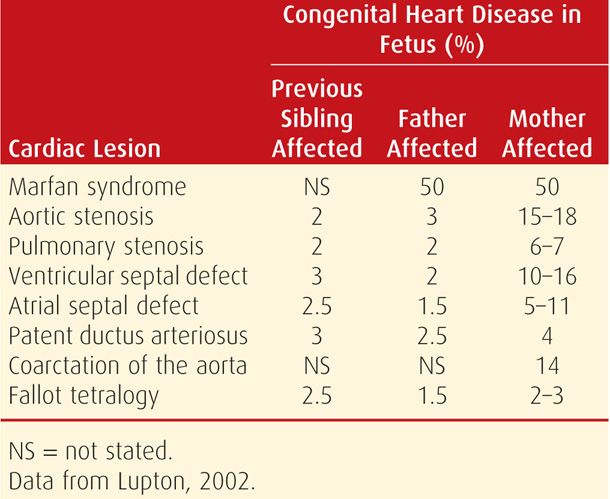
Many congenital heart lesions appear to be inherited as polygenic characteristics, which are discussed in Chapter 13 (p. 274). Because of this, some women with congenital heart lesions give birth to similarly affected infants, the risk of which varies widely as shown in Table 49-4. Environmental factors are also important. One example is a study from Tibet in which the prevalence of fetal heart disease was increased among women living at higher altitudes (> 3600 meters) and was presumably due to lower oxygen concentrations (Chen, 2008).
PERIPARTUM MANAGEMENT CONSIDERATIONS
In most instances, management involves a team approach with an obstetrician, cardiologist, anesthesiologist, and other specialists as needed. With complex lesions or with women who are especially at risk, evaluation by a multidisciplinary team is recommended early in pregnancy. During consultation, cardiovascular changes likely to be poorly tolerated by an individual woman are identified, and plans are formulated to minimize these. In some, pregnancy termination may be advisable. Maxwell (2010) has provided an in-depth checklist that considers detailed management options. The four changes that affect management that are emphasized by the American College of Obstetricians and Gynecologists (1992) include decreased vascular resistance, increased blood volume and cardiac output and their fluctuations peripartum, and hypercoagulability. Within this framework, both prognosis and management are influenced by the type and severity of the specific lesion and by the patient’s functional classification.
With rare exceptions, women in NYHA class I and most in class II negotiate pregnancy without morbidity. Special attention should be directed toward both prevention and early recognition of heart failure as discussed on page 990. Infection with sepsis syndrome is an important factor in precipitating cardiac failure. Moreover, bacterial endocarditis is a deadly complication of valvular heart disease (p. 990). Each woman should receive instructions to avoid contact with persons who have respiratory infections, including the common cold, and to report at once any evidence for infection. Pneumococcal and influenza vaccines are recommended.
Cigarette smoking is prohibited, because of both its cardiac effects and its propensity to cause upper respiratory infections. Illicit drug use may be particularly harmful, an example being the cardiovascular effects of cocaine or amphetamines. In addition, intravenous drug use increases the risk of infective endocarditis.
Fortunately, cases of NYHA class III and IV are uncommon today. In the Canadian study, only 3 percent of approximately 600 pregnancies were complicated by NYHA class III heart disease, and no women had class IV when first seen (Siu, 2001b). In a Turkish study, 8 percent of pregnancies in women with cardiac diseases were NYHA class III or IV (Madazli, 2010). An important question in these women is whether pregnancy should be undertaken. If women make that choice, they must understand the risks, and they are encouraged to be compliant with planned care. In some, prolonged hospitalization or bed rest is often necessary with continued pregnancy.
 Labor and Delivery
Labor and Delivery
In general, vaginal delivery is preferred, and labor induction is usually safe (Oron, 2004). Cesarean delivery is limited to obstetrical indications, and considerations are given for the specific cardiac lesion, overall maternal condition, and availability of experienced anesthesia personnel and general support facilities. Some of these women tolerate major surgical procedures poorly and are best delivered in a unit experienced with management of complicated cardiac disease. In some women, pulmonary artery catheterization may be indicated for hemodynamic monitoring (Chap. 47, p. 941). In our experiences, however, invasive monitoring is rarely indicated.
Based on her review, Simpson (2012) recommends cesarean delivery for women with the following: (1) dilated aortic root > 4 cm or aortic aneurysm; (2) acute severe congestive heart failure; (3) recent myocardial infarction; (4) severe symptomatic aortic stenosis; (5) warfarin administration within 2 weeks of delivery; and (6) need for emergency valve replacement immediately after delivery. Although we agree with most of these, we have some caveats. For example, we prefer aggressive medical stabilization of pulmonary edema followed by vaginal delivery if possible. Also, warfarin anticoagulation can be reversed with vitamin K, plasma, or prothrombin concentrates.
During labor, the mother with significant heart disease should be kept in a semirecumbent position with lateral tilt. Vital signs are taken frequently between contractions. Increases in pulse rate much above 100 bpm or respiratory rate above 24 per minute, particularly when associated with dyspnea, may suggest impending ventricular failure. If there is any evidence of cardiac decompensation, intensive medical management must be instituted immediately. It is essential to remember that delivery itself does not necessarily improve the maternal condition and in fact, may worsen it. Moreover, emergency operative delivery may be particularly hazardous. Clearly, both maternal and fetal status must be considered in the decision to hasten delivery under these circumstances.
Analgesia and Anesthesia
Relief from pain and apprehension is important. Although intravenous analgesics provide satisfactory pain relief for some women, continuous epidural analgesia is recommended for most. The major problem with conduction analgesia is maternal hypotension (Chap. 25, p. 514). This is especially dangerous in women with intracardiac shunts in whom flow may be reversed. Blood passes from right to left within the heart or aorta and thereby bypasses the lungs. Hypotension can also be life-threatening if there is pulmonary arterial hypertension or aortic stenosis because ventricular output is dependent on adequate preload. In women with these conditions, narcotic conduction analgesia or general anesthesia may be preferable.
For vaginal delivery in women with only mild cardiovascular compromise, epidural analgesia given with intravenous sedation often suffices. This has been shown to minimize intrapartum cardiac output fluctuations and allows forceps or vacuum-assisted delivery. Subarachnoid blockade is not generally recommended in women with significant heart disease. For cesarean delivery, epidural analgesia is preferred by most clinicians with caveats for its use with pulmonary arterial hypertension (p. 987). Finally, general endotracheal anesthesia with thiopental, succinylcholine, nitrous oxide, and at least 30-percent oxygen has also proved satisfactory.
Intrapartum Heart Failure
Cardiovascular decompensation during labor may manifest as pulmonary edema with hypoxia or as hypotension, or both. The proper therapeutic approach depends on the specific hemodynamic status and the underlying cardiac lesion. For example, decompensated mitral stenosis with pulmonary edema due to fluid overload is often best approached with aggressive diuresis. If precipitated by tachycardia, heart rate control with β-blocking agents is preferred. Conversely, the same treatment in a woman suffering decompensation and hypotension due to aortic stenosis could prove fatal. Unless the underlying pathophysiology is understood and the cause of the decompensation is clear, empirical therapy may be hazardous. Heart failure is discussed in more detail on page 990.
 Puerperium
Puerperium
Women who have shown little or no evidence of cardiac compromise during pregnancy, labor, or delivery may still decompensate postpartum when fluid mobilization into the intravascular compartment and reduction of peripheral vascular resistance place higher demands on myocardial performance. Therefore, it is important that meticulous care be continued into the puerperium (Keizer, 2006; Zeeman, 2006). Postpartum hemorrhage, anemia, infection, and thromboembolism are much more serious complications with heart disease. Indeed, these factors often act in concert to precipitate postpartum heart failure (Cunningham, 1986). In addition to increased cardiac work, many of these—for example, sepsis and severe preeclampsia—cause or worsen pulmonary edema because of endothelial activation and capillary-alveolar leakage (Chap. 47, p. 947).
 Sterilization and Contraception
Sterilization and Contraception
If indicated, tubal sterilization is performed at cesarean delivery. If it is to be performed after vaginal delivery, the procedure can be delayed up to several days to ensure that the mother has become hemodynamically near normal and that she is afebrile, not anemic, and ambulating normally. Other women are given detailed contraceptive advice. Special considerations for contraception in women with various cardiac disorders are discussed in some of the following sections and in Table 38-3 (p. 698).
SURGICALLY CORRECTED HEART DISEASE
Most clinically significant congenital heart lesions are repaired during childhood. Examples of those frequently not diagnosed until adulthood include atrial septal defects, pulmonic stenosis, bicuspid aortic valve, and aortic coarctation (Brickner, 2000). In some cases, the defect is mild and surgery is not required. In others, a significant structural anomaly is amenable to surgical correction. With successful repair, many women attempt pregnancy. In some instances, surgical corrections have been performed during pregnancy.
 Valve Replacement before Pregnancy
Valve Replacement before Pregnancy
Numerous reports describe subsequent pregnancy outcomes in women who have a prosthetic mitral or aortic valve. The type of valve is paramount, and pregnancy is undertaken only after serious consideration in women with a prosthetic mechanical valve. This is because anticoagulation is requisite, and at least when not pregnant, warfarin is necessary. As shown in Table 49-5, a number of serious complications can develop with mechanical valves. Both thromboembolisms involving the prosthesis and hemorrhage from anticoagulation are of extreme concern. This is in addition to deterioration in cardiac function. Overall, the maternal mortality rate is 3 to 4 percent with mechanical valves, and fetal loss is common.
TABLE 49-5. Outcomes Reported Since 1990 in Pregnancies Complicated by a Heart-Valve Replacement
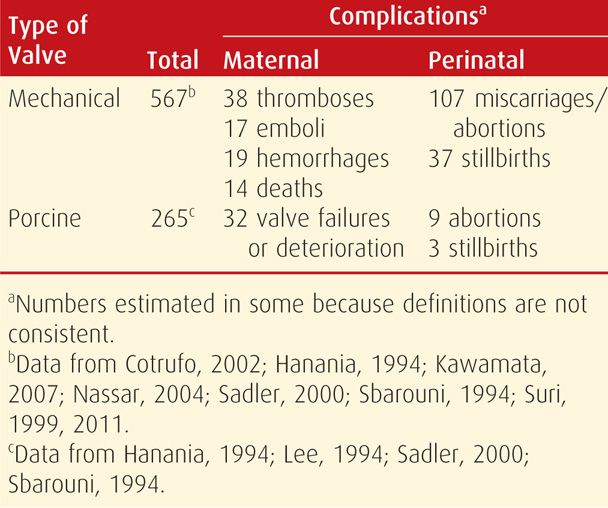
Porcine tissue valves are much safer during pregnancy, primarily because thrombosis is rare and anticoagulation is not required (see Table 49-5). Despite this, valvular dysfunction with cardiac deterioration or failure is common and develops in 5 to 25 percent of involved pregnancies. Another drawback is that bioprostheses are not as durable as mechanical ones, and valve replacement longevity averages 10 to 15 years. Based on their longitudinal study of 100 reproductive-aged women with a biological heart valve prosthesis, Cleuziou and colleagues (2010) concluded that pregnancy does not accelerate the risk for replacement.
Anticoagulation
This is critical for women with mechanical prosthetic valves. Unfortunately, warfarin is the most effective anticoagulant for preventing maternal thromboembolic complications but causes significant fetal morbidity and mortality. Anticoagulation with heparin is less hazardous for the fetus, however, the risk of maternal thromboembolic complications is much higher (McLintock, 2011).
As noted, warfarin, despite its effective anticoagulation, is teratogenic and causes miscarriage, stillbirths, and fetal malformations (Chap. 12, p. 252). In one study of 71 pregnancies in women given warfarin throughout pregnancy, the rates of miscarriage were 32 percent; stillbirth, 7 percent; and embryopathy, 6 percent (Cotrufo, 2002). The risk was highest when the mean daily dose of warfarin exceeded 5 mg. From a systematic review, Chan and coworkers (2000) concluded that the best maternal outcomes were achieved with warfarin anticoagulation but with a 6.4-percent embryopathy rate. And although heparin substituted before 12 weeks’ gestation eliminated embryopathy, thromboembolic complications during that time were increased significantly.
In examining heparin, anticoagulation for mechanical valves using low-dose unfractionated heparin is definitely inadequate and has a high associated maternal mortality rate (Chan, 2000; Iturbe-Alessio, 1986). Even full anticoagulation with either unfractionated heparin (UFH) or one of the low-molecular-weight heparins (LMWH) is associated with valvular thrombosis (Leyh, 2002, 2003; Rowan, 2001). Thus, many authorities recommend warfarin. However, the American College of Chest Physicians has recommended use of any of several regimens that include adjusted-dose UFH or LMWH heparin given throughout pregnancy as subsequently discussed (Bates, 2012).
Recommendations for Anticoagulation. A number of different treatment options—none of which are completely ideal—have been proposed and are principally based on consensus opinion. For this reason, they differ and allow more than one scheme. For example, and as shown in Table 49-6, the most recent guidelines of the American College of Chest Physicians for the management of pregnant women with mechanical prosthetic valves offer several different treatment options.
TABLE 49-6. American College of Chest Physicians Guidelines for Anticoagulation of Pregnant Women with Mechanical Prosthetic Valves
Any one of the following anticoagulant regimens is recommended:
Adjusted-dose LMWH twice daily throughout pregnancy. The doses should be adjusted to achieve the manufacturer’s peak anti-Xa level 4 hours after subcutaneous injection
Adjusted-dose UFH administered every 12 hours throughout pregnancy. The doses should be adjusted to keep the midinterval aPTT at least twice control or attain an anti-Xa level of 0.35 to 0.70 U/mL
LMWH or UFH as above until 13 weeks’ gestation, then warfarin substitution until close to delivery when LMWH or UFH is resumed
In women judged to be at very high risk of thromboembolism and in whom concerns exist about the efficacy and safety of LMWH or UFH as dosed above—some examples include older-generation prosthesis in the mitral position or history of thromboembolism—warfarin treatment is suggested throughout pregnancy with replacement by UFH or LMWH (as above) close to delivery. In addition, low-dose aspirin—75 to 100 mg daily—should be orally administered
Heparin is discontinued just before delivery. If delivery supervenes while the anticoagulant is still effective, and extensive bleeding is encountered, then protamine sulfate is given intravenously. Anticoagulant therapy with warfarin or heparin may be restarted 6 hours following vaginal delivery, usually with no problems. Following cesarean delivery, full anticoagulation is withheld, but the duration is not exactly known. The American College of Obstetricians and Gynecologists (2011b) advises resuming unfractionated or low-molecular-weight heparin 6 to 12 hours after cesarean delivery. It is our practice, however, to wait at least 24 hours, and preferably 48 hours, following a major surgical procedure.
Because warfarin, low-molecular-weight heparin, and unfractionated heparin do not accumulate in breast milk, they do not induce an anticoagulant effect in the infant. Therefore, these anticoagulants are compatible with breast feeding (American College of Obstetricians and Gynecologists, 2011b).
Contraception
Because of their possible thrombogenic action, estrogen-progestin oral contraceptives are relatively contraindicated in women with prosthetic valves. Because these women are generally fully anticoagulated, however, any increased risk is speculative. Contraceptive options are discussed in Chapters 38 and 39. Sterilization should be considered because of the serious pregnancy risks faced by women with significant heart disease.
 Cardiac Surgery During Pregnancy
Cardiac Surgery During Pregnancy
Although usually postponed until after delivery, valve replacement or other cardiac surgery during pregnancy may be lifesaving. Several reviews confirm that such surgery is associated with major maternal and fetal morbidity and mortality. Sutton and associates (2005) found that maternal mortality rates with cardiopulmonary bypass are between 1.5 and 5 percent. Although these are similar to those for nonpregnant women, the fetal mortality rate approaches 20 percent. In a longitudinal study from the Mayo Clinic, John and coworkers (2011) reported the outcomes of 21 pregnant women who underwent cardiothoracic surgery requiring cardiopulmonary bypass between 1976 and 2009. The procedures included eight aortic valve replacements, six mitral valve repairs or replacements, two myxoma excisions, two aortic aneurysm repairs, one patent foramen ovale closure, one prosthetic aortic valve thrombectomy, and one septal myectomy. Median cardiopulmonary bypass time was 53 minutes, with a range of 16 to 185 minutes. One woman died two days after surgery, and three other deaths occurred 2, 10, and 19 years postoperatively. Three fetuses died, and 52 percent delivered before 36 weeks. To optimize outcomes, Chandrasekhar and coworkers (2009) recommend the following: surgery done electively when possible, pump flow rate maintained > 2.5 L/min/m2, normothermic perfusion pressure > 70 mm Hg, pulsatile flow used, and hematocrit kept > 28 percent.
Mitral Valvotomy During Pregnancy
Tight mitral stenosis that requires intervention during pregnancy was previously treated by closed mitral valvotomy (Pavankumar, 1988). More recently, however, percutaneous transcatheter balloon dilatation of the mitral valve has largely replaced surgical valvotomy during pregnancy (Fawzy, 2007). Rahimtoola (2006) summarized outcomes of 36 women—25 of whom were NYHA class III or IV—who underwent balloon commissurotomy at an average gestational age of 26 weeks. Surgery was successful in 35 women, and left atrial and pulmonary artery pressures were reduced as the mitral valve area was increased from 0.74 to 1.59 cm2. Esteves and associates (2006) described similarly good outcomes in 71 pregnant women with tight mitral stenosis and heart failure who underwent percutaneous valvuloplasty. At delivery, 98 percent were either NYHA class I or II. At a mean of 44 months, the total event-free maternal survival rate was 54 percent, however, eight women required another surgical intervention. The 66 infants who were delivered at term all had normal growth and development.
 Pregnancy after Heart Transplantation
Pregnancy after Heart Transplantation
The first successful pregnancy in a heart-transplant recipient was reported 25 years ago by Löwenstein and associates (1988). Since that time, more than 50 pregnancies in heart-transplant recipients have been described. Key (1989) and Kim (1996) and their colleagues provide detailed data to show that the transplanted heart responds normally to pregnancy-induced changes. Despite this, complications are common during pregnancy (Dashe, 1998).
Armenti (2002) from the National Transplantation Pregnancy Registry and Miniero (2004), each with their coworkers, described outcomes of 53 pregnancies in 37 heart recipients. Almost half developed hypertension, and 22 percent suffered at least one rejection episode during pregnancy. They were delivered—usually by cesarean—at a mean of 37 to 38 weeks. Three fourths of infants were liveborn. At follow-up, at least five women had died more than 2 years postpartum. From Scandinavia, Estensen and associates (2011) detailed the outcomes of 19 women who had received a heart transplant and six who received both a heart and lung transplant. These 25 women had 42 pregnancies, and there were no maternal deaths. Major complications included two rejections during the early puerperium, two cases of renal failure, and 11 spontaneous abortions. Five women died 2 to 12 years after delivery. Ethical considerations of counseling and caring for such women related to pregnancy were summarized by Ross (2006).
VALVULAR HEART DISEASE
Rheumatic fever is uncommon in the United States because of less crowded living conditions, availability of penicillin, and evolution of nonrheumatogenic streptococcal strains. Still, it remains the chief cause of serious mitral valvular disease (Roeder, 2011).
 Mitral Stenosis
Mitral Stenosis
Rheumatic endocarditis causes most mitral stenosis lesions. The normal mitral valve surface area is 4.0 cm2, and when stenosis narrows this to < 2.5 cm2, symptoms usually develop (Desai, 2000). The contracted valve impedes blood flow from the left atrium to the ventricle. The most prominent complaint is dyspnea due to pulmonary venous hypertension and edema. Fatigue, palpitations, cough, and hemoptysis are also common.
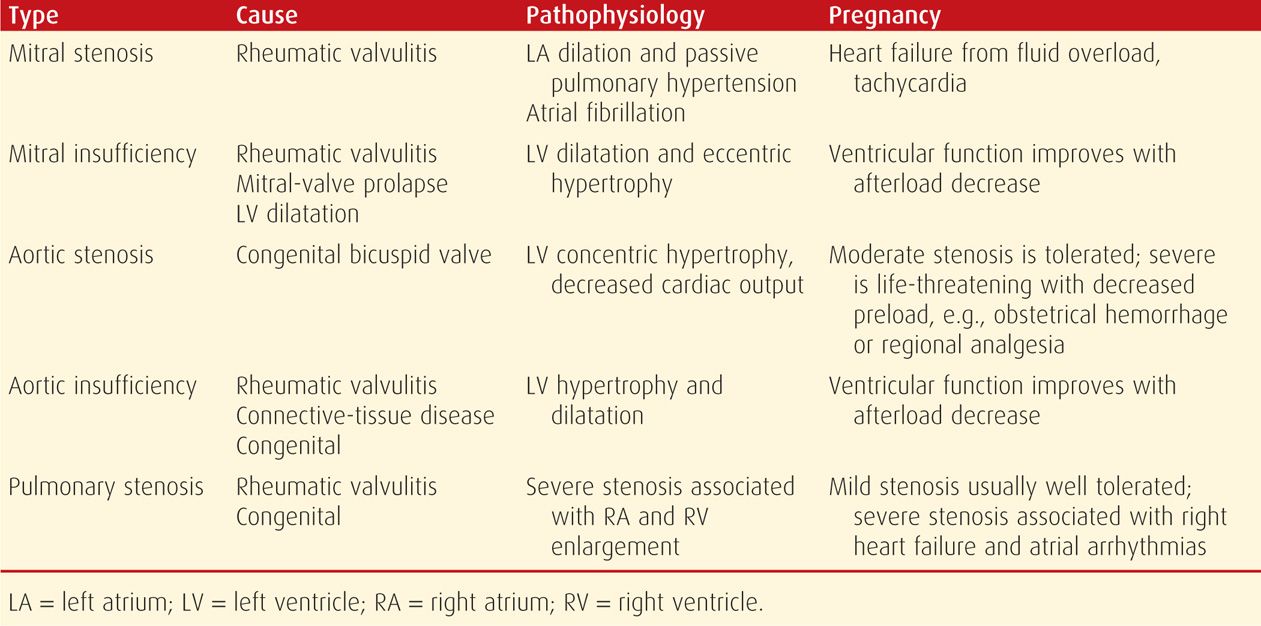
With more severe stenosis, the left atrium dilates, left atrial pressure is chronically elevated, and significant passive pulmonary hypertension develops (Table 49-7). These women have a relatively fixed cardiac output, and thus the increased preload of normal pregnancy, as well as other factors that increase cardiac output, may cause ventricular failure and pulmonary edema. Indeed, a fourth of women with mitral stenosis have cardiac failure for the first time during pregnancy (Caulin-Glaser, 1999). Because the murmur may not be heard in some women, this clinical picture at term may be confused with idiopathic peripartum cardiomyopathy (Cunningham, 1986, 2012).
TABLE 49-7. Major Cardiac Valve Disorders
Also with significant stenosis, tachycardia shortens ventricular diastolic filling time and increases the mitral gradient. This raises left atrial as well as pulmonary venous and capillary pressures and may result in pulmonary edema. Thus, sinus tachycardia is often treated prophylactically with β-blocking agents. Atrial tachyarrhythmias, including fibrillation, are common in mitral stenosis and are treated aggressively. Atrial fibrillation also predisposes to mural thrombus formation and cerebrovascular embolization that can cause stroke (Chap. 60, p. 1192). Atrial thrombosis can develop despite a sinus rhythm, and Hameed (2005) reported three such women. One suffered an embolic stroke, and another had pulmonary edema causing maternal hypoxemia leading to fetal encephalopathy.
Pregnancy Outcomes
In general, complications are directly associated with the degree of valvular stenosis. Recall that investigators from the large Canadian study found that women with a mitral-valve area < 2 cm2 were at greatest risk. In another study, Hameed (2001) described 46 pregnant women with mitral stenosis—43 percent developed heart failure and 20 percent developed arrhythmias. Fetal-growth restriction was more common in those women with a mitral valve area < 1.0 cm2.
Prognosis is also related to maternal functional capacity. Among 486 pregnancies complicated by rheumatic heart disease—predominantly mitral stenosis—Sawhney (2003) reported that eight of 10 maternal deaths were in women in NYHA classes III or IV.
Management
Limited physical activity is generally recommended in women with mitral stenosis. If symptoms of pulmonary congestion develop, activity is further reduced, dietary sodium is restricted, and diuretics are given (Siva, 2005). A β-blocker drug is usually given to slow the ventricular response to activity (Maxwell, 2010). If new-onset atrial fibrillation develops, intravenous verapamil, 5 to 10 mg, is given, or electrocardioversion is performed. For chronic fibrillation, digoxin, a β-blocker, or a calcium-channel blocker is given to slow ventricular response. Therapeutic anticoagulation with heparin is indicated with persistent fibrillation. Some recommend heparin anticoagulation for those with severe stenosis even if there is a sinus rhythm (Hameed, 2005).
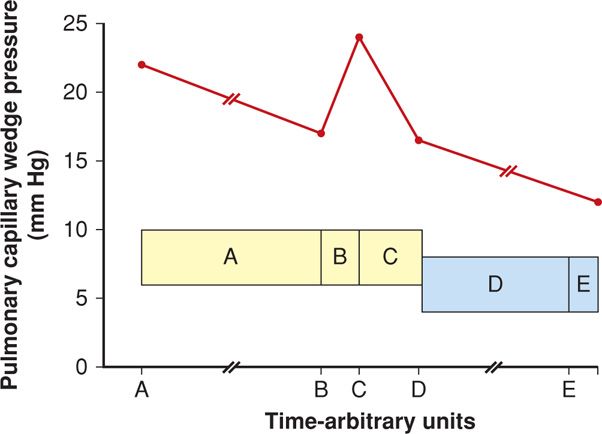
Labor and delivery are particularly stressful for women with symptomatic mitral stenosis. Uterine contractions increase cardiac output by increasing circulating blood volume. Pain, exertion, and anxiety cause tachycardia with possible rate-related heart failure. Epidural analgesia for labor is ideal, but with strict attention to avoid fluid overload. Abrupt increases in preload may increase pulmonary capillary wedge pressure and cause pulmonary edema. The effects of labor on pulmonary pressures in women with mitral stenosis are shown in Figure 49-3. Wedge pressures increase most immediately postpartum. Clark and colleagues (1985) hypothesize that this is likely due to loss of the low-resistance placental circulation along with the venous “autotransfusion” from a now-empty uterus and from the lower extremities and pelvis.
FIGURE 49-3 Mean pulmonary capillary wedge pressure measurements (red graph line) in eight women with mitral valve stenosis. Shaded yellow and blue boxes are mean (± 1 SD) pressures in nonlaboring normal women at term. A. First-stage labor. B. Second-stage labor 15 to 30 minutes before delivery. C. Postpartum 5 to 15 minutes. D. Postpartum 4 to 6 hours. E. Postpartum 18 to 24 hours. (Data from Clark, 1985, 1989.)
Most consider vaginal delivery to be preferable in women with mitral stenosis. Elective induction is reasonable so that labor and delivery are attended by a scheduled, experienced team. With severe stenosis and chronic heart failure, insertion of a pulmonary artery catheter may help guide management.
 Mitral Insufficiency
Mitral Insufficiency
A trivial degree of mitral insufficiency is found in most normal patients (Maxwell, 2010). But if there is improper coaptation of mitral valve leaflets during systole, abnormal degrees of mitral regurgitation may develop. This is eventually followed by left ventricular dilatation and eccentric hypertrophy (see Table 49-7). Chronic mitral regurgitation has a number of causes, including rheumatic fever, mitral valve prolapse, or left ventricular dilatation of any etiology—for example, dilated cardiomyopathy. Less common causes include a calcified mitral annulus, possibly some appetite suppressants, and in older women, ischemic heart disease. Mitral valve vegetations—Libman-Sacks endocarditis—are relatively common in women with antiphospholipid antibodies (Roldan, 1996; Shroff, 2012). These sometimes coexist with systemic lupus erythematosus (Chap. 59, p. 1170). In contrast, acute mitral insufficiency is caused by chordae tendineae rupture, papillary muscle infarction, or leaflet perforation from infective endocarditis.
In nonpregnant patients, symptoms from mitral valve incompetence are rare, and valve replacement is seldom indicated unless infective endocarditis develops. Likewise, mitral regurgitation is well tolerated during pregnancy, probably because decreased systemic vascular resistance results in less regurgitation. Heart failure only rarely develops during pregnancy, and occasionally tachyarrhythmias require treatment.
Mitral Valve Prolapse
This diagnosis implies the presence of a pathological connective tissue disorder—often termed myxomatous degeneration—which may involve the valve leaflets themselves, the annulus, or the chordae tendineae. Mitral insufficiency may develop. Most women with mitral valve prolapse are asymptomatic and are diagnosed by routine examination or while undergoing echocardiography. The small percentage of women with symptoms have anxiety, palpitations, atypical chest pain, dyspnea with exertion, and syncope (Guy, 2012).
Pregnant women with mitral valve prolapse rarely have cardiac complications. Hypervolemia may even improve alignment of the mitral valve, and women without pathological myxomatous change generally have excellent pregnancy outcomes (Leśniak-Sobelga, 2004; Rayburn, 1987). In a study of 3100 women in the Taiwanese Birth Registry with mitral valve prolapse, however, the preterm birth rate was 1.2 times higher than among controls (Chen, 2011).
For women who are symptomatic, β-blocking drugs are given to decrease sympathetic tone, relieve chest pain and palpitations, and reduce the risk of life-threatening arrhythmias. According to the American College of Obstetricians and Gynecologists (2011a), mitral valve prolapse is not considered an indication for infective endocarditis prophylaxis.
 Aortic Stenosis
Aortic Stenosis
Usually a disease of aging, aortic stenosis in women younger than 30 years is most likely due to a congenital lesion. This stenosis is less common since the decline in incidences of rheumatic diseases, and the most frequent cause in this country is a bicuspid valve (Friedman, 2008). A normal aortic valve has an area of 3 to 4 cm2, with a pressure gradient of less than 5 mm Hg. If the valve area is < 1 cm2, there is severe obstruction to flow and a progressive pressure overload on the left ventricle (Carabello, 2002; Roeder, 2011). Concentric left ventricular hypertrophy follows, and if severe, end-diastolic pressures become elevated, ejection fraction declines, and cardiac output is reduced (see Table 49-7). Characteristic clinical manifestations develop late and include chest pain, syncope, heart failure, and sudden death from arrhythmias. Life expectancy averages only 5 years after exertional chest pain develops, and valve replacement is indicated for symptomatic patients.
Pregnancy
Clinically significant aortic stenosis is infrequent during pregnancy. Mild to moderate degrees of stenosis are well tolerated, however, severe disease is life threatening. The principal underlying hemodynamic problem is the fixed cardiac output associated with severe stenosis. During pregnancy, a number of events acutely decrease preload further and thus aggravate the fixed cardiac output. These include vena caval occlusion, regional analgesia, and hemorrhage. Importantly, these also decrease cardiac, cerebral, and uterine perfusion. It follows that severe aortic stenosis may be extremely dangerous during pregnancy. From the large Canadian multicenter study cited above, there were increased complications if the aortic valve area was < 1.5 cm2 (Siu, 2001b). And in the report by Hameed and associates (2001) described earlier, the maternal mortality rate with aortic stenosis was 8 percent. Women with valve gradients exceeding 100 mm Hg appear to be at greatest risk.
Management
For the asymptomatic woman with aortic stenosis, no treatment except close observation is required. Management of the symptomatic woman includes strict limitation of activity and prompt treatment of infections. If symptoms persist despite bed rest, valve replacement or valvotomy using cardiopulmonary bypass must be considered. In general, balloon valvotomy for aortic valve disease is avoided because of serious complications, which exceed 10 percent. These include stroke, aortic rupture, aortic valve insufficiency, and death (Reich, 2004). In rare cases, it may be preferable to perform valve replacement during pregnancy (Datt, 2010).
For women with critical aortic stenosis, intensive monitoring during labor is important. Pulmonary artery catheterization may be helpful because of the narrow margin separating fluid overload from hypovolemia. Women with aortic stenosis are dependent on adequate end-diastolic ventricular filling pressures to maintain cardiac output and systemic perfusion. Abrupt decreases in end-diastolic volume may result in hypotension, syncope, myocardial infarction, and sudden death. Thus, the management key is avoidance of decreased ventricular preload and the maintenance of cardiac output. During labor and delivery, such women should be managed on the “wet” side, maintaining a margin of safety in intravascular volume in anticipation of possible hemorrhage. In women with a competent mitral valve, pulmonary edema is rare, even with moderate volume overload.
During labor, narcotic epidural analgesia seems ideal, thus avoiding potentially hazardous hypotension, which may be encountered with standard conduction analgesia techniques. Easterling and coworkers (1988) studied the effects of epidural analgesia in five women with severe stenosis and demonstrated immediate and profound effects of decreased filling pressures. Xia and associates (2006) emphasize slow administration of dilute local anesthetic agents into the epidural space. Forceps or vacuum delivery is used for standard obstetrical indications in hemodynamically stable women. Late cardiac events include pulmonary edema, arrhythmias, cardiac interventions, and death, which were identified within 1 year of delivery in 70 pregnancies (Tzemos, 2009).
 Aortic Insufficiency
Aortic Insufficiency
Aortic valve regurgitation or insufficiency allows diastolic flow of blood from the aorta back into the left ventricle. Frequent causes of abnormal insufficiency are rheumatic fever, connective-tissue abnormalities, and congenital lesions. With Marfan syndrome, the aortic root may dilate, resulting in regurgitation. Acute insufficiency may develop with bacterial endocarditis or aortic dissection. Aortic and mitral valve insufficiency have been linked to the appetite suppressants fenfluramine and dexfenfluramine and to the ergot-derived dopamine agonists cabergoline and pergolide (Gardin, 2000; Schade, 2007; Zanettini, 2007). With chronic insufficiency, left ventricular hypertrophy and dilatation develop and are followed by slow-onset fatigue, dyspnea, and edema, although rapid deterioration usually follows (see Table 49-7).
Aortic insufficiency is generally well tolerated during pregnancy. Like mitral valve incompetence, diminished vascular resistance is thought to improve hemodynamic function. If symptoms of heart failure develop, diuretics are given and bed rest is encouraged.
 Pulmonic Stenosis
Pulmonic Stenosis
The pulmonary valve is affected by rheumatic fever far less often than the other valves. Instead, pulmonic stenosis is usually congenital and also may be associated with Fallot tetralogy or Noonan syndrome. The clinical diagnosis is typically identified by auscultating a systolic ejection murmur over the pulmonary area that is louder during inspiration.
Increased hemodynamic burdens of pregnancy can precipitate right-sided heart failure or atrial arrhythmias in women with severe stenosis. Surgical correction ideally is done before pregnancy, but if symptoms progress, a balloon angioplasty may be necessary antepartum (Maxwell, 2010; Siu, 2001a). In a study of 81 pregnancies in 51 Dutch women with pulmonic stenosis, cardiac complications were infrequent (Drenthen, 2006). NYHA classification worsened in two women, and nine experienced palpitations or arrhythmias. No changes in pulmonary valvular function or other adverse cardiac events were reported. However, noncardiac complications were increased—17 percent had preterm delivery; 15 percent had hypertension; and 4 percent developed thromboembolism. Interestingly, two of the offspring were diagnosed with pulmonic stenosis, and another had complete transposition and anencephaly.
CONGENITAL HEART DISEASE
The incidence of congenital heart disease in the United States is approximately 8 per 1000 liveborn infants. Approximately a third of these have critical disease that requires cardiac catheterization or surgery during the first year of life. Others require surgery in childhood, and it is currently estimated that there are nearly 1 million adults in this country with congenital heart disease (Bashore, 2007).
According to an analysis from the Nationwide Inpatient Sample discharge database, more than 30,000 women admitted for delivery between 1998 and 2007 had congenital heart disease—a rate of 71.6 per 100,000 deliveries (Opotowsky, 2012). After statistical adjustments, women with congenital heart disease were found to be eight times more likely to sustain an adverse cardiovascular event that included death, heart failure, arrhythmia, and cerebrovascular or embolic event. Of these, arrhythmia was the most common, and the rate of maternal death was approximately 1.5 per 1000. Thompson and coworkers (2014) found similar risks.
 Septal Defects
Septal Defects
Atrial Septal Defects
After bicuspid aortic valve, these are the most frequently encountered adult congenital cardiac lesions. Indeed, a fourth of all adults have a patent foramen ovale (Kizer, 2005). Most are asymptomatic until the third or fourth decade. The secundum-type defect accounts for 70 percent, and associated mitral valve myxomatous abnormalities with prolapse are common. Most recommend repair if discovered in adulthood. Pregnancy is well tolerated unless pulmonary hypertension has developed, but this is uncommon (Maxwell, 2010; Zuber, 1999). Treatment is indicated for congestive heart failure or an arrhythmia. Based on their review, Aliaga and associates (2003) concluded that the risk of endocarditis with an atrial septal defect is negligible.
With the potential to shunt blood from right to left, a paradoxical embolism, that is, entry of a venous thrombus through the septal defect and into the systemic arterial circulation, is possible and may cause an embolic stroke (Chap. 60, p. 1192). Erkut and associates (2006) described a woman who developed an entrapped thrombus in a patent foramen ovale postpartum. In asymptomatic women, thromboembolism prophylaxis is problematic, and recommendations include either observation or antiplatelet therapy such as low-dose aspirin (Kizer, 2005; Maxwell, 2010). Compression stockings and prophylactic heparin for a pregnant woman with a septal defect and concurrent immobility or other risk factors for thromboembolism have also been recommended (Head, 2005).
Ventricular Septal Defects
These lesions close spontaneously during childhood in 90 percent of cases. Most defects are paramembranous, and physiological derangements are related to lesion size. In general, if the defect is less than 1.25 cm2, pulmonary hypertension and heart failure do not develop. If the effective defect size exceeds that of the aortic valve orifice, symptoms rapidly develop. For these reasons, most children undergo surgical repair before pulmonary hypertension develops. Adults with unrepaired large defects develop left ventricular failure and pulmonary hypertension and have a high incidence of bacterial endocarditis (Brickner, 2000; Maxwell, 2010).
Pregnancy is well tolerated with small to moderate left-to-right shunts. If pulmonary arterial pressures reach systemic levels, however, there is reversal or bidirectional flow—Eisenmenger syndrome (p. 985). When this develops, the maternal mortality rate is significantly increased, and thus, pregnancy is not generally advisable. Bacterial endocarditis is more common with unrepaired defects, and antimicrobial prophylaxis is often required (p. 991). As shown in Table 49-4, up to 15 percent of offspring born to these women also have a ventricular septal defect.
Atrioventricular Septal Defects
These account for approximately 3 percent of all congenital cardiac malformations and are distinct from isolated atrial or ventricular septal defects. An atrioventricular (AV) septal defect is characterized by a common, ovoid AV junction. This defect is associated with aneuploidy, Eisenmenger syndrome, and other malformations, but still, some of these women become pregnant. Compared with simple septal defects, complications are more frequent during pregnancy. In a review of 48 pregnancies in 29 such women, complications included persistent deterioration of NYHA class in 23 percent, significant arrhythmias in 19 percent, and heart failure in 2 percent (Drenthen, 2005b). Congenital heart disease was identified in 15 percent of the offspring.
 Persistent (Patent) Ductus Arteriosus
Persistent (Patent) Ductus Arteriosus
The ductus connects the proximal left pulmonary artery to the descending aorta just distal to the left subclavian artery. Functional closure of the ductus from vasoconstriction occurs shortly after term birth (Akintunde, 2011). The physiological consequences of persistence of this structure are related to its size. Most significant lesions are repaired in childhood, but for individuals who do not undergo repair, the mortality rate is high after the fifth decade (Brickner, 2000). In some younger women with an unrepaired ductus during pregnancy, however, pulmonary hypertension, heart failure, or cyanosis will develop if systemic blood pressure falls and leads to shunt reversal of blood from the pulmonary artery into the aorta (Maxwell, 2010). A sudden blood pressure decline at delivery—such as with conduction analgesia or hemorrhage—may lead to fatal collapse. Therefore, hypotension should be avoided whenever possible and treated vigorously if it develops. Prophylaxis for bacterial endocarditis may be indicated at delivery for unrepaired defects (p. 991). As shown in Table 49-4, the incidence of inheritance is approximately 4 percent.
 Cyanotic Heart Disease
Cyanotic Heart Disease
When congenital heart lesions are associated with right-to-left shunting of blood past the pulmonary capillary bed, cyanosis develops. The classic and most commonly encountered lesion in adults and during pregnancy is the Fallot tetralogy (Maxwell, 2010). It is characterized by a large ventricular septal defect, pulmonary stenosis, right ventricular hypertrophy, and an overriding aorta that receives blood from both the right and left ventricles. The magnitude of the shunt varies inversely with systemic vascular resistance. Hence, during pregnancy, when peripheral resistance decreases, the shunt increases and cyanosis worsens. Women who have undergone repair and who do not have a recurrence of cyanosis do well in pregnancy.
Some women with Ebstein anomaly with a malpositioned, malformed tricuspid valve may reach reproductive age. Right ventricular failure from volume overload and appearance or worsening of cyanosis are common during pregnancy. In the absence of cyanosis, these women usually tolerate pregnancy well.
Women with cyanotic heart disease generally do poorly during pregnancy. With uncorrected Fallot tetralogy, for example, maternal mortality rates approach 10 percent. Moreover, any disease complicated by severe maternal hypoxemia is likely to lead to miscarriage, preterm delivery, or fetal death. There is a relationship between chronic hypoxemia, polycythemia, and pregnancy outcome. When hypoxemia is intense enough to stimulate a rise in hematocrit above 65 percent, pregnancy wastage is virtually 100 percent.
Pregnancy after Surgical Repair
Some of the more complex lesions cannot be successfully repaired. But with satisfactory surgical correction of cyanotic lesions before pregnancy, maternal and fetal outcomes are much improved (Maxwell, 2010).
Fallot Tetralogy. Balci (2011) and Kamiya (2012) and their associates described a total of 197 pregnancies in 99 women with surgically corrected Fallot tetralogy. Pregnancy was usually well tolerated, and there were no maternal deaths. Still, almost 9 percent of pregnancies were complicated by adverse cardiac events including new onset or worsening of arrhythmias and heart failure. For women with a pulmonary valve replacement, Oosterhof and coworkers (2006) reported that pregnancy did not adversely affect graft function.
Transposition of the Great Vessels. Pregnancy following surgical correction of transposition also has risks. Canobbio (2006) and Drenthen (2005a), each with their colleagues, described outcomes of 119 pregnancies in 68 women—90 percent had a Mustard procedure and 10 percent a Senning procedure. During pregnancy, a fourth had arrhythmias. Twelve percent developed heart failure, and one of these patients subsequently required cardiac transplantation. One woman died suddenly a month after delivery, and another died 4 years later. A third of the newborns were delivered preterm, but no infant had heart disease. In a more recent study, Metz and associates (2011) reported that five of 14 pregnancies resulting in live births were complicated by symptomatic intracardiac baffle obstruction, which required postpartum stenting. In review, baffles are surgically constructed conduits that redirect anomalous cardiac blood flow and are integral to initial transposition correction.
Successful—although eventful—pregnancies in women with previously repaired truncus arteriosus and double-outlet right ventricle have also been described (Drenthen, 2008; Hoendermis, 2008).
Single Functional Ventricle. Feinstein and associates (2012) recently reviewed the remarkably improved treatments for patients with hypoplastic left heart syndrome. Almost 70 percent of these women are now expected to survive into adulthood and frequently become pregnant. Those who have undergone a Fontan repair are at particularly high risk for complications, which include atrial arrhythmias and peripartum heart failure (Nitsche, 2009). In a report of four pregnancies post-Fontan repair, there were no maternal deaths, but complications were frequent (Hoare, 2001). All were delivered preterm, two had supraventricular arrhythmias, and two developed ventricular failure. Similarly high complication rates were described by Jain and coworkers (2011) in 15 women with a systemic right ventricle, that is, one in which the right ventricle rather than the left pumps blood to the support systemic circulation.
Eisenmenger Syndrome
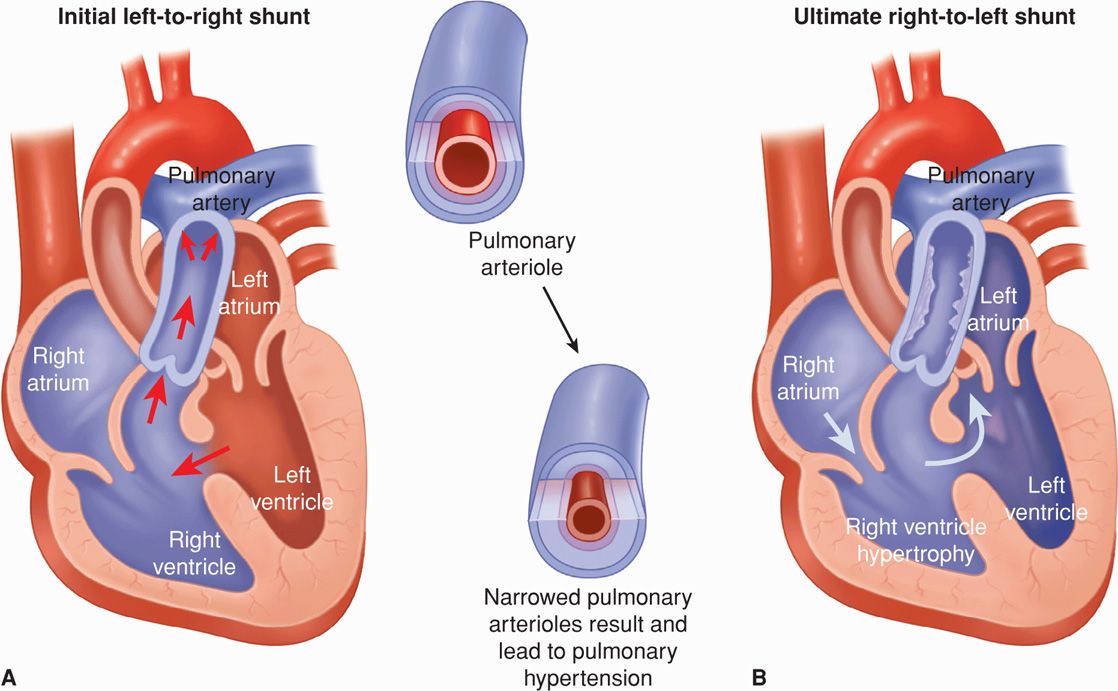
This describes secondary pulmonary hypertension that develops from any cardiac lesion. The syndrome develops when pulmonary vascular resistance exceeds systemic resistance and leads to concomitant right-to-left shunting. The most common underlying defects are atrial or ventricular septal defects and persistent ductus arteriosus (Fig. 49-4). Patients are asymptomatic for years, but eventually pulmonary hypertension becomes severe enough to cause right-to-left shunting, and few persons survive into the fifth decade (Makaryus, 2006; Maxwell, 2010).
FIGURE 49-4 Eisenmenger syndrome due to a ventricular septal defect (VSD). A. Substantial left-to-right shunting through the VSD leads to morphological changes in the smaller pulmonary arteries and arterioles. Specifically, medial hypertrophy, intimal cellular proliferations, and fibrosis lead to narrowing or closure of the vessel lumen. These vascular changes create pulmonary hypertension and a resultant reversal of the intracardiac shunt (B). With sustained pulmonary hypertension, extensive atherosclerosis and calcification often develop in the large pulmonary arteries. Although a VSD is shown here, Eisenmenger syndrome may also develop in association with a large atrial septal defect or patent ductus arteriosus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree