Malia S. Q. Murphy, BScH, PhD (c)
Kara A. Nerenberg, MD
PREGNANCY COMPLICATIONS AND CARDIOVASCULAR DISEASE IN WOMEN
PREPREGNANCY CARDIOVASCULAR RISK FACTORS
WHEN SHOULD FOLLOW-UP BE CARRIED OUT?
HOW SHOULD THESE WOMEN BE FOLLOWED UP?
LONG-TERM BENEFITS OF THE MATERNAL HEALTH CLINIC
INTRODUCTION
Pregnancy has been likened to a cardiovascular stress test in that the development of certain common pregnancy complications have the potential to reveal a woman’s vascular or metabolic susceptibility for future diseases.1 Indeed, the degree to which a woman “fails” the pregnancy stress test and the number of times the woman “fails” it, in all likelihood reflects not only her risk for future cardiovascular disease (CVD) but the timeframe over which it is likely to develop.2 The 2011 update to the American Heart Association’s Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women3 now includes certain common pregnancy complications (eg, preeclampsia, gestational hypertension, gestational diabetes, etc) as key components when screening women at risk for heart disease and stroke. Importantly, the development of these pregnancy complications not only identifies women at increased risk for future CVD but also can accurately identify women who already have underlying, often unrecognized, cardiovascular risk factors (CVRs).4,5 As such, we have coined the term “Pregnancy-related cardiovascular risk indicators”6 (Table 38-1), the development of which provides an early window of opportunity for postpartum CVR identification and interventions that could lead to a reduction in risk of future CVD and potentially improved outcomes in subsequent pregnancies.2
Pregnancy-Related Cardiovascular Risk Indicators |

Although women with pregnancy complications are also at increased risk of other future diseases (ie, heart failure and cardiac dysrhythmias,7 venous thromboembolism,1 renal disease,8 and neurocognitive abnormalities9), given the public health burden of CVD and opportunities for CVD prevention, this chapter will discuss the justifications for postpartum cardiovascular risk screening in women. Furthermore, this chapter will present an approach to setting up and operating such a program. This guide will be based on the success and data garnered from our own clinic, developed specifically for postpartum cardiovascular risk assessment; The Maternal Health Clinic.6
PREGNANCY COMPLICATIONS AND CARDIOVASCULAR DISEASE IN WOMEN
It is now scientifically well established that women who have had an adverse obstetrical outcome (see Tables 38-1 and 38-2)10–15—preeclampsia, gestational hypertension, gestational diabetes, idiopathic preterm birth, clinically significant placental abruption, or delivering an intrauterine growth restricted (IUGR) or low birth weight baby—are at an increased risk [presented as relative risk (RR) (95% confidence interval (CI)] of hypertension [3.70 (2.70-5.05)],16 ischemic heart disease [2.16 (1.86-2.52)],16 major stroke [RR (95% CI), 1.81 (1.45-2.27)],16 and premature cardiovascular death [RR (95% CI), 1.49 (1.05-2.14)]16 compared with women with uncomplicated pregnancies.17–19 Women who deliver an IUGR baby prematurely in conjunction with severe preeclampsia, appear to be at significantly greater risk, especially for premature CVD [RR (95% CI), 8.12 (4.31-15.33)].16,17,20,21 Numerous retrospective analyses comparing women who had one or more of these adverse obstetrical outcomes to healthy pregnancy controls have demonstrated differences in CVRs including increased blood pressure, lipids and body mass index (BMI).10 In addition, these women exhibit evidence of insulin resistance, residual microalbuminuria, and are more likely to meet the criteria for the Metabolic Syndrome (MetS) at various time points in the postpartum period.17,18,22,23 MetS is a composite of CVRs which, when present, increases one’s risk of developing CVD and diabetes mellitus. Based on the International Diabetes Federation24 criteria, women are diagnosed with MetS if they have three or more of: elevated blood pressure, abdominal obesity, elevated triglycerides, decreased HDL cholesterol, and elevated fasting serum glucose. Of note, the International Diabetes Federation24 and American Heart Association25 criteria appear to be more sensitive26 than that of the NCEP ATP III27 in identifying future cardiometabolic risk.
Traditional and Pregnancy-Related CVRs and Risk for Future Cardiovascular Disease |
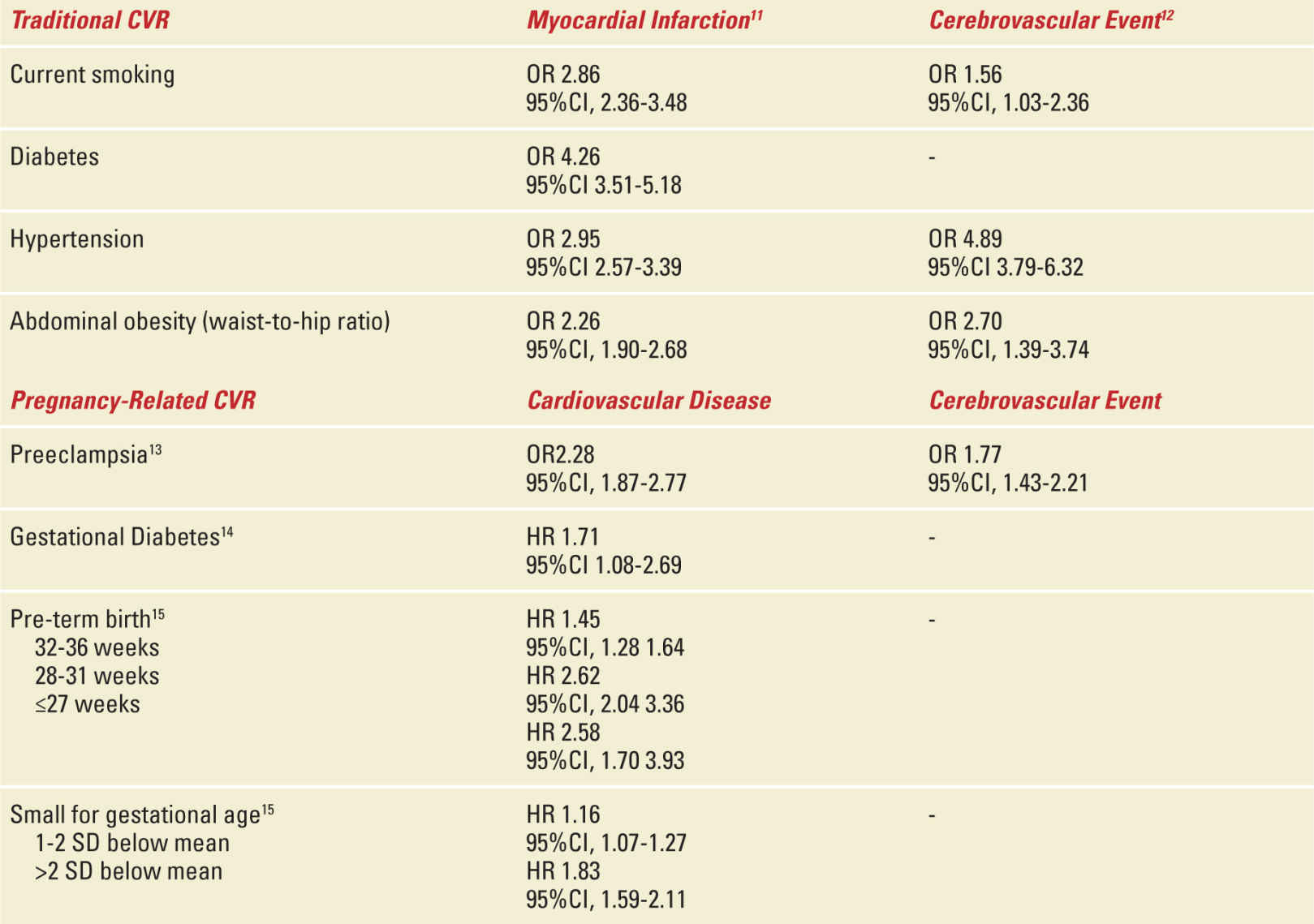
Our own work demonstrates that by twelve months postpartum, women who had developed preeclampsia had significantly increased blood pressure, total cholesterol, LDL cholesterol, triglycerides, BMI, insulin resistance, and microalbuminuria compared with women with uncomplicated pregnancies.4 Incorporating these data with other markers of obesity, we also identified that the prevalence of MetS in women who developed preeclampsia was 18.2% at 1 year and 21.9% at 3 years postpartum almost three times higher than control women in whom the prevalence of MetS remained stable at approximately 6.7%.28 In addition, there was no significant trend in women who did/did not have preeclampsia either recovering from or developing MetS over the study period. That is, women with MetS at 1 year postpartum tended to remain in the disease state of MetS at 3 years postpartum whereas women without MetS at 1 year tended to remain disease free by 3 years postpartum. Based upon these findings, we recommend that CVRs screening should take place within the first year postpartum for young women with pregnancy complications, as waiting up to 3 years does not significantly increase the number of CVRs detected and may potentially decrease opportunities for preventative interventions.
An increasing number of American (36.2%) and Canadian (23.9%) females between the ages of 20 and 79 fall into BMI categories of 30 kg/m2 and higher—a nearly 10% increase in the prevalence of obesity in North America over the past 20 years.29 Alarmingly, these weight increases have been greatest among North American women of child-bearing age. Relative body weight in women is a particularly strong independent risk factor for the future development of CVD and death30 as each unit of BMI increase accounts for nearly a 10% increase in an individual’s risk for CVD. A woman’s lifetime weight trajectory can be influenced by pregnancy and the early postpartum period. As it relates to pregnancy, recent data from the United States demonstrate that approximately 40% of normal weight and 60% of overweight women gain excessive weight during pregnancy based on Institute of Medicine recommendations.31 In long-term follow-up studies, gestational weight gain is significantly associated with weight change from early pregnancy to 1 year,32,33 10 years,34 and 15 years postpartum.35–37 Excess gestational weight gain has also been associated with long-term postpartum weight retention and obesity in pregnant adolescents.38 Just as concerning, excess maternal weight gain during pregnancy is also correlated with the weight of the offspring at three years of age and its impact is greater among overweight and obese women than it is in women with a normal prepregnancy BMI.39
By 6 months postpartum, 20% to 30% of women will weigh 5 kg (11 pounds) or more compared to their prepregnancy weight.40 This reflects not only retention of weight gained during pregnancy but also weight gained early postpartum.41,42 Approximately 75% of women attending an obesity clinic in Sweden identified pregnancy and the postpartum periods as the primary trigger for marked weight gain and/or onset of their obesity.41 As such, the American College of Obstetrics and Gynecology43 and Health Canada40 guidelines recommend that women with pregnancy weight retention by 6 months to 1 year postpartum should receive appropriate follow-up by health practitioners/educators in order to assist in lifestyle modifications. These recommended lifestyle modifications include “…healthy eating and active living, which will limit further weight gain and/or encourage a return to a stable lower weight and BMI.” Unfortunately, there are no specific recommendations for how this should be achieved. Although there are many factors that play a role in weight retention/weight gain postpartum, the strongest factor is excess weight gain during pregnancy, although smoking cessation, a sedentary lifestyle, and socioeconomic factors also contribute.44 Pregnancy and the postpartum provides us with a “teachable moment” for weight management and obesity prevention.45 Therefore, while reducing prepregnancy BMI may have a beneficial impact on pregnancy and long-term maternal and child outcomes, prevention of excess gestational weight gain and facilitation of postpartum weight loss for all women appears to be an simple, feasible strategy to address maternal obesity and ultimately CVR reduction and CVD prevention.46,47
CVD, variably defined as having a myocardial infarction or stroke, remains the leading cause of death among women in the United States,48 in Canada,49 and around the world.50 In fact, CVD is becoming increasingly prevalent among younger women who are now exhibiting higher rates of heart disease, hypertension, type 2 diabetes, and obesity.51 In general, the underlying contributors of many cardiovascular events (eg, hypertension, diabetes, and dyslipidemia) are often present for years if not decades before the onset of clinical symptoms; therefore, the presence of these risk factors in early life (ie, 20s and 30s) may contribute significantly to the risk of premature CVD (ie, before age 65 in women). With over 88% of women over the age of 20 exhibiting at least one CVR,49 early-onset heart disease, hypertension, and diabetes are likely to pose substantial burdens on health care resources as these individuals age and require longer term and more intensive treatment. The American Heart Association projects that total direct costs of all CVD in the United States will increase by 200% over the next 20 years.52 Unfortunately, while these statistics demonstrate that mortality and morbidity rates of CVD in women are increasing around the world, this trend remains largely under recognized by physicians and the public.53,54
Women often present with atypical symptoms of CVD compared with men,55 and are more likely to be misdiagnosed upon presentation.56 As a result, women are less likely to receive optimal evidence-based treatments and are more likely to experience poorer outcomes including death following a major cardiovascular event.57–59 Considering that up to 80% of all CVD disease is preventable with early diagnosis, lifestyle modification, and therapeutic intervention,54 innovative CVD screening strategies are especially important for women given the increased morbidity and mortality outlined above.54 The postpartum period may represent a novel time for CVD screening, as pregnancy is a time when women access the health-care system on a regular basis, often for the first time. The postpartum period offers a time during which many forms of health screening, including CVR identification and interventions could be efficiently carried out for health preservation and disease prevention. Unfortunately, despite a well-established link between pregnancy complications and future CVD, obstetrical care and primary care providers are largely unaware of this association and as such, rarely implement any type of follow-up or screening.60,61
It is widely accepted that age, sex, hypertension, smoking, dyslipidemia, and diabetes are major risk factors contributing to the development of CVD and that these risk factors often cluster and interact to compound vascular risk.62 This has led to the development of short-term (10-year)62 and medium-term (30-year)63 CVD risk prediction models used to guide clinical decision-making regarding potential benefits and risks of preventive pharmacotherapy.64 These cardiovascular prediction algorithms have traditionally relied on risk factors in men that may not be accurate for risk estimation in women. In addition, the risk prediction models assign a high score to age in predicting absolute risk so that typically only older patients exceed the thresholds set for initiation of treatment in the clinical practice guidelines. As such, these risk prediction models may underestimate the risk of CVD in young women with pregnancy complications.65 In addition, the emphasis on age may obscure the important contributions that modifiable risk factors make in young adults.64 Furthermore, modestly elevated risk factors in young adults may have little effect on short-term risk scores, but can substantially elevate longer term risk for CVD. In clinical practice, preventative measures against CVD often take the form of waiting until either an individual manifests CVRs or when the calculated 10-year or 30-year risk estimates exceed 10%. This approach, however, typically results in treating older adults who have exhibited risk factors over longer periods of time and as such have a potentially greater burden of atherosclerosis. Although this approach does represent a form of primary prevention, prevention of this nature does not reduce cardiovascular risk to the same extent as maintaining favorable risk factor levels throughout adulthood.64 A lifetime CVD risk estimate,66 based on the stratification of risk factors (ie, optimal vs nonoptimal vs elevated vs major) is an important adjunct to short-term (10-year) and medium-term (30-year) risk estimations that should be used to identify treatment-eligible young adults. The variables used for the different risk score estimates can be found in Table 38-3.
Variables Used in Each Method of Cardiovascular Risk Estimation. (Used with Permission of the Society of Obstetricians and Gynaecologists of Canada.) |
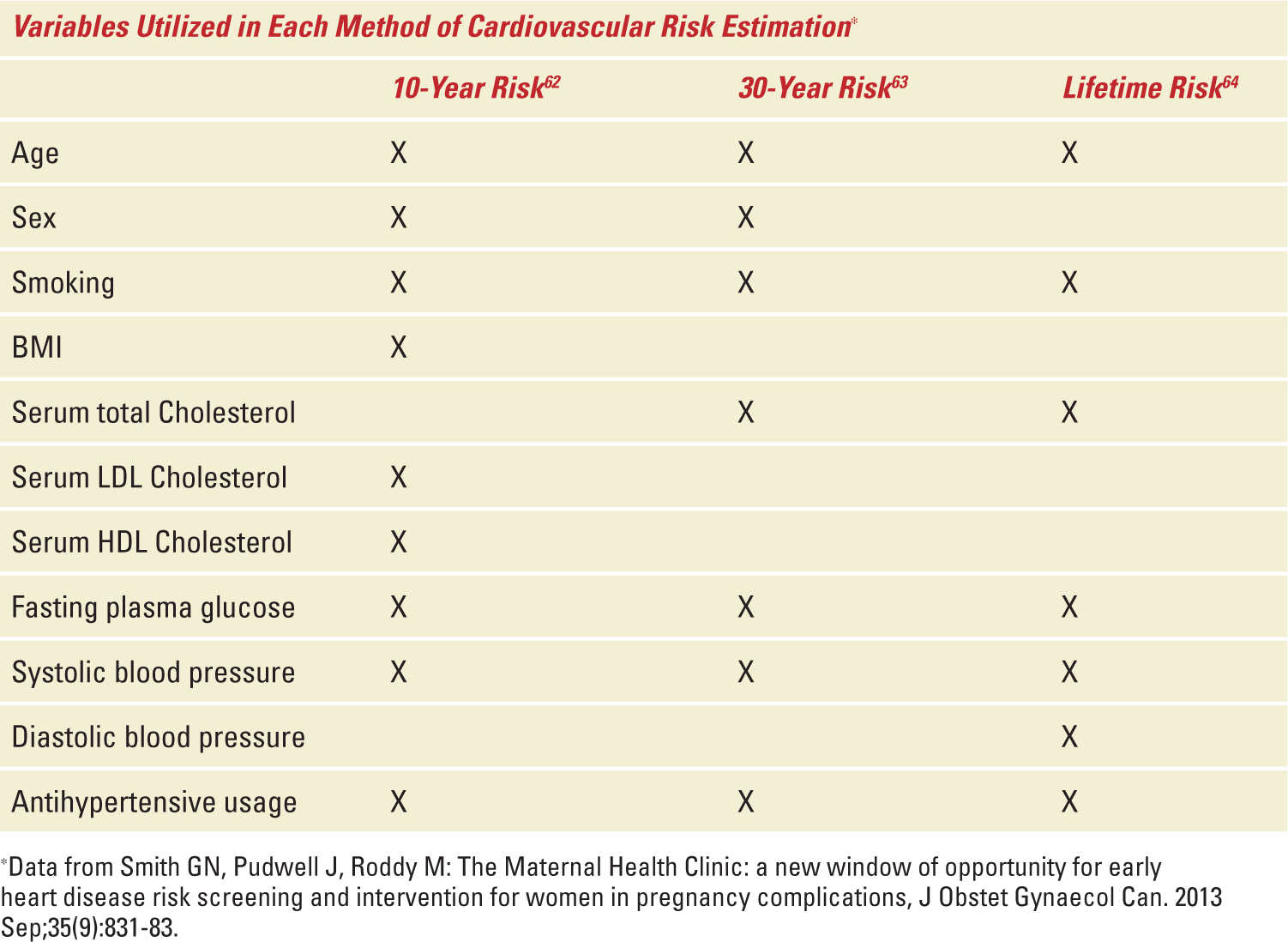
If younger women can be identified as being at high risk of CVD based upon either a high 10-year or 30-year risk score (≥10%) or a low 10-year or 30-year risk score (<10%) coupled with a high lifetime risk (≥39%), the risks and costs of long-term therapy may be more widely acceptable given the potential health benefits.64,67
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


