Fig. 2.1
(a) Ovarian cyst with surface involvement by friable papillary excrescences. They appear to cover most of the ovarian cyst surface. (b) The inner lining of the same ovarian cystic mass is mainly smooth. However, there are areas showing irregular friable vegetating masses
Typical SBT
Typical SBT makes up the majority of SBT or (90 %). A diagnosis of SBT/LMP is based on three main characteristics : (1) epithelial stratification and cellular budding where the tumor cells become detached from the papillae and appear to float in the cystic lumen with no fibrovascular core. (2) The tumor cells have mild to moderate cytologic atypia. (3) There is lack of stromal invasion. Microscopically, the papillae are lined by stratified cuboidal to columnar epithelial cells. These papillae show branching and complex structure. The epithelial cells have high nuclear-to-cytoplasmic ratio (N/C), and the nuclei are hyperchromatic with prominent nucleoli. Mitotic figures are frequently present (Fig. 2.2a–c). While the histologic criteria may suggest that a diagnosis of serous LMP is straightforward, sometimes the diagnosis of serous LMP can be challenging as these diagnoses are subject to numerous pitfalls, including the following:
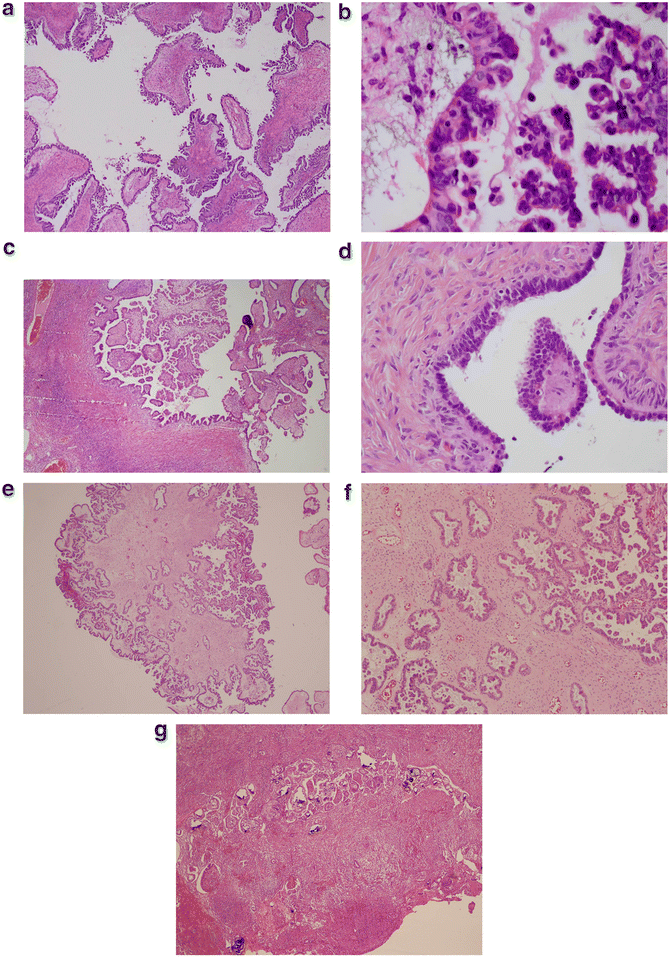

Fig. 2.2
(a) Cut section of these vegetating masses reveals papillary structure with fibrovascular stalks. These structures are lined by stratified cuboidal to columnar epithelial cells. (b) There is stratification of tumor cells which they start getting detached and float in the lumen. These cells exhibit moderate cellular atypia with high nuclear/cytoplasmic ratio. (c) The main characteristic feature of ovarian serous borderline tumor is the absence of ovarian stromal invasion. (d) Cut surface of benign serous cystadenoma. The cyst is lined by cuboidal epithelium. There are few areas where the cells appear to be stratified. However, due to the lack of cytologic atypia, this mass is considered as benign and this pseudo-stratification is due to tangential section. (e) Lower magnification showed tumor cells that seem to infiltrate fibrous stroma. (f) Higher magnification, these cells seemed to invade the stalk of the papillae and not the ovarian stroma which can be a major pitfall. (g) Microscopic features of autoimplantations are very similar to the features of desmoplastic noninvasive implant. They are defined by clusters of tumor cells in a background of extensive hemorrhage, fibrosis, and acute chronic inflammation. Frequent psammoma bodies are seen. These autoimplantations are usually seen on the surface of the ovary
Serous LMP May Have Variants
Some SBTs present with intracystic mucin and can mimic mucinous adenocarcinoma. The key to make the diagnosis is that the mucin is intracystic, not intracytoplasmic, as usually seen in mucinous tumors. The second variant is that the tumor can have a cribriform pattern and can mimic endometrioid tumor. While these variants do not carry any significance on prognosis, they can create a diagnostic challenge for pathologists.
Tangential Cut
Caution should be practiced when one sees what appears to be epithelial proliferation without cytologic atypia, because tangential sectioning of the lining of a benign serous cystadenoma can give the impression of proliferation of the epithelial lining (Fig. 2.2d).
Stromal Invasion
By definition, SBT lacks stromal invasion . This is a major criterion to differentiate SBT from serous adenocarcinoma. Therefore, invasion of the stalk of the papillae should not be considered as ovarian stromal invasion as illustrated in Fig. 2.2e, f.
Autoimplantation
Another pitfall is the failure to differentiate between stromal invasion and autoimplantation, which is the invagination of the tumor on itself creating the illusion of a stromal invasion, as shown in Fig. 2.2g. Grossly, serous LMP tumors exist as well-demarcated plaques on the surface of the ovary. It is essential to mention that autoimplantations are localized superficially on the surface of the ovary and are morphologically similar to desmoplastic noninvasive implants with disorganized groups of tumor cells embedded in dense stroma with hemorrhage, chronic inflammation, mesothelial proliferation, and massive necrosis.
Micropapillary SBT
Micropapillary SBT (MSBT) accounts for 5–10 % of all SBTs. The significance of this subtype is debated among pathologists. Some authors have found a close association between MSBT and invasive implants and urged to call this entity as “micropapillary serous carcinoma” [7, 8]. Others preferentially use the term MSBT, avoiding the use of the term of “carcinoma,” to minimize the possibility of over-treating patients [7, 8]. The general agreement on the significance of micropapillary architecture in SBTs is that there is a significant increase in incidence of invasive peritoneal implants [9]. Molecular studies show that MSBT has a similar gene expression profile as low-grade serous carcinoma and distinct from typical SBT [10]. MSBT is the only surface epithelial stromal tumor with a well-defined adenoma-carcinoma sequence, where LGSC is thought to arise in a stepwise fashion from a benign cystadenoma through BST to an invasive low-grade serous carcinoma [11]. Microscopically, MSBTs show highly complex micropapillary growth in a filigree pattern, growing in a nonhierarchical fashion from stalk which has been aptly described as a “Medusa head”-like appearance. Micropapillae are at least five times as long as they are wide [12] (Fig. 2.3a–c). Micropapillary foci should occupy an area of at least 5 mm, since micropapillary foci of less than 5 mm have no bearing on clinical outcome [12].
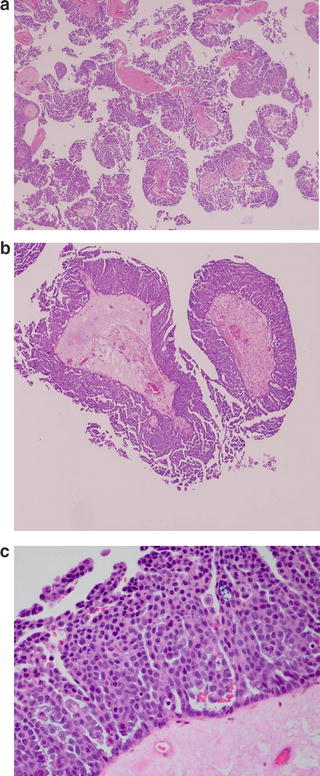

Fig. 2.3
(a) There is highly complex micropapillary growth in a filigree pattern, growing in a nonhierarchical fashion from stalk. These are described as a “Medusa head”-like appearance. (b) Micropapillae should be at least five times as long as they are wide. (c) Cytologically, tumor cells are somewhat bland looking exhibiting mild atypia and very infrequent mitotic figures
SBT with Microinvasion
Microinvasion is defined as single cells or few clusters of cells similar to those seen in the overlying SBT that infiltrate the stroma. One or more foci may be present but none should exceed 10 mm2 or not exceeding 3 mm or 5 mm. SBT with microinvasion appears to have no significance on disease outcome, with 10-year survival rate of 86 % [12].
Peritoneal Implants
Peritoneal implants are classified into epithelial invasive and noninvasive implants and desmoplastic noninvasive implants. Implants are a heterogeneous group of lesions and various types may coexist; therefore, multiple biopsies of numerous foci of suspicious lesions at the time of surgery and extensive tumor sampling by the pathologist are essential for the accurate evaluation of peritoneal implants. Differentiating invasive and noninvasive implants can be challenging, but given the increased probability of tumor recurrence for invasive implants, accurate diagnoses have a significant impact on patient prognosis and clinical management of the case.
Epithelial noninvasive implants are characterized by the presence of papillae within cystic spaces exhibiting mild cytologic atypia. There are frequent psammoma bodies and no stromal reaction or destruction with mild degree of inflammatory cells (Fig. 2.4a, b). SBTs with noninvasive implants are considered indolent, with 5-year survival rates of 95 % and recurrence rates are typically low, ranging from 8 % to 32 % [13].
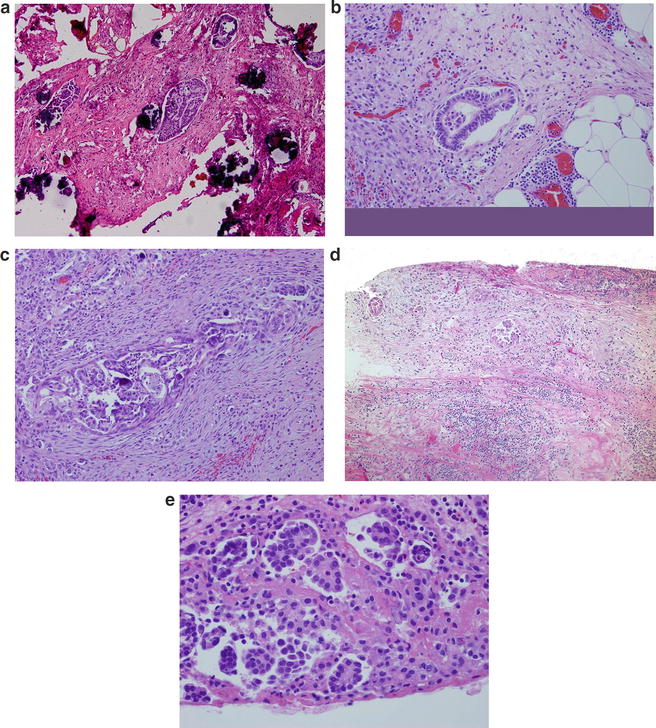

Fig. 2.4
(a) Noninvasive implants are characterized by cluster of tumor cells embedded in a fibrous tissue with no desmoplastic reaction. Psammoma bodies are frequently present. (b) Empty spaces are seen surrounding these clusters. (c) Invasive implants are characterized by complex papillae structures that seemed to infiltrate the stroma. There is extensive desmoplastic reaction with proliferation of fibrous tissue and chronic inflammation. Psammoma bodies are usually infrequent. (d) Desmoplastic non invasive implants are defined by clusters of papillae usually seen on the surface. These papillae are seen in a background of fibrotic stroma with extensive chronic inflammation. (e) Close r magnification shows very bland-looking cells surrounded by empty spaces
Epithelial invasive implants are characterized by haphazardly distributed glands and clusters of branching papillae infiltrating the adipose tissue and stroma. The epithelial cells have moderate to marked cytologic atypia. Psammoma bodies are sparsely distributed throughout the tumor, and the associated stroma is composed of dense fibrous tissue with mild degree of inflammation (Fig. 2.4c). Patients with SBT with invasive implants have higher chances of developing low-grade carcinomas many years after initial diagnosis [14].
Desmoplastic noninvasive implants are defined by clusters of irregular glands tumor cells exhibiting mild cytologic atypia. Frequent psammoma bodies are seen (Fig. 2.4d, e). There is no stromal reaction; on the contrary, the stroma is loose and may have granulation tissue-like features with neutrophilic infiltrates and hemorrhage.
Ovarian Grading Systems and Low-Grade Ovarian Serous Carcinoma
Before we discuss the molecular characteristics of low-grade ovarian serous carcinoma (LGSC), it is worth discussing the grading system for epithelial ovarian cancer. There are at least five grading systems that are in use by pathologists worldwide. The most commonly used around the world are from the International Federation of Gynecology Oncology (FIGO) and the World Health Organization (WHO). The FIGO grading system [15] is based on the ratio of glandular or papillary pattern to solid growth of the tumor: grade 1 tumors when <5 % is solid growth, grade II when 5–50 % is solid growth, and grade 3 tumors when >50 % is solid growth. The WHO system is more subjective, as it depends on the impression of the pathologist assessing the tumor architecture and cytologic features. It is considered an intuitive method where there are no actual objective criteria for grading. The other system used commonly in the United States is the Gynecologic Oncology Group (GOG) grading system [16]. Basically, the GOG system borrows the grading system from cancer occurring in other sites, depending on the histologic type; for example, the FIGO system for grading endometrial cancer will be used when the tumor is endometrioid type, and when the tumor is transitional cell type, the same grading system as for transitional cell carcinoma of the bladder is used. Clear cell carcinoma is not graded at all.
Of particular importance for basic and clinical research is the lack of reproducibility of the three grading systems and the frequent disparities between diagnoses by different pathologists using the same grading system [17, 18]. As a result, the significance of tumor grade to prognosis varies in the literature. It is clear that classification of EOC histological subtype and grading based on molecular markers would significantly improve reproducibility of diagnoses and enable more accurate clinical studies to be performed.
An additional grading system that is commonly used is Silverberg’s grading system [19]. Silverberg and his colleagues tried to create a grading system using the Nottingham grading system of the breast, which is based on architecture, cytologic atypia, and mitotic counts. Each is given a number and then they added to a score. As the criteria for this system are very defined and very objective, it is not surprising that this system shows a high degree of reproducibility among pathologists. In addition, using this grading system, tumor grade was shown to be a predictive factor for survival, with lower tumor grade associated with a more favorable outcome [20]. Lastly, the MD Anderson two-tier grading system grades each tumor as low grade or high grade [21]. Low-grade tumors are defined as tumors with mild atypia and a low frequency of mitotic figures (<12 mitoses/10 high-power fields), whereas high-grade tumors are tumors with moderate to severe atypia and high mitotic rates (Fig. 2.5a, b). This final grading system is only applied to serous carcinoma and again shows good intra-observer reproducibility [22]. Moreover, the two-tier system reveals prognostic associations that are consistent with those seen when using the Silverberg’s grading system [22].
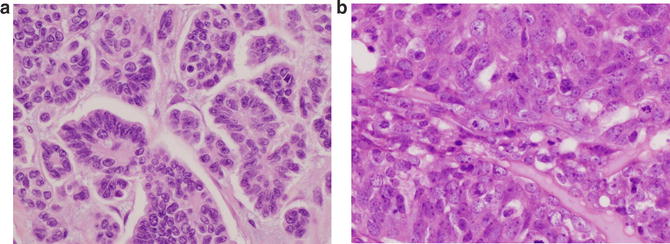

Fig. 2.5
(a) Low-grade serous carcinoma shows tumor cells with mild atypia and very few mitotic. (b) High-grade serous carcinoma is defined by tumor cells with moderate to severe atypia and high mitotic rate
Accurate grading of serous carcinomas as low-grade or high-grade is crucial for multiple reasons: (1) LGSCs and HGSCs are associated with markedly different prognoses, (2) LGSCs are usually cisplatinum resistant and so may often not receive standard chemotherapy, and (3) LGSCs may benefit from novel therapeutics designed to interrupt signaling pathways activated in this tumor type specifically.
Cellular Origins
Ovarian LGSCs are relatively rare tumors, which makes investigating the origins challenging. LGSCs can arise de novo but others clearly evolve in a stepwise manner beginning with a benign serous cystadenoma which progresses to a serous borderline tumor (SBT) which then develops into an invasive LGSC, as described above [23]. Not all borderline tumors will develop into invasive cancer but the proportion that do tend to have invasive implants upon presentation. LGSCs are a distinct entity to high-grade serous counterparts and are associated with distinct somatic alterations, clinical characteristics, and epidemiological risk factors. Although some case reports identified low-grade and high-grade components within the same tumor, this appears to be a rare occurrence and the distinct somatic profiles of LGSC and HGSC most strongly support the hypothesis that the two entities are different diseases and LGSC is not a precursor of HGSC [23]. The majority of HGSCs appear to originate from secretory cells in the fimbrial portion of the fallopian tube [4, 24–26]. Although recent pathological evidence has suggested a fallopian origin for at least a subset of LGSCs, classically it has been thought that LGSCs originate from ovarian surface epithelial cells (OSECs) . A third model for LGSC origins is the endometrial model. Each of these three cell-of-origin models is discussed in more detail below.
Ovarian Epithelial Cells
Historically it was thought that the majority of LGSCs arise from ovarian epithelial cells, a layer of simple, cuboidal, mesothelial-type epithelial cells covering the surface of the ovary. OSEC-type cells can also line simple cysts within the ovarian cortex, termed cortical inclusion cysts (CICs) . CICs arise from invaginations of the ovarian surface that occur following ovulation. Invaginations that fuse at the top create OSEC cysts, where OSECs are in close proximity to the mitogenic environment of the ovarian stroma. Interestingly, there is a relationship between body mass index (BMI) and number of CICs [27]. BMI is associated with borderline and low-grade serous cancer risk, but not HGSC risk [28], consistent with an ovarian origin for the former histological subgroup, but not for the latter.
In this model the microenvironment of the ovarian stroma plays a key role in the early genesis of LGSC by promoting Müllerian differentiation of OSECs. Evidence shows OSECs exhibit marked phenotypic plasticity, which some argue enables the cells to differentiate into the histologically diverse subtypes of EOC during cancer development [29]. However, theories supporting OSECs as cells of origin for serous ovarian cancer have recently come under scrutiny and have been heavily criticized. The lack of expression of EOC markers in OSECs, the divergent embryological origins of OSECs and Müllerian-type epithelium, and the scant evidence of early-OSEC-derived neoplastic lesions have all been used to question the validity of the OSEC as a precursor cell for serous EOCs.
Fallopian Epithelial Cells
Recent pathological evidence, as well as data from in vitro and in vivo models, has demonstrated that a significant proportion of high-grade serous ovarian cancers (HGSCs) originate from secretory epithelial cells located in the epithelium of the fallopian tube fimbriae [4, 24–26, 30]. This has led researchers to look more closely into whether LGSCs could also have a tubal origin. A key observation is the morphological similarity of LGSCs to the fallopian tube: LGSCs can contain both secretory and ciliated epithelia that closely resemble the morphology and immunohistochemical staining profile of normal tubal epithelium. The ratio of ciliated to secretory cells in fallopian-type inclusion cysts and serous cystadenomas is similar, with an increase in the proportion of secretory cells in borderline tumors progressing to a near absence of ciliated cells in LGSC [31]. Extensive sectioning and examination of fallopian tubes from patients with LGSC has identified regions of papillary tubal hyperplasia occurring more commonly in women with atypical proliferative serous tumors than in unaffected women [32]. Moreover, chronic salpingitis has been identified in association with ovarian serous borderline tumors, and secretory cell outgrowths (considered to be a precursor lesion) are more common in fallopian tubes from women with serous borderline tumors compared to controls [33]. Finally, mutational analyses have identified identical mutations in the KRAS proto-oncogene in serous borderline tumors and endosalpingiosis, suggesting co-occurrence of the two represent different stages of the disease continuum [34].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


