Fig. 13.1
HSIL/CIN3 and atypical mitotic figure. Nuclear atypia extends through the full epithelial thickness without any cytoplasmic maturation in the superficial epithelial layers. Note the nuclear size and nuclear/cytoplasmic ratio and the intraepithelial mitotic activity. Most cases of squamous intraepithelial neoplasia show increased mitotic activity, including atypical forms (inset)
Criteria for the Diagnosis of LSIL/CIN1/Mild Dysplasia including HPV-Associated Changes [88, 89]
Not all of the abnormalities that are typical of low-grade squamous intraepithelial neoplasia are found in every case, although nuclear atypia is essential for the diagnosis (Fig. 13.2). Some degree of nuclear abnormality extends through the full thickness of the squamous epithelium, but atypical nuclei are most prominent in the basal third, in proliferating basal and parabasal cells. Cytoplasmic maturation is present in the upper two-thirds of the epithelium although the nuclear/cytoplasmic ratio is increased. Mild nuclear hyperchromasia is usual, and sometimes irregularities are visible in the nuclear membrane. A well-defined nuclear halo with a sharp or “hard” edge may be present in squamous cells in the upper third of the epithelial thickness; if this feature is associated with the nuclear abnormalities described above, the abnormality is designated HPV effect, koilocytosis or koilocytotic atypia [90]. Usually there is acanthosis, papillomatosis, hyperkeratosis, or parakeratosis at the epithelial surface, and individual cell keratinization may be present [91]. Normal mitotic figures may be increased. Atypical mitoses are uncommon and if present support a diagnosis of LSIL/CIN1. LSIL may be identified in immature and atrophic squamous epithelium and is often seen in association with HPV infection, as too is multinucleation.
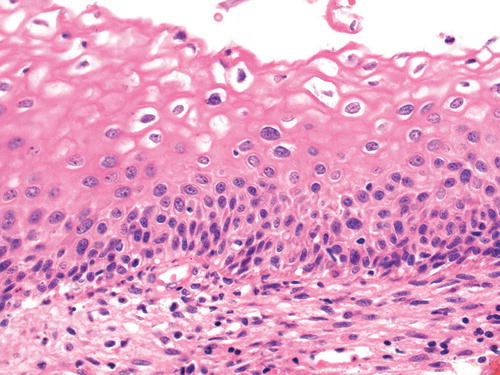

Fig. 13.2
LSIL/CIN1. Some degree of nuclear abnormality extends through the full epithelial thickness, but atypical nuclei are most prominent in the basal third of the epithelium, in the basal and parabasal cells. Koilocytosis (HPV effect) is present in the squamous cells in the upper third of the epithelium. The koilocytes have a well-defined nuclear halo with a sharp or “hard” edge and tend to have a smaller crenated nucleus—sometimes described as “raisinoid”
LSIL must be distinguished from HSIL/CIN2/CIN3 and benign mimics of LSIL, including a range of inflammatory processes and infections . Immunohistochemistry (see section “Histogenesis and Immunophenotype” below) may be helpful.
Criteria for the Diagnosis of HSIL (CIN2 and CIN3)
There is poor interobserver variability for the diagnosis of CIN2 [79] and in follow-up excision specimens, over a half of patients who had CIN2 biopsies are found to have CIN3 [92]. The intermediate state of CIN2 is felt to be a “mix of biological CIN1 and CIN3” [38].
In both HSIL (CIN2) (Fig. 13.3) and HSIL (CIN3) (Fig. 13.1), nuclear atypia extends through the full thickness of the epithelium and is more marked than in LSIL/CIN1. There is increased nuclear size and nuclear/cytoplasmic ratio and irregularity of the nuclear outline. Cytoplasmic maturation is present in the upper third of the epithelium in HSIL (CIN2) but usually absent or confined only to the superficial epithelial layers in HSIL (CIN3). Mitotic figures (including abnormal forms) are increased and found at all levels of the epithelium.
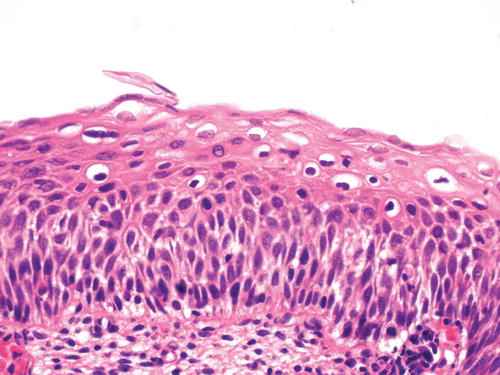

Fig. 13.3
HSIL/CIN2. Nuclear atypia extends through the full thickness of the epithelium but is most prominent in the lower half to two-thirds. There is some cytoplasmic maturation in the upper third of the epithelium, where koilocytes are also seen
Nucleolation is not usually a feature of high-grade dysplasia and if present should prompt the exclusion of an under-sampled squamous carcinoma, reparatory changes, or severe inflammatory atypia. p16 expression is not present in reparative or inflammatory atypia [38].
The 2014 WHO fascicle [50] describes three variants of HSIL: thin HSIL, keratinizing HSIL, and papillary squamous carcinoma in situ. Thin HSIL (Fig. 13.4) represents a high-grade squamous intraepithelial lesion that is <10 cells thick and may be differentiated from immature metaplasia and cervical atrophy by immunohistochemistry. Keratinizing HSIL (Fig. 13.5) is usually found on the ectocervix and resembles HPV-associated HSILs at cutaneous sites such as the vulva. Keratinizing HSIL has a thick layer of keratinization on the surface and markedly pleomorphic and severely atypical, dyskeratotic cells within the squamous epithelium. Papillary squamous carcinoma in situ should be diagnosed only if the lesion has been completely excised and the absence of stromal invasion confirmed. This is a papillary lesion with a squamotransitional morphology: the papillae are fine and are overlain by severely dysplastic epithelium, which resembles neoplastic urothelium [93, 94].
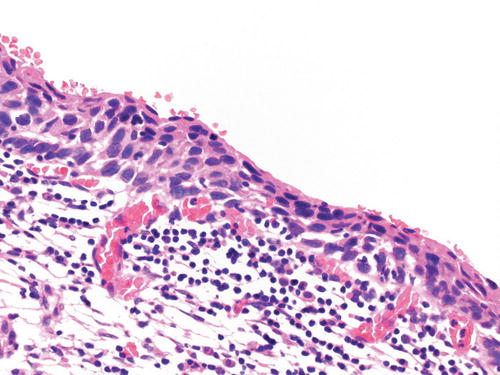
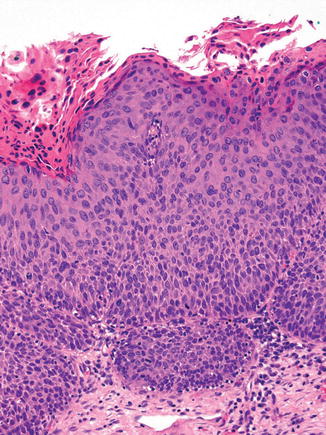

Fig. 13.4
Thin HSIL. This high-grade squamous intraepithelial lesion is <10 cells thick and shows full thickness nuclear atypia with an increase in nuclear size and nuclear/cytoplasmic ratio

Fig. 13.5
Keratinizing HSIL. This variant of HSIL has a thick layer of keratinization on the surface and markedly pleomorphic and severely atypical cells throughout the squamous epithelium
Histogenesis and Immunophenotype
Histogenesis
Both LSILs and HSILs develop as a result of HPV infection. Over 80 % of LSILs result from infection by high-risk (HR) subtypes of HPV [95, 96]. The remainder—true LSILs—result from infection by low-risk (LR) HPV types. Over 90 % of HSILs are caused by infection with HR HPV [97]. Traditionally, squamous intraepithelial lesions (SILs) were thought to progress in a stepwise fashion from LSIL to HSIL. However, this is controversial. Although some studies have shown that HSIL may arise de novo without progression from LSIL [98] and that specific HPV subtypes are associated with individual SILs [48, 82], variations in tissue sampling and histological interpretation of biopsy material [99] have clouded the issue.
Human papillomaviruses (HPVs) typically infect basal cells in the stratified squamous epithelium of the cervical transformation zone. It is likely that HPV infection of the basal layer is acquired through mild abrasion or “microtrauma” of the squamous epithelium [100]. The basal cells are sometimes referred to as reserve cells or stem cells because they and the contiguous parabasal cells are the only cervical epithelial cells capable of cell division and are therefore responsible for maintenance and regeneration of the squamous epithelium. They have the potential to differentiate along one or more epithelial lines: squamous, glandular, or neuroendocrine. When basal cells are committed to squamous differentiation, they mature throughout the epithelial thickness in an orderly fashion. This maturation takes place at both morphological and molecular levels. If morphologically normal basal cells become infected with HPV, the infection is typically nonproductive with only maintenance levels of gene expression (see above). There is normally close regulation of productive HPV gene expression, and this takes place only in cells that have begun squamous maturation and lost their proliferative capacity [101–104]. Early virus proteins are expressed in the parabasal zone, and as squamous differentiation proceeds, there is viral DNA synthesis and induction of all viral genes, with production and assembly of virions in the squamous cells just beneath the epithelial surface. Morphologically, such lesions manifest as LSILs and are characterized by koilocytic atypia, nuclear enlargement, and hyperchromasia. These features are the result of viral proteins that affect DNA synthesis and the structure of intermediate filaments in the host cell cytoplasm. However, the absence of koilocytes in cytology samples and the absence of koilocytic atypia in histological specimens should not be interpreted as an absence of HPV gene expression.
In HSILs which are usually associated with HR HPV infection, coordination is lost between cellular differentiation and viral early gene expression, and the viral oncogenes E6 and E7 are inappropriately expressed in a population of immature-looking squamous cells that retain the capacity to divide and initiate unregulated cell proliferation [48, 105]. With the continued proliferation of this population of cells, the normal epithelium is overrun by epithelial cells that show disorderly squamous maturation and have a basal cell-like morphology, i.e., the epithelium shows the morphological features of HSIL (CIN2 and 3).
Immunophenotype
Immunohistochemistry is helpful to confirm a diagnosis of CIN—the morphological changes of LSIL/CIN1 may be subtle in thin metaplastic epithelium, and distinguishing HSILs from benign mimics such as immature squamous metaplasia, transitional metaplasia, and atrophy may be problematic. Reactive/inflammatory changes, morphological changes associated with repair and regeneration, and tangential sectioning can also mimic both HSIL and LSIL. Finally, immunohistochemistry may be of value in identifying CIN at resection margins of loop biopsies where there is significant electrothermal/cautery artifact.
The immunohistochemical markers most commonly used to confirm a diagnosis of CIN are those that corroborate HPV infection (p16 and ProEx C) and confirm proliferation in the atypical cells (MIB-1) [106, 107].
The p16 gene , CDKN2A, (previously P16INK4A) [108] is a tumor suppressor gene that encodes a cyclin-dependent kinase inhibitor, a protein involved in cell cycle regulation. p16 acts with the retinoblastoma protein, another tumor suppressor, in a negative feedback loop to control cell proliferation. When HR HPV DNA integrates into the host cell genome, the viral oncoprotein E7 binds to the retinoblastoma protein and inactivates it, with resultant loss of negative feedback, and p16 overexpression (see above). p16 is therefore a surrogate marker of HR HPV infection. Significant p16 expression manifests as diffuse, strong, cytoplasmic, and nuclear immunopositivity (“block” immunopositivity) in squamous lesions associated with HR HPV infection, and the extent of labeling correlates with the grade of the histological abnormality [109]. In one study, over 99 % of cases with histologically confirmed HSIL (CIN 3) were p16-positive [110]. According to recommendations of the LAST project [38], the labeling should be present in the basal layer and extend upward to involve at least one-third of the epithelium (Fig. 13.6). The “one-third” condition has been suggested to add specificity but is arbitrary. Full-thickness labeling or extension into the upper third or half of the epithelium is not required to call a specimen positive.
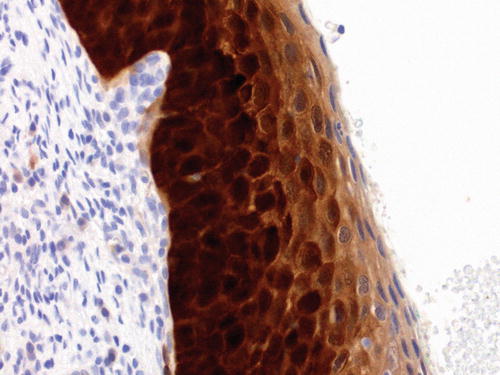

Fig. 13.6
Immunolabeling of p16 in HSIL. The labeling is both nuclear and cytoplasmic and extends from the basal layer upward through most of the thickness of the epithelium. According to the recommendations of the LAST project, this pattern of “block” staining should be present in the basal epithelium and involve at least one-third of the epithelial thickness
Interpretation of p16 immunolabeling can be problematic as the intensity and distribution of the staining may be variable, particularly when associated with LR HPV infection and a range of lesions in the cervix that may mimic CIN. Focal or patchy nuclear staining is regarded as nonspecific and may be present in reactive conditions as well as LSIL and immature metaplasia (Fig. 13.7). p16 may help in the diagnosis of CIN2 but is not recommended if the differential diagnosis on H&E morphology is between LSIL (CIN1) and normal epithelium, because LSIL (CIN1) can be p16-negative; only 30 % of cases of CIN1 are p16-positive [110–113]. p16 immunohistochemistry is recommended when assessing biopsy specimens that appear morphologically low grade but associated with high-grade antecedent cytology, when there is a high risk of missed high-grade disease.
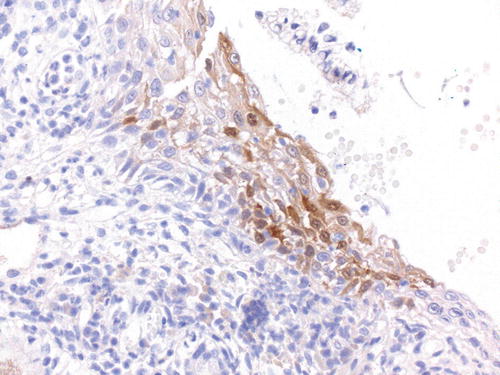

Fig. 13.7
Immunolabeling p16 in LSIL. This example illustrates focal or patchy nuclear and cytoplasmic p16 in LSIL, but the labeling pattern is regarded as nonspecific and may also be present in reactive conditions as well as immature metaplasia
It is important that the immunohistochemistry is interpreted in an adequate biopsy, in which a precancerous lesion is morphologically under consideration. Tiny, disrupted epithelial fragments, detached single epithelial cells, and other material of a suboptimal nature may lead to misinterpretation of p16 immunohistochemistry. The LAST study concluded that p16 is the most reliable biomarker of the activation of E6/E7-driven cell proliferation in the context of HPV infection, and its use is therefore recommended in the assessment of lower anogenital tract SILs [38].
ProEx C , a cocktail of antibodies against topoisomerase II-alpha and minichromosome maintenance two proteins, is overexpressed in CIN and cervical carcinoma [114, 115]. Only nuclear positivity in the upper two-thirds of the epithelium is considered significant. Several studies have confirmed that ProEx C is comparable to p16 and MIB-1 in identifying HSIL in formalin-fixed tissue and in its differentiation from benign mimics [116–118]. However, a literature review carried out as part of the LAST project [38] found insufficient data to recommend ProEx C (or MIB-1) for use, alone or in combination, in the diagnosis of CIN. The LAST report acknowledged t hat some pathologists or institutions might choose to use these other markers as an adjunct, within a broader panel or in selected cases when p16 labeling is equivocal. Since both ProEx C and MIB-1 show very specific nuclear staining, the immunohistochemistry may be easier to interpret.
MIB-1 is a monoclonal antibody against Ki-67, a nuclear cell proliferation-associated antigen that is expressed in all active parts of the cell cycle (G1, S, G2/M) [119–121]. Expression of MIB-1 is usually confined to basal and parabasal cells. Detection of this antigen in the middle and upper thirds of the squamous epithelium is therefore significant. HSIL (CIN2 and CIN3) usually shows widespread nuclear MIB-1-positivity in all layers of the epithelium [122]. In LSIL (CIN1), staining is not widespread and only small clusters of immunopositive squamous cells may be present in the upper two-thirds of the epithelium [123]. Tangential sectioning and intraepithelial lymphocytes associated with inflammatory changes may give the impression that MIB-1-immunopositive cells are in the upper two-thirds of the squamous epithelium, and pathologists should be mindful of this diagnostic pitfall.
Patient Outcomes
LSIL
In histologically confirmed LSIL, regression occurs within 12 months on average [50]. The risk of progression correlates with HPV type (HPV 16 infection is associated with a high risk of progression), immunosuppression, smoking, and older age. The biopsy procedure itself affects the natural history of LSIL—regression occurs in up to one-third of cases, and this reduces the predictive value of biomarkers [92]. Even after colposcopy and a histological diagnosis of LSIL, there is 10 % chance that HSIL has been missed by the biopsy [124–126]. In some studies, the risk of progression was found to be increased when LSIL was associated with p16 expression [127, 128]. However, to date, no combination of biomarkers has been shown reliably to predict whether a LSIL lesion will regress, persist, or progress.
HSIL
Testing for HPV DNA 12 months after treatment is the best predictor of recurrent or residual disease [129]. The size of the lesion and the presence of margin involvement are also predictive of recurrence. There are no significant differences in outcome based on the modality of treatment (loop electrosurgical excision, conization, laser ablation, or cryotherapy). To date, no biomarkers have proven reliable in predicting which HSILs are most likely to progress.
References
1.
Berman JJ, Albores-Saavedra J, Bostwick D, Delellis R, Eble J, Hamilton SR, et al. Precancer: a conceptual working definition – results of a Consensus Conference. Cancer Detect Prev. 2006;30:387–94.PubMed
2.
Williams J. On cancer of the uterus: being the Harveian Lectures for 1886. London: H.K. Lewis; 1888.
3.
Cullen TS. Cancer of the uterus. New York, NY: Appleton; 1900.
4.
Papanicolaou GN. Diagnosis of pregnancy by cytologic criteria in catheterized urine. Proc Soc Exp Biol Med. 1948;67:247–9.PubMed
5.
Papanicolaou GN. Diagnostic value of exfoliated cells from cancerous tissues. J Am Med Assoc. 1946;131:372–8.PubMed
6.
Papanicolaou GN. A general survey of the vaginal smear and its use in research and diagnosis. Am J Obstet Gynecol. 1946;51:316–28.PubMed
7.
Nelson Jr JH, Masterson JG. Confirmatory diagnostic procedures and definitive treatment following a positive cervical smear. CA Cancer J Clin. 1964;14:46–58.PubMed
8.
Papanicolaou GN. A survey of the actualities and potentialities of exfoliative cytology in cancer diagnosis. Ann Intern Med. 1949;31:661–74.PubMed
9.
Buckley CH, Butler EB, Fox H. Cervical intraepithelial neoplasia. J Clin Pathol. 1982;35:1–13.PubMedCentralPubMed
10.
International Committee on Histological Definitions, 1961. Acta Cytol. 1962;6:235–6.
13.
Poulsen HE, Taylor CW, Sobin LH. Histological typing of female genital tract tumours. International Histological Classification of Tumours No 13. Geneva: World Health Organisation; 1975.
14.
Fu YS, Reagan JW. Precursors of cervical cancer. Cancer Surv. 1983;2:359–82.
16.
Cocker J, Fox H, Langley FA. Consistency in the histological diagnosis of epithelial abnormalities of the cervix uteri. J Clin Pathol. 1968;21:67–70.PubMedCentralPubMed
17.
Kirkland JA. Atypical epithelial changes in the uterine cervix. J Clin Pathol. 1963;16:150–4.PubMedCentralPubMed
18.
Govan AD, Haines RM, Langley FA, Taylor CW, Woodcock AS. The histology and cytology of changes in the epithelium of the cervix uteri. J Clin Pathol. 1969;22:383–95.PubMedCentralPubMed
19.
Richart RM. Cervical intraepithelial neoplasia. Pathol Annu. 1973;8:301–28.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


