Chapter 27
Postoperative and Chronic Pain
Systemic and Regional Analgesic Techniques
Pamela Flood MD, Pedram Aleshi MD
Chapter Outline
MECHANISMS AND PREVALENCE OF PAIN
NON-NEURAXIAL REGIONAL ANALGESIC TECHNIQUES
NONPHARMACOLOGIC INTERVENTIONS
IMPACT OF PAIN AND ANALGESIC TREATMENT ON BREAST-FEEDING
Mechanisms and Prevalence of Pain
Pregnancy is associated with increased excitability of mechanosensitive afferent nerve fibers innervating the uterine cervix and lower uterine corpus. This change in sensitivity of the nerve fibers is likely due, at least in part, to elevated estrogen levels during pregnancy.1,2 Whether skin and visceral nociception are transmitted by different nerve fiber subgroups or discharge patterns is controversial.3 The uterine (visceral) afferent fibers stimulated by pressure and vasoconstriction primarily include C fibers and some A-delta fibers. By contrast, the majority of afferent fibers that relay nociceptive stimuli from the skin are A-delta fibers.
Postoperative pain results from direct trauma to tissue and subsequent inflammation. Local and systemic inflammatory cytokines act to sensitize the peripheral nerves and enhance pain perception.4 Inflammation likely plays a significant role in pain after delivery because inflammatory cytokines are elevated as a part of the normal labor and delivery process.5,6 Additionally, after cesarean delivery, cytokines are present in the wound; their concentration is positively correlated with analgesic drug consumption.7
Multimodal therapy has long been advocated for postoperative analgesia in nonobstetric patients, and it clearly provides benefit to obstetric patients. Local anesthetics, nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, gabapentin, and epidural clonidine are efficacious adjuvants to opioid analgesia after cesarean delivery.8–13
Pain is less severe immediately after vaginal delivery than after cesarean delivery (Figure 27-1).14 In one multicenter study, patients reported an average pain score of 3.3 of a possible 10 during the first 24 hours after vaginal delivery and an average score of 4.7 after cesarean delivery; however, there was significant variability in both groups. Forceps-assisted vaginal delivery was associated with more pain than spontaneous vaginal delivery, likely owing to local tissue damage from the forceps or factors related to the indication for forceps delivery. Mode of delivery was not associated with risk for persistent pain 8 weeks after delivery.14
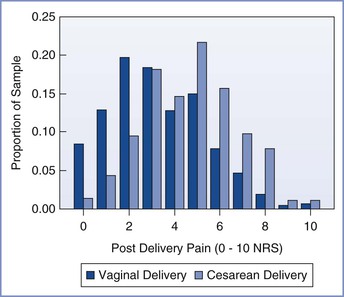
FIGURE 27-1 Distribution of numerical rating scale (NRS) scores (0, no pain; 10, worst pain imaginable) of average pain for the first 24 hours after vaginal and cesarean delivery. (From Eisenach JC, Pan PH, Smiley R, et al. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain 2008; 140:87-94.)
Intrapartum and postpartum pain was previously considered a reality of life that was not discussed as a complication of childbirth. The idea that acute pain during and immediately after delivery may have long-term consequences was not evaluated until the early 2000s, when investigators noted that severe pain after some types of surgery was associated with a high incidence of persistent chronic pain.14,15
Pain after Cesarean Delivery
In a prospective study, the prevalence of persistent pain 8 weeks after cesarean delivery was 9.2%; the risk for experiencing pain at 8 weeks was associated with the severity of acute postpartum pain.14 In a retrospective survey, the incidence of persistent pain 1 year after delivery was 10% after vaginal delivery and 18% after cesarean delivery.16 Another retrospective study reported a 6% incidence of daily or almost daily pain 6 to 18 months after cesarean delivery.17 In these studies,16,17 the incidence of persistent pain after cesarean delivery was comparable to that reported 4 months after a hysterectomy.18 By contrast to the results of retrospective studies,16,17 a prospective study observed that the incidence of persistent pain 1 year after either cesarean or vaginal delivery was less than 1%.19
Pain after Vaginal Delivery
In the absence of analgesia, almost all patients experience severe pain at some point during labor and vaginal delivery (see Chapter 20).20,21 The severity of acute pain after vaginal delivery is highly variable.14 Most patients experience some degree of cramping pain from uterine involution. Severe pain may result from episiotomy, perineal lacerations, and/or perineal hematoma. This pain is transmitted primarily via the pudendal and iliohypogastric nerves and the sacral and lumbar plexuses. Such pain should be treated aggressively because it significantly distracts from the activities of daily living for a new mother. First-line treatment of pain after vaginal delivery typically consists of NSAIDs, supplemented by opioids if necessary. In a prospective study, the prevalence of persistent pain 8 weeks after vaginal delivery was 10%.14
Predictors of Pain
Intrinsic Patient Factors
The likelihood of developing chronic pain after childbirth may be influenced by multiple factors. There is evidence for heritability in labor pain. Studies of nonpregnant patients have shown that patients who carry a common polymorphism in the μ-opioid receptor gene (OPRM1: A118G [substitution of guanine for adenine at nucleotide position 118; Rs 1799971]) have an altered response to exogenous opioids.22 Landau et al.23 studied the ED50 (median effective dose) of intrathecal fentanyl for labor analgesia in women who were homozygous for the wild-type allele compared with women who carried the A118G polymorphism. Women who carried at least one copy of the G-allele had a lower ED50 and requested analgesia at a greater cervical dilation than women with two copies of the wild-type allele.23 However, in another study, the duration of intrathecal fentanyl analgesia did not vary according to the OPRM1 genotype.24
In contrast to the findings of Landau et al.,23 in which women with the G-allele had a lower ED50 for intrathecal fentanyl for labor analgesia, Sia et al.25 observed that women who carried the G-allele self-administered a higher dose of morphine to treat breakthrough pain after intrathecal morphine administration for postcesarean analgesia. The reasons for these seemingly disparate results have yet to be elucidated.26
Elevated concentrations of endorphins and enkephalins are found in the plasma and cerebrospinal fluid (CSF) of parturients, and opioid antagonists abolish pregnancy-induced analgesia to visceral stimulation in experimental animals (see Chapter 2). Given our current knowledge of the influence of genetic variability on response to exogenous opioids, it would not be surprising if some of the variability in labor pain and acute postpartum pain is a result of differing responses to endogenous and exogenous opioids. Other genes may also play a role. β2-Adrenergic receptor polymorphisms have been associated with preterm labor27 and labor progress.28,29 Slower development of pain during labor may be associated with slower labor progress.28
Long-standing psychological factors and mental preparation for labor influence labor pain or its expression during labor. Catastrophizing (i.e., the unfounded belief that something will be worse than it is) has been associated with labor pain and request for treatment.30,31 There is evidence that even the way that the practitioner asks about pain influences the response. When the negative word “pain” was used in a postcesarean visit, 54% of women reported pain. By comparison, when patients were asked, “Are you comfortable,” only 28% reported pain directly or indirectly (P < .001).32 Preexisting depression and anxiety impact the outcome after surgery; affected patients report more severe pain and are at increased risk for analgesic drug abuse.33
Women who abuse illegal drugs are at increased risk for adverse pregnancy outcomes.34 These patients have particularly high risk for inadequate treatment of intrapartum and postpartum pain. Although a large proportion of pregnant women who abuse drugs deny doing so,35 it is important to identify these patients antenatally to devise an analgesia plan. Buprenorphine and methadone are the most common drugs used for treatment of opioid addiction during pregnancy. Both drugs are effective and are not associated with respiratory depression in the neonate, although these infants usually require treatment of opioid withdrawal.36 Women who use opioids, cocaine, and/or amphetamines during pregnancy require more analgesia during labor than nonusers.37 Patients on opioid maintenance programs should receive their normal maintenance dose of opioid and should receive additional medication to treat postpartum pain. A multimodal analgesia strategy that includes NSAIDs is beneficial in this setting.
Women with preexisting chronic pain syndromes may have significant anxiety regarding childbirth pain. Anxiety itself is a predictor of labor pain.38 Patients with chronic pain syndromes who are treated with chronic opioid therapy are likely to develop opioid tolerance similar to patients who use opioids recreationally. Thus, patients with chronic pain syndromes should be maintained on their therapeutic regimen throughout the course of labor, delivery, and recovery; additional medication will likely be necessary to treat postpartum pain.
Environmental Factors
Sleep deprivation accentuates responses to noxious stimuli. Research subjects who sleep less than 6.5 hours per day have greater areas of secondary hyperalgesia in response to a heat test after capsaicin treatment. Additionally, those deprived of sleep have a lower threshold for pressure pain.39 Sleep deprivation accentuates pain, and pain interrupts sleep. This association creates a vicious cycle that commonly accentuates pain after cesarean delivery. Prolonged labor may precede cesarean delivery. Afterward, care of the newborn requires frequent interruptions in sleep. It is important to facilitate sleep as a therapeutic intervention as much as possible by decreasing environmental stimuli and reducing light during the evening.
Stress is also an important factor that can worsen postoperative pain and can facilitate conversion to chronic pain. Antepartum stressors induced by fear of surgery, parenting, or other changes that are associated with childbirth and cesarean delivery may accentuate postoperative pain. In patients undergoing back surgery, preoperative report of worry or intrusive memories was associated with chronic preoperative pain, failed back syndrome, and chronic postoperative pain.40 Further, biochemical evidence of preoperative stress, as measured by abnormal reactivity of the hypothalamic-pituitary-adrenal axis, was also associated with chronic pain after surgery.40 Evidence specific to obstetric surgery is limited, but there is no reason to expect that the common stressors that accompany a significant life change such as childbirth, as well as specific individual stressors, would not have a similar impact on postcesarean pain and conversion to chronic pain.
In summary, the presence of intrinsic and extrinsic factors predicts severe acute pain after cesarean delivery. Similarly, conversion to chronic pain is associated with the presence of these factors and the presence of severe acute postoperative pain. Patients may have an intrinsic tendency toward severe pain in response to injury. This tendency may be inherited as a genetic or epigenetic phenomenon, or it may be acquired through life experiences. Assessment of these predictive factors with validated scales may identify a subset of women for whom aggressive multimodal treatment of acute postoperative pain may prevent conversion of acute to chronic pain.41
Depression, anxiety, sleep deprivation, and disability are also predictable consequences of severe pain, thus creating a positive reinforcing cycle. Interruption of this cycle by predicting and treating severe postcesarean pain may help prevent the development of chronic pain. In addition, identification and treatment of coexisting psychological distress and sleep disorders may help prevent conversion of acute pain to chronic pain.
In 2001, The Joint Commission announced new pain management standards.42 These standards recognize the right of patients to receive appropriate assessment and management of pain. The standards require accredited organizations to screen patients for pain during the initial assessment, to reassess pain periodically, and to educate patients about pain management options. Fulfillment of these requirements in the obstetric setting is challenging because pain is expected during childbirth. Nonetheless, the mandate for pain assessment is not controversial. As occurs with all other types of surgery, obstetric patients should be assessed for postpartum pain at regular intervals and offered treatment. Multimodal pharmacologic and nonpharmacologic treatment for pain is the optimal approach and should be offered whenever feasible and medically indicated.
Systemic Opioid Analgesia
Neuraxial opioid administration currently represents the “gold standard” for providing effective postcesarean analgesia. Intrathecal and epidural opioid administration provide analgesia that is superior to intramuscular opioid and intravenous opioid patient-controlled analgesia (PCA) after cesarean delivery (see Chapter 28, Figure 28-1).43–45 A 2010 systematic review found that a single bolus dose of epidural morphine provides better analgesia than parenteral opioids after cesarean delivery.46 In the United States, most women who undergo cesarean delivery with neuraxial anesthesia receive neuraxial opioids for postcesarean analgesia. Multimodal analgesic strategies are used to augment the analgesic effect of neuraxial opioids in this setting. Some women may have breakthrough pain using this analgesia strategy and may require augmentation with systemic opioids.
Although pain after vaginal delivery is usually less severe than pain after cesarean delivery, its severity is highly variable. A large episiotomy with a third- or fourth-degree perineal laceration may warrant administration of a single dose of a neuraxial opioid for postpartum analgesia.47
The analgesic effect of opioids can vary significantly among patients; one study observed a fivefold difference in the maximum blood drug concentration still associated with pain (MCP).48 The MCP decreases over time after a surgical procedure. The goal of opioid administration is to achieve a minimum effective analgesic concentration (MEAC) with minimal side effects.49 Because both the MEAC and the MCP vary greatly among patients, an individualized approach to pain control is required. There are no maximum allowable doses for specific opioids; the primary limiting factor is the occurrence of side effects (Table 27-1).
TABLE 27-1
Management of Opioid Side Effects*
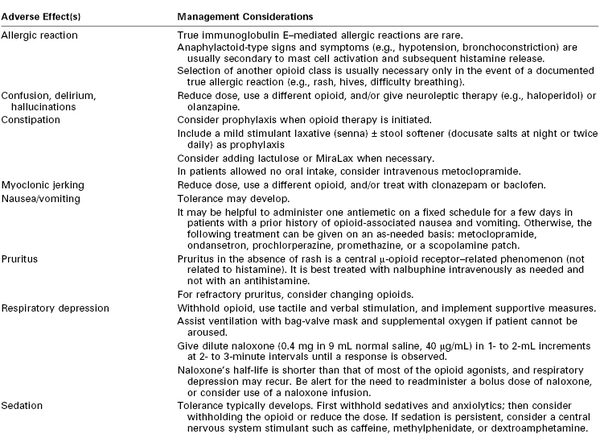
* Nonopioid analgesic options should be considered to limit opioid-related side effects. All adverse events should be carefully evaluated to rule out other potential causes.
Courtesy of the Dana Farber Cancer Institute Pain and Palliative Care Program and the Brigham and Women’s Hospital Pain Committee. Modified with permission from Bridget C. Fowler, Pharm. D., Clinical Pharmacy Manager, Dana Farber Cancer Institute.
Patients with hepatic or renal dysfunction (which may occur with severe preeclampsia), morbid obesity, and/or obstructive sleep apnea are particularly susceptible to the respiratory depressant effects of opioids. Patients who have never received opioids may be especially prone to the occurrence of side effects. It is important to monitor the respiratory rate and sedation level before giving an additional dose or adjusting the bolus dose that the patient can self-administer. After adjusting the dose, the clinician must document the respiratory rate and pattern, sedation level, and analgesic response. The use of a multimodal analgesic approach helps provide adequate analgesia while limiting opioid-related side effects (see later discussion).
Intravenous Patient-Controlled Analgesia
In the past, opioids were commonly administered intramuscularly or subcutaneously; these simple routes of administration do not require the postoperative return of bowel activity or the use of sophisticated equipment. Intramuscular and subcutaneous medications are inexpensive, easy to administer, and associated with a long history of safety. Disadvantages of this approach include the need for repeated painful injections, a delayed (and sometimes erratic) absorption of drug, and an inconsistent analgesic response due to variations in plasma opioid concentrations.
A number of studies have compared intravenous opioid PCA to traditional nurse-administered parenteral analgesia. A 2006 meta-analysis concluded that intravenous PCA provided better postoperative analgesia and greater patient satisfaction than conventional nurse-administered opioid analgesia.50 Although the use of intravenous PCA was associated with greater opioid use and a higher incidence of pruritus, the incidence of other side effects was not different between groups.50 The American Society of Anesthesiologists (ASA) Task Force for Acute Pain Management in the Perioperative Setting51 recommends that “these modalities [epidural or intrathecal opioids, systemic opioid PCA, and peripheral regional techniques] should be used in preference to intramuscular opioids ordered ‘as needed.’ ”
PCA has been used with intravenous and epidural routes of administration after cesarean delivery. In a study of intravenous versus epidural meperidine PCA, higher pain scores with rest and movement, greater sedation, and lower patient satisfaction were observed with the intravenous route of administration.52 Moreover, plasma meperidine and normeperidine concentrations were almost double with the intravenous route.52 Similarly, another study that compared intravenous versus epidural fentanyl PCA after cesarean delivery reported higher pain scores and greater fentanyl consumption with the intravenous route, although patient satisfaction ratings were similar in the two groups.53 Another study compared intravenous versus epidural hydromorphone PCA after cesarean delivery.54 Hydromorphone requirements were threefold to fourfold higher in the intravenous group. The two groups had similar pain and sedation scores, but patients in the intravenous group reported more frequent drowsiness and less pruritus.54
Several studies have compared intravenous morphine PCA to single-shot epidural morphine for postcesarean analgesia.45,55,56 Although the incidence of pruritus was higher with epidural morphine than with intravenous morphine PCA, analgesia and patient satisfaction were better with epidural morphine.45,55,56 The incidence of nausea was not different between groups45,55,56; sedation was greater in the intravenous PCA group.45
Choice of Opioid
Overall, factors that affect the choice of opioid are speed of onset, duration of action, overall efficacy, and the type and frequency of side effects (Table 27-2). If side effects prevent adequate analgesia, other opioids or nonopioid adjuvants should be used. Patient preferences based on past experiences and desired analgesia should also be considered.
Historically, meperidine has been a popular opioid for postoperative analgesia. It is the least potent of the opioids used clinically, and it has a long-acting active metabolite, normeperidine, that is excitotoxic to the central nervous system. In the past two decades a concerted effort has been made to decrease the use of meperidine for postoperative analgesia.57 In 1999, the American Pain Society stated that “meperidine should not be used for more than 48 hours for acute pain…[it] should be reserved for brief courses in otherwise healthy patients who have demonstrated an unusual reaction or allergic response to other opioids.”58 The American College of Obstetricians and Gynecologists (ACOG)59 has discouraged the use of meperidine because of the accumulation of normeperidine in the neonate and its subsequent effect on neurobehavioral scores.
Safety
Health professionals who use PCA should (1) be able to evaluate candidates for PCA (e.g., mental state, level of consciousness, patient understanding); (2) know drug selection criteria, dosing schedules, lockout periods, and infusion devices; (3) be able to provide patient education on pain management and the use of PCA; (4) understand when to alter PCA settings and when to give or withhold additional (rescue) doses of medications; and (5) be able to respond to side effects and adverse events.
In December 2004, The Joint Commission60 issued a Sentinel Event Alert on PCA “by proxy” (i.e., when other individuals, including family members, become involved in drug administration). The Joint Commission acknowledged that PCA is a safe and effective method of controlling pain when used as prescribed; however, serious adverse events, including oversedation, respiratory depression, and death, can result when analgesia is delivered “by proxy.” The Joint Commission60 made the following recommendations: (1) develop criteria for selecting appropriate candidates for PCA, (2) carefully monitor patients, (3) teach patients and family members about the proper use of PCA and the dangers of others’ pressing the button for the patient, (4) alert staff to the dangers of administering a dose outside a prescribed protocol, and (5) consider placing warning tags on all PCA delivery pendants stating, “Only the patient should press this button.”
When intravenous PCA is used for postoperative analgesia, guidelines for safe administration should be employed and documented. At many institutions two registered nurses must verify all pump settings when they are entered or changed and during communications associated with all patient transfers or nurse shift changes. The amount of opioid administered is recorded from the pump every 2 hours and when a drug cartridge is changed. The PCA settings (drug, demand dose, lockout interval, 4-hour limit, and the rate of continuous infusion, if used) are documented on a flow sheet. Any changes in PCA settings are clearly documented. In our institution, the use of a continuous background infusion is discouraged except in patients who were taking opioids preoperatively or in patients in whom nonstandard dosing requirements have already been demonstrated.
Infusion Pump Settings
A number of PCA parameters must be considered, including drug choice, incremental dose, maximum dose, and lockout interval (Table 27-3). Owen et al.48,61–63 performed a number of investigations of PCA in patients undergoing abdominal surgery. In an assessment of PCA morphine demand bolus doses (0.5, 1, or 2 mg with a 5-minute lockout interval), more patients in the 0.5-mg group had inadequate pain relief, whereas those in the 2-mg group had more side effects, including respiratory depression (i.e., respiratory rate < 10 breaths/min).62 These results correlated with the total dose of self-administered morphine. Although the role of the lockout interval was not addressed in this study, the investigators suggested that inadequate analgesia could be produced by lockout intervals that were too long or demand doses that were too small. By contrast, larger doses or shorter lockout intervals might lead to more opioid-related side effects. Therefore, longer lockout intervals typically require larger bolus doses, whereas smaller bolus doses typically require shorter lockout intervals.
Although patients who experience inadequate analgesia would be expected to make more PCA demands, this is often not the case.62 Patients may be afraid to administer too much opioid or anticipate more severe side effects. Additionally, it has been suggested that patients are discouraged by an inadequate analgesic effect or they may expect a delayed response.62 For this reason, a hydrophilic opioid (e.g., morphine) with a longer latency may be preferred over a lipophilic opioid (e.g., fentanyl).
The amount of opioid delivered in a continuous basal (background) infusion may or may not alter the analgesic efficacy of patient-controlled bolus doses. One study compared PCA bolus doses of morphine (0.4, 0.7, or 1.0 mg) combined with a continuous infusion of morphine at 1.5 mg/h after gynecologic surgery.61 The number of demand bolus doses and the quality of analgesia did not vary among groups despite the overall higher use of morphine in the group that received the largest bolus dose. In addition, there were no differences among groups in patient satisfaction or side effects. In an earlier study, Owen et al.63 observed that the patient-administered morphine dose did not differ in patients randomized to receive patient-administered bolus morphine compared with patient-administered bolus morphine with a continuous basal infusion (morphine 1.5 mg/h). Moreover, the quality of analgesia was similar in the two groups despite a twofold higher total dose of morphine in the group receiving a continuous infusion.
Controversy exists regarding the use of a continuous basal infusion during administration of intravenous opioid PCA. Studies of patients undergoing gynecologic surgery do not support the use of a continuous background infusion to provide better postoperative analgesia. Parker et al.64 evaluated 230 women who had undergone an abdominal hysterectomy; one group received a demand bolus dose of morphine (1 to 2 mg) without a continuous infusion, and the other three groups received a continuous infusion of morphine (0.5, 1, or 2 mg/h) in addition to the demand bolus dose. No differences in the number of demand or delivered doses per hour, pain scores, or overall morphine consumption were observed, except that an overall higher dose of morphine was administered in the 2 mg/h continuous infusion group than in the demand bolus–only group. A subsequent study by the same investigators in a similar patient population compared a group receiving an intravenous PCA morphine (2 mg demand bolus dose) regimen with a group receiving the same intravenous PCA regimen and a nighttime continuous infusion of morphine (1 mg/h).65 There were no differences between groups in postoperative pain, sleep patterns, demand or delivered bolus doses per hour, opioid consumption, or recovery from surgery. The investigators reported that the use of a continuous infusion resulted in six errors during the programming of the device and that three patients required discontinuation of the continuous infusion because of significant oxyhemoglobin desaturation.65
Thus, a continuous basal infusion is often avoided in opioid-naive patients because of concern about the risks of oversedation and respiratory depression. The ASA Task Force on Acute Pain Management51
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree