Maternal intravascular volume changes
| pH | 7.35–7.45 |
| PaO2 | 9.3–13.3 kPa (80–100 mmHg) |
| PaCO2 | 4.7–6.0 kPa (35–45 mmHg) |
| HCO3– | 22–26 mmol/l |
| Base excess | –3 to +3 mmol/l |

Maternal cardiovascular changes
Overall, from 8 to 36 weeks, systolic blood pressure falls by approximately 5 mmHg and diastolic blood pressure by around 10 mmHg. Blood pressure reaches its nadir at approximately 24 weeks, rising again as term approaches, often reaching nonpregnant values by 40 weeks. If a pregnant woman in the third trimester lies supine, the gravid uterus may compress the inferior vena cava against her spine, impeding venous return and leading to a fall in cardiac output. She may experience a marked fall in blood pressure and may feel faint, dizzy and nauseous. This is known as supine hypotensive syndrome; it is quickly relieved if the woman moves to the lateral position. Supine hypotensive syndrome reduces the uterine blood supply, which might cause fetal distress.
Electrocardiographic Changes
The increase in cardiac output in pregnancy causes hypertrophy and dilation of the left ventricle and atrium. However, there is no change in contractility. As a result of the upward displacement of the diaphragm by the enlarging uterus, the axis of the heart is shifted anteriorly and to the left. These changes explain the typical features of the electrocardiogram in pregnancy: heart rate 10–15% faster than normal; left axis deviation by approximately 15 degrees; inverted T wave in lead III; a Q wave in leads III and aVF.
Respiratory system
Ventilation increases by approximately 40% owing to stimulation of the respiratory centre by progesterone both directly and indirectly (by increasing the sensitivity of the respiratory centre to carbon dioxide). This rise begins in the first trimester and the increased ventilation results in a mild fall in PCO2 to 4.1 kPa and increase in PCO2 to 14 kPa during the third trimester. Towards term, PCO2 falls a little again to 13.5 kPa, as the increased cardiac output is unable to compensate for the increase in oxygen consumption. The mild decrease in PCO2 leads to a slightly alkaline pH. In response, there is an increase in bicarbonate excretion by the kidney, leading to a fall in bicarbonate levels to approximately 19–20 mEq/l. This is associated with a fall in sodium levels and in osmolarity (by 10 mmol/l). The changes in maternal respiration are illustrated in Figures 31.3–31.5.
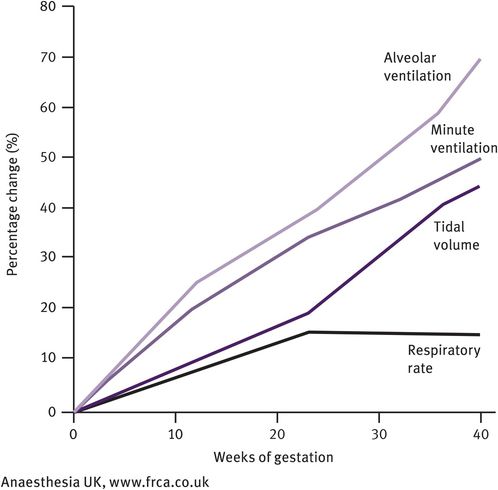
Maternal respiratory changes
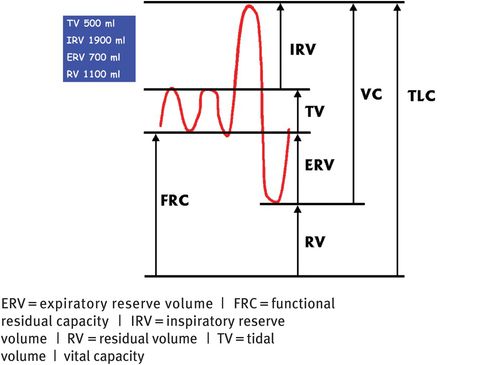
Lung volumes and capacities
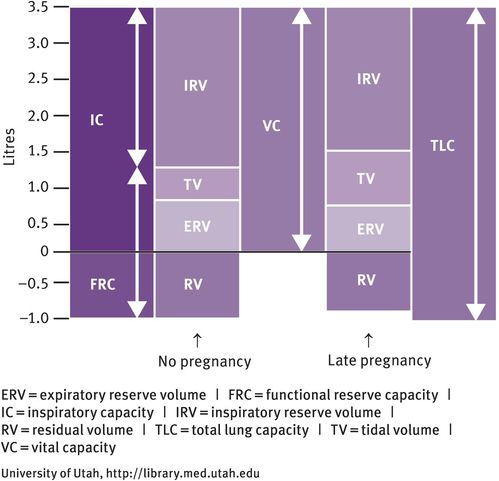
Differences in lung volumes between pregnant and nonpregnant women
Progesterone also relaxes bronchial smooth muscle; the calibre of the tracheobronchial tree increases and resistance falls. During pregnancy, breathing is more diaphragmatic than thoracic. Tidal volume increases by approximately 40%, but there is no change in the respiratory rate. The residual volume (the volume of gas that remains at the end of forced expiration, which is normally 1500 ml in the nonpregnant state) decreases by 200 ml, leading to a reduction in expiratory reserve volume and inspiratory reserve volume. Total lung capacity (5 litres in the nonpregnant individual) decreases by 200 ml. However, there is no change in the forced expiratory volume or peak flow rate.
Vital capacity (the volume of gas that can be inhaled from forced expiration to forced inspiration) is unchanged at about 3.5 litres as the reduction in the expiratory reserve and inspiratory volumes are compensated for by the increase in tidal volume. Lung compliance is unaffected, but chest compliance is reduced, especially if the woman is in the lithotomy position. Seventy percent of pregnant women experience subjective dyspnoea. Oxygen consumption increases by 50 ml/minute at term. This increased demand comes from the fetus (20 ml/minute), increased cardiac output (6 ml/minute), increase in renal work (6 ml/minute) and increase in metabolic rate (18 ml/minute). During pregnancy, the increase in ventilation is greater than the increase in oxygen consumption. In pregnancy, there is also an increased risk of pulmonary embolism (see below).
Urinary system
In pregnancy, the kidneys increase in size by approximately 1 cm. The ureters dilate, partly from the effect of progesterone on the smooth muscle in their walls and partly from obstruction caused by pressure from the growing uterus. As a result, women are more prone to urinary tract infections in pregnancy and, if they have a urinary tract infection, are more likely to develop pyelonephritis. Both renal blood flow and glomerular filtration rate increase in pregnancy. These rises start in the first trimester and, by term, both are 50–60% higher than before pregnancy, the glomerular filtration rate reaching 140–170 ml/minute.
As a result of these changes, the urea level in pregnancy falls from 4.3 to 3.1 mmol/l and serum creatinine falls from 73 to 47 micromol/l. Urate and bicarbonate levels also fall. In pregnancy, there is mild glycosuria and proteinuria. Plasma osmolality decreases because of the combined effect of increased progesterone and changes in the renin–angiotensin–aldosterone pathway.
Gastrointestinal tract
The nausea and vomiting that commonly occur in early pregnancy are largely explained by increasing levels of human chorionic gonadotrophin and/or progesterone. Gastrointestinal tone and motility are reduced by the high levels of progesterone, leading to delayed gastric emptying, particularly during labour. Progesterone also relaxes the oesophageal sphincter, which means that acid reflux is common; at term, 81% of pregnant women complain of heartburn. More importantly, these changes make pregnant women more prone to aspiration of gastric contents, particularly during induction of general anaesthesia. Furthermore, because gastric contents are more acidic in pregnancy, if aspiration occurs, the resulting pneumonitis (Mendelson syndrome) may be severe and even fatal. Some of the dyspepsia in pregnancy can also be explained by reduced motility of the gallbladder, again most likely due to the effects of progesterone.
Small and large bowel motility is decreased. Stools become firmer as transit time is longer, allowing more time in the colon for reabsorption of water. Consequently, constipation is a common problem in pregnancy.
Changes Affecting the Liver
In pregnancy, plasma alkaline phosphatase increases up to three times the normal level, because it is also produced by the placenta. Gallbladder contractility decreases as cholecystokinin secretion falls. As a result, pregnant women are more prone to develop gallstones during pregnancy.
Obstetric cholestasis is a pregnancy-specific hepatic condition, characterised by itching in pregnancy and accompanied by raised liver transaminases and bile acids. This condition is associated with an increased risk of intrauterine fetal death and fetal distress in labour. One hypothesis proposes that obstetric cholestasis is caused by an interaction between inherited and acquired abnormalities in bile acid transport. Women who experience obstetric cholestasis in pregnancy may have a similar reaction to the combined oral contraceptive pill.
Haematological system
In pregnancy, plasma volume expands by around 45% (from 2600 ml to 3800 ml). This expansion begins early in pregnancy (around 6–8 weeks of gestation) and reaches a peak at 32 weeks. Red cell mass increases steadily until term (from 1400 ml to 1700 ml), reaching a maximum of 20–30% above nonpregnant levels. This increase in erythro-poiesis is probably mainly explained by an increase in erythropoietin and, to a lesser extent, by an increase in human placental lactogen. Because the rise in plasma volume is proportionately greater than the rise in red cell mass, haemoglobin concentration and haematocrit fall, averaging approximately 11.5 g/100 ml and 34%, respectively, by 30 weeks. This dilution of haemoglobin and haematocrit levels in pregnancy is known as ‘physiological anaemia’ and is most marked at 32 weeks of gestation. The definition of anaemia must therefore be changed during pregnancy.
Other permanent elements in the blood are changed to a lesser extent in pregnancy. There is a modest rise in leucocyte count, although in the weeks after delivery the count may reach as high as 25 000 leucocytes/mm3. This rise is primarily in neutrophils.
The effect of pregnancy on the platelet count is debated, but in some women there may be a modest decline by term, perhaps by as much as 25%. This fall is believed to be due to increased destruction of platelets not caused by immune factors and is termed gestational thrombocytopenia. Platelet size increases, indicating less mature platelets in the circulation.
Iron Metabolism
Iron demand increases in pregnancy; the total extra requirement is around 1000 mg (500 mg for the increased red cell mass, 300 mg to meet the needs of the fetus and placenta and 200 mg to cover the obligatory excretion of iron). The extra requirement comes to approximately 4 mg/day (increased from 2.8 mg/day in a nonpregnant woman to 6.6 mg/day by the end of pregnancy).
In pregnancy, iron absorption is increased, owing to erythroid hyperplasia. Absorption is dependent on iron stores (represented by ferritin levels and iron binding saturation) and the amount of iron in the diet (including any supplements), but only 10–20% of ingested iron is absorbed. Absorption increases in the second and third trimesters, when the need is greatest, but may still not meet the needs of pregnancy and the puerperium. Consequently, iron deficiency anaemia is the most common haematological problem in pregnancy, with symptoms of dyspnoea, tiredness and occasionally faintness. Investigations usually reveal serum iron below 12 mmol/l and saturation of the total iron-binding capacity less than 15%. Thalassaemia trait may have a similar presentation to iron deficiency anaemia, so this possibility must be considered and excluded. Fortunately, maternal iron deficiency does not appear to reduce iron transport to the fetus.
Haemostasis in Pregnancy
Pregnancy is known to be a hypercoagulable state. This is due to an increase in coagulation factors and a decrease in fibrinolytic activity, changes that can be demonstrated from the third month of pregnancy. The concentrations of most of the coagulation factors (VII, VIII, X) increase in pregnancy, but factors XI and XIII do not. The rise in fibrinogen levels leads to an accelerated erythrocyte sedimentation rate, which, in late pregnancy, reaches double nonpregnant levels.
Routine coagulation screening in pregnancy is essentially normal; the activated partial thromboplastin time is unchanged or slightly shortened and the bleeding and clotting times are unaffected. Levels of antithrombin III (the main physiological inhibitor of thrombin and factor Xa) change little although, as plasma volume expands, production is increased to maintain this normal concentration. The activity of the fibrinolytic system remains low in pregnancy and labour, but returns to normal within one hour of delivery of the placenta. During the third stage of labour, myometrial contraction and increased coagulability of the blood combine to stop bleeding after delivery of the placenta.
Implications of maternal physiological changes on therapeutic drug administration
Absorption of drugs from the gastrointestinal tract may be impaired by gastric stasis, poor gut motility and, for some drugs, lower gastric pH. The increase in plasma volume means that the volume of distribution of the drug increases, so concentrations may be lower than expected. This is particularly important in women taking anticonvulsant drugs or thyroxine. Because of the increased glomerular filtration rate, excretion of drugs that are mainly excreted by the kidneys will be accelerated. These changes often require doses of a drug given during pregnancy to be adjusted.
Physiology of lactation
Development of human breasts takes place around the time of puberty (that is, prior to pregnancy). From then on, the adult breasts require surprisingly little hormonal stimulation to begin milk secretion: 14 days’ exposure to estrogen followed by stimulation of prolactin secretion may be enough to establish milk production; this has been used to encourage lactation in women who wish to breastfeed an adopted baby. Prolactin is a long-chain polypeptide hormone and is essential for successful lactation. In early pregnancy, there is hyperplasia of the alveolar cells and lactiferous ducts, which is followed in later pregnancy by alveolar cell hypertrophy and the initiation of secretion. These changes are stimulated by the increased levels of prolactin and human placental lactogen. During pregnancy, the high levels of estrogen and progesterone hold this process in check and full milk production is achieved only after delivery, when progesterone and estrogen levels fall rapidly. Similarly, women who take estrogen while breastfeeding (for example, in the combined oral contraceptive pill) will find that milk production is decreased.
After delivery, the colostrum (or early milk) has a high concentration of protein relative to the concentration of lactose. However, over the following three days or so, the concentration of lactose increases sharply and the concentration of protein falls. However, the main reason for this fall in protein concentration is actually dilution. In order to maintain ionic equilibrium, water is drawn into the breast, causing an increase in milk volume while the total amount of protein in the milk remains unchanged.
Milk production averages 500–1000 ml/day and is highly dependent on continued suckling, which causes release of both prolactin and oxytocin. In women who do not suckle, milk production gradually falls and may persist for 3–4 weeks postpartum. In breastfeeding mothers, equilibrium is reached after around three weeks whereby milk production is tailored to the amount taken by the baby. Interestingly, mothers who are breastfeeding twins produce twice as much milk. A breastfeeding woman requires 2950 kcal/day. The recommended daily calorie intake is 2700 kcal (2200 kcal for the nonlactating nonpregnant requirement plus 500 kcal towards the energy requirement of the milk); an extra 250 kcal/day required for milk should come from maternal fat stores laid down during pregnancy.
The suckling stimulus sends afferent impulses to the hypothalamus that lead to a surge of prolactin release. This surge reaches a peak around 30 minutes after the baby is put to the breast and gradually declines to basal levels by 120 minutes. The control of prolactin release from the anterior pituitary is primarily via prolactin inhibitory factors from the hypothalamus that are secreted into the pituitary portal blood system. The most important prolactin inhibitory factor is dopamine. In fact, dopamine agonists, such as bromocriptine and cabergoline, can be used in the early puerperium to suppress milk production in women who do not wish to breastfeed. Conversely, dopamine antagonists such as metoclopramide increase prolactin levels and are sometimes used in breastfeeding women to stimulate milk production. Thyrotrophin-releasing hormone may also play a role in stimulating prolactin production. After the sixth week postpartum, both basal prolactin levels and the peak level following suckling gradually decline; the greater the frequency and duration of suckling, the slower the decline.
Suckling also stimulates oxytocin release. The afferent impulses trigger the synthesis of this octapeptide hormone from specialised neurons in the supraoptic and paraventricular nuclei of the hypothalamus and its release from the posterior pituitary gland. This neuroendocrine reflex can also be initiated by the mother hearing her baby cry or even thinking about breastfeeding. In this way, the release of oxytocin, which typically occurs in short one‐minute bursts, may begin even before the baby is put to the breast. Oxytocin binds to specific receptors on the myoepithelial cells, which surround the alveolar (milk-producing) cells in the breasts and are longitudinally arranged in the walls of the milk ducts. Contraction of the myoepithelial cells forces the milk into the ducts; contraction of the longitudinally arranged cells in the duct walls causes the ducts to dilate and allows milk to flow more easily towards the nipple. Both prolactin and oxytocin are necessary for successful breastfeeding; in general terms, prolactin stimulates the production of milk, while oxytocin stimulates its ejection (or let down).
Table 31.3 shows the typical composition of breast milk. The composition varies from woman to woman, over time in an individual woman and even differs between the beginning and end of the same feed. The most important factor, however, is the time since the birth, suggesting that the milk is adapted in a very sensitive way to the changing needs of the baby. Thus, any statements about the composition of human breast milk are averages; these are the basis for the composition of artificial feeds.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


