Physiologic Basis of Pulmonary Function
Daniel J. Weiner
The respiratory system is composed of many different cell and tissue types, including airways, lung parenchyma, respiratory muscles, nerves, and blood vessels. These structures must operate in an integrated way to serve the main functions of the system, that is, to deliver oxygen to tissues and remove carbon dioxide. Herein we will examine the component parts of the respiratory system, as well as explore techniques for assessing the function of these components in health and disease.
RESPIRATORY DRIVE
 STRUCTURES/PHYSIOLOGY: RESPIRATORY CONTROL CENTERS
STRUCTURES/PHYSIOLOGY: RESPIRATORY CONTROL CENTERS
The coordination of breathing, which occurs 24 hours per day, requires a careful integration of automatic respiratory centers with multiple inputs that allow for changes in breathing patterns necessary to accommodate speech, swallowing, and other activities. The automatic control centers are located primarily in the medulla of the brainstem, although other brainstem centers in the pons contribute to finer tuning of inspiration and expiration times. These automatic control centers allow for very fine control over ventilation, maintaining Pco2 and pH in a very narrow range. There are a variety of mechanoreceptors in the upper airway that can be stimulated by airflow or swallowing, as well as receptors in the lung parenchyma that can be stimulated by rapid stretch or deflation. These receptors can modulate an increase or decrease in ventilation.
 TESTING: P0.1, Vco2 RESPONSE, POLYSOMNOGRAPHY
TESTING: P0.1, Vco2 RESPONSE, POLYSOMNOGRAPHY
Assessing the function of the respiratory control centers is not straightforward. The measurement of airway opening pressure very early (100 ms) in inspiration (P0.1 or P100) has been used as an assessment of respiratory drive; depressed drive caused, for example, by narcotics, lowers this pressure. Polysomnography measures a wide variety of physiologic variables during sleep, including nasal and oral airflow, chest and abdominal wall motion, oxygen saturation (SpO2), end-tidal (exhaled) pCO2, and electrocardiogram. Sleep state is monitored by electroencephalogram (EEG), electrooculogram, and electromyo-gram. Applications of polysomnography will be discussed in more full detail elsewhere (Chakravorty), but abnormalities of respiratory drive (especially central apneas and periodic breathing) can be detected with this testing.
RESPIRATORY PUMP
 STRUCTURES/PHYSIOLOGY CHEST WALL, RESPIRATORY MUSCLES
STRUCTURES/PHYSIOLOGY CHEST WALL, RESPIRATORY MUSCLES
The respiratory muscles, notably the diaphragm and intercostal muscles, are the “pump” of this system and are responsible for generation of negative intrathoracic pressure during inspiration. Respiratory muscle weakness can manifest in primary neuromuscular diseases (ie, muscular dystrophy, spinal muscular atrophy, myasthenia gravis) or secondarily due to malnutrition or hyperinflation (which alters the length–tension ratio of the diaphragm).
Most chronic respiratory failure in children results from pump, not lung, failure. The pump is responsible for maintenance of ventilation by their force-generating capacity to overcome the elastic and resistive loads of the lungs and chest wall. Respiratory “success” depends not on pump function alone, but on a balance between pump function and the magnitude of the loads upon which the pump is acting. In many obstructive lung diseases (asthma, cystic fibrosis), the predominant load is due to elevated airways resistance. In contrast, respiratory failure in neuromuscular diseases results from primary failure of the pump. Additionally, patients with neuromuscular disease may have altered pump function as a result of scoliosis, as the force generated by downward displacement of the diaphragm or contraction of intercostals muscles is no longer parallel to the spinal axis.
 TESTING: STRENGTH (MIP, MEP, SNIP), ENDURANCE/FATIGUE (TENSION-TIME INDEX)
TESTING: STRENGTH (MIP, MEP, SNIP), ENDURANCE/FATIGUE (TENSION-TIME INDEX)
The respiratory muscles (including the diaphragm, intercostals muscles, and others) contract intermittently 24 hours per day to perform the work of ventilation. Many diseases can affect the strength of these muscles, putting patients at risk for hypoventilation, impaired airway clearance of secretions, and respiratory insufficiency. These conditions include primary muscular disorders (muscular dystrophy, myopathy), conditions affecting nerve transmission to the muscles (neuropathies, disorders of the neuromuscular junction), malnutrition, and stretch of the muscles beyond their optimal length-tension relationship (which can occur in hyperinflation).
Typically, maximal expiratory pressure (MEP, PEmax) is measured by having the subject inhale maximally to total lung capacity (TLC) and blow out as hard as possible into a mouthpiece connected to a pressure transducer, and with an occluded distal end. Similarly, maximal inspiratory pressure (MIP, PImax) is measured by having the subject exhale completely to residual volume (RV) and inhale rapidly against the occluded tube. Usually several repeated maneuvers are required to elicit the maximal effort. Several sets of standardized values have been published, but they vary considerably. Nasal sniff pressures (SNIP) have also been used as a measure of inspiratory muscle strength, and may be particularly useful in patients who have facial muscle weakness and difficulty forming a seal around a mouthpiece.1
Breathing is an endurance task, as it must be done 24 hours a day. Therefore, measurements of fatigue or endurance, rather than strength, may be critical to assess respiratory pump function.
The tension-time index (TTI), and its noninvasive analogue, tension-time index of the inspiratory muscles (TTmus) is one way of assessing fatigability.2 The TTmus is defined as (Pi/MIP) × (Ti/Ttot), where Pi is the average pressure generated at the airway opening during a breath, MIP is the maximal inspiratory pressure, Ti is the time spent in inspiration, and Ttot is the total breath duration.3 This can be thought of as a fraction of maximal effort that the diaphragm performs during its contraction time. The TTI predicts the likelihood of respiratory muscle fatigue. In adults, when the TTI is less than 0.1, it is unlikely that diaphragmatic fatigue will occur. When the TTI exceeds 0.2, it is highly likely that fatigue will occur.2 However, there are no data in infants or children to determine whether these threshold values of TTI are applicable in these groups.
RESPIRATORY SYSTEM–INTEGRATED
Mechanics: Compliance, Resistance
In an oversimplified way, the respiratory system is sometimes considered as a balloon (lungs) at the end of a tube (the airways). As pressure is increased inside the balloon (eg, blowing through the tube), the balloon will inflate. It is the difference in pressures from inside to outside (the transmural pressure) that will create a change in volume of the balloon. If we measure the volume of the balloon at different transmural pressures, we can plot a pressure-volume curve for the balloon (or lung) (Fig. 503-1).
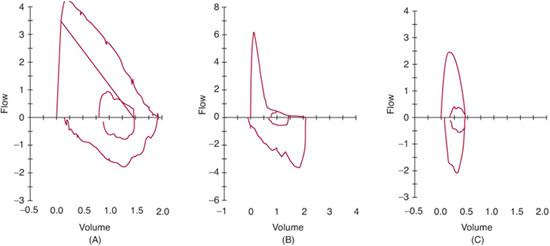
FIGURE 503-1. Flow-volume curves. The normal curve (A) has a smooth, linear decrease in flow from near total lung capacity (to the left) to residual volume (to the right). Note that time is not represented in these curves but is recorded in a separate volume-time curve (not shown). The patient with obstructive lung disease (B) will demonstrate a curve that is concave towards the volume access, such that for any given lung volume, measured flow is lower than predicted. Restrictive lung diseases (C) result in a curve that has a small exhaled-lung volume and is narrow, but without concavity.
The slope of this pressure-volume curve, where V is volume and P is pressure, is called (C), and can be thought of as the distensibility of the lung. If the compliance is high, small changes in pressure will create large changes in volume (easy to blow up the balloon). Low lung compliance can be seen in many disease states, including fibrosis and pulmonary edema.
Measurement of lung mechanics requires the ability to measure the transmural pressure across the lung, that is, alveolar pressure and pleural pressure. The latter is difficult to measure, but can be estimated from esophageal pressure with a balloon catheter. However, the compliance of the entire respiratory system can be more easily measured; here, the relevant pressure change is the pressure at the airway opening (measured at the mouth or end of endotracheal tube) referenced to atmospheric pressure.
Although not straightforward to measure, lung mechanics can have useful application in the clinical setting. For example, monitoring of lung compliance in a patient with respiratory failure can provide an objective assessment of treatments such as diuretics or monitoring recovery of lung function. Similarly, elevated airway resistance would provide a rationale for bronchodilators or evaluation for obstructing lesions. These measurements can be made at the bedside in mechanically ventilated children, or with a facemask in spontaneously breathing children.4
AIRWAYS
 STRUCTURES/PHYSIOLOGY
STRUCTURES/PHYSIOLOGY
Conducting Airways, Concept of Dead Space, Partitioning of Resistance
The conducting airways (trachea, main bronchi, lobar bronchi, segmental bronchi, bronchioles, and terminal bronchioles) do not participate in gas exchange and therefore constitute the anatomic deadspace. The volume of the anatomic deadspace is approximately 2 cc/kg, or 150 cc in an adult. The terminal bronchioles continue dividing into smaller airways, and alveoli can be found budding off of respiratory bronchioles. All of the structures distal to a single terminal bronchiole comprise an acinus.
Pressure is needed to generate the flow of air through the airways, depending in part on the flow rate and in part on the resistance. Laminar flow occurs at low flow rates (eg, in the very small airways) and is characterized by orderly parallel streams of gas flow. The rate of flow is determined according to Poiseuille’s law: 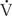 = Pπr4/8 nl, and therefore determined by the radius of the tube (r), the driving pressure (P), the length of the tube (l), and viscosity of the gas (n). Because resistance (R) is the pressure required to achieve a given flow (P/
= Pπr4/8 nl, and therefore determined by the radius of the tube (r), the driving pressure (P), the length of the tube (l), and viscosity of the gas (n). Because resistance (R) is the pressure required to achieve a given flow (P/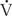 , resistance is governed by these same factors (R = 8 nl/πr4). The reciprocal of resistance (
, resistance is governed by these same factors (R = 8 nl/πr4). The reciprocal of resistance (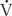 /P is termed conductance (denoted G). As airways dilate and resistance decreases with increasing lung volume, these measures are frequently normalized to lung volume (and termed specific resistance, sRaw, and specific conductance, sGaw).
/P is termed conductance (denoted G). As airways dilate and resistance decreases with increasing lung volume, these measures are frequently normalized to lung volume (and termed specific resistance, sRaw, and specific conductance, sGaw).
Several important points can be noted from these relationships. First, small changes in radius generate huge changes in resistance. For example, consider the difference in resistance resulting from changing from a 2.5 mm endotracheal tube to a 3.0 mm tube. The smaller tube has twice the resistance of the larger tube despite only a 0.5 cm difference in radius! Second, changes in length of the tube are not as significant, but long endotracheal tubes (or long tubing connecting the ventilator) add resistance. Third, changes in the viscosity of the gas also affect resistance. 
The above equations define resistance to flow through a single airway. Because the respiratory tree is composed of many airways that are connected in parallel, the resistance is divided amongst many airways. Therefore, the total resistance at any generation of airways is dependent upon the number of branches in that generation and the total cross sectional area. Thus, the proportion of resistance in the first generation (trachea) is much higher than the resistance in the 19th generation airways (terminal bronchioles) because there are hundreds of thousands of bronchioles and the total cross sectional area is much higher in generation 19. Thus, doubling the resistance in the peripheral airways, as might occur in bronchiolitis or asthma, might have only a small effect on the total airways resistance.
Airway Function: Airway caliber (maximal flows, spirometry, RV-RTC/iPFT), reactivity (challenge testing including exercise), airway resistance/conductance (Raw, Gaw, IOS)
Airway resistance can be measured by several techniques, most commonly by using a body plethysmograph, with the subject making small-volume panting efforts initially with a shutter open and then with a shutter closing. The plethysmograph allows measurement of alveolar pressure, and subsequently calculation of the pressure gradient generated to achieve the flow measured by the pneumotachometer, a device to measure gas flow. Resistance can also be measured using other techniques, including the interrupter technique and impulse oscillometry.5 The latter technique involves application of multiple sound frequencies to the airway opening through a mouthpiece, and assessment of the airway response (termed impedance, of which resistance is one component). The technique is potentially attractive in young children as the measurement is made during tidal breathing (and thus does not require specialized maneuvers on the child’s part). Change in resistance measurements after inhalation of a bronchoconstrictor (eg, methacholine) or bronchodilator is one way of assessing airway reactivity.
Spirometry is the measurement of airflow during a maximally forced exhalation.6 The test is informative because airflow rates are inversely proportional to the fourth power of the radius of the airway as described above; small or obstructed airways result in reduced airflow rates. Many common pediatric lung diseases (asthma and cystic fibrosis) are obstructive in nature, and are characterized by reduced airflows. Furthermore, in the smaller airways, minimal effort is needed to achieve a maximal flow rate. The tests are reproducible within subjects, making them useful for assessing response to treatment over time.
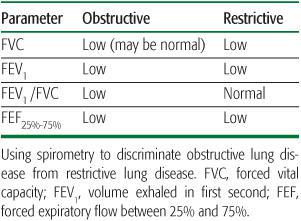
The subject breathes through a mouthpiece connected to a pneumotachometer while wearing nose clips. After inhalation to total lung capacity, the subject is coached to exhale rapidly and forcefully until the lungs have emptied. Subjects are coached to exhale for a minimum of 6 sec, although shorter exhalations (3 sec) are acceptable for younger children (younger than age 10 years). Several parameters can be calculated from these maneuvers. First, the total exhaled volume is termed the forced vital capacity (FVC). The volume exhaled in the first second is termed the FEV1. The forced expiratory flow between 25% and 75% of the exhaled volume is termed the FEF25%-75% (or occasionally the maximal mid-expiratory flow, MMEF). Other instantaneous flow rates can also be calculated with reference to a different exhaled volume (eg, FEF50, FEF75). The pattern of these parameters can suggest an obstructive defect or a restrictive defect (Table 503-1). The FEV1/FVC ratio may help distinguish obstructive from restrictive diseases, although measurement of lung volumes is required to accurately diagnose restrictive disease.
When flow is plotted against exhaled volume (Fig. 503-1A), the resultant curve can help locate the site of any flow limitation. Intrathoracic obstructive defects (such as those seen in asthma, cystic fibrosis) result in a curve that is concave to the volume axis (Fig. 503-1B). Less common restrictive defects (eg, pulmonary hypoplasia) usually have a normally shaped curve that is narrower because of a lower exhaled volume (Fig. 503-1C). Central airway obstructions can manifest as flattening of the inspiratory limb if the lesion is variable and extrathoracic (eg, tra-Fixed obstructions (eg, intraluminal tumors, subglottic stenosis) can result in flattening of the inspiratory and expiratory portions of the curve.
If an obstructive defect is documented, reversibility can also be assessed utilizing spirometry. Following administration of the bronchodilator, testing is repeated after 15 to 20 minutes. Commonly, a 12% increase in the FEV1 is considered indicative of a significant response. Similarly, in patients in whom airway hyperreactivity is suspected, spirometry can be performed before and after provocative agents, including methacholine, cold air, or exercise.7 Similarly, graded exercise (using a treadmill or cycle) or hyperventilation with cold air can be used to provoke a bronchospasm response. The specificity of exercise testing is usually higher than its sensitivity, that is, an abnormal test usually is associated with airway reactivity, but a normal test does not exclude it.
Similarly, graded exercise (using a treadmill or cycle) or hyperventilation with cold air can be used to provoke a bronchospasm response. The specificity of exercise testing is usually higher than its sensitivity, that is, an abnormal test usually is associated with airway reactivity, but a normal test does not exclude it.
Most of the tests described above have been adapted to infants, with the obvious challenge being that maximal efforts cannot be elicited voluntarily. Infants are usually sedated using chloral hydrate, and placed supine with a mask over mouth and nose to measure airflow and pressure at the mouth. These techniques require specialized equipment not available in most pulmonary function laboratories. 
GAS EXCHANGE
 STRUCTURES/PHYSIOLOGY
STRUCTURES/PHYSIOLOGY
Alveoli, Pulmonary Vasculature, V/Q Matching
Gases move from the mouth to the respiratory bronchioles by bulk flow, but diffusion is the process by which the gases traverse the alveolus and enter into the blood. The rate of diffusion across a membrane is governed by Fick’s law: V α (sol/mw) × (A/T) × (P1-P2), and thus determined by cross-sectional area (A), thickness of the membrane (T), and partial pressures across the membrane (P1, P2), molecular weight (mw) and solubility (sol) of the gas. Because CO2 is much more soluble than oxygen, it diffuses approximately 20 times as fast. Although unusual in pediatrics, disease states that increase the membrane thickness (pulmonary fibrosis) or decrease the surface area (pulmonary hypoplasia) will decrease the ability of gases to diffuse into the blood. 
Effective delivery of oxygen to the body’s cells requires a precise balance between ventilation of lung units and blood flow to those units. Imbalances in these relationships, termed ventilation-perfusion (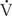 /
/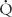 ) ratios, are the most common cause of hypoxemia in patients. There are two extremes of
) ratios, are the most common cause of hypoxemia in patients. There are two extremes of 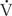 /
/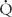 imbalance: shunt and dead space. Shunt refers to situations where there is blood flow (
imbalance: shunt and dead space. Shunt refers to situations where there is blood flow (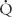 > 0) that comes into contact with nonventilated lung units (
> 0) that comes into contact with nonventilated lung units (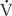 = 0) or does not come into contact with the lung at all; in these situations,
= 0) or does not come into contact with the lung at all; in these situations, 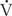 /
/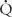 = 0. Shunts can be “irreversible” or “fixed,” such as intracardiac shunts or intra-pulmonary shunts (eg, arteriovenous malformations). They are designated “irreversible” or “fixed” because they do not decrease in response to hyperoxia. “Reversible” shunts are really areas of very low
= 0. Shunts can be “irreversible” or “fixed,” such as intracardiac shunts or intra-pulmonary shunts (eg, arteriovenous malformations). They are designated “irreversible” or “fixed” because they do not decrease in response to hyperoxia. “Reversible” shunts are really areas of very low 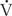 /
/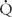 ; in response to hyperoxia, more oxygen is taken up from the alveoli, and the very low oxygen tension in blood draining these areas is increased considerably. This gives the effect of reducing or eliminating the “shunt.” Dead space refers to situations in which airflow (
; in response to hyperoxia, more oxygen is taken up from the alveoli, and the very low oxygen tension in blood draining these areas is increased considerably. This gives the effect of reducing or eliminating the “shunt.” Dead space refers to situations in which airflow (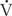 > 0) comes into contact with nonperfused lung units (
> 0) comes into contact with nonperfused lung units (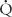 = 0). Overdistension of alveoli with positive airway pressure or pulmonary embolus is an example of processes that create dead space.
= 0). Overdistension of alveoli with positive airway pressure or pulmonary embolus is an example of processes that create dead space.
Most alterations in ventilation or perfusion result in some imbalance in 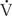 /
/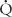 ratios. For example, acute airway obstruction (eg, a mucous plug in a bronchus) decreases ventilation to the distal alveoli while leaving their associated capillary perfusion unchanged; this results in a decreased
ratios. For example, acute airway obstruction (eg, a mucous plug in a bronchus) decreases ventilation to the distal alveoli while leaving their associated capillary perfusion unchanged; this results in a decreased 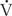 /
/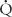 ratio and causes end-capillary PO2 to be decreased and PCO2 to be increased. The gas tensions in the alveolus and end-capillary blood will thereby approach that of mixed venous blood. In contrast, in an area with ventilation but no perfusion the oxygen and CO2 tensions in the affected alveolus will approach those in the inspired gas.
ratio and causes end-capillary PO2 to be decreased and PCO2 to be increased. The gas tensions in the alveolus and end-capillary blood will thereby approach that of mixed venous blood. In contrast, in an area with ventilation but no perfusion the oxygen and CO2 tensions in the affected alveolus will approach those in the inspired gas.
Function: Diffusing Capacity, A-a Gradient
Quantifying 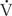 /
/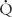 relationships is very difficult. The simplest estimation of imbalances in
relationships is very difficult. The simplest estimation of imbalances in 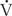 /
/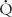 can be obtained by calculation of the difference in oxygen tensions in the alveolus (estimated from the alveolar gas equation) and the arterial blood (the A-a gradient). This gradient is usually very small (< 15 torr) when someone is breathing room air at sea level. The A-a gradient will be elevated if there is a block in diffusion,
can be obtained by calculation of the difference in oxygen tensions in the alveolus (estimated from the alveolar gas equation) and the arterial blood (the A-a gradient). This gradient is usually very small (< 15 torr) when someone is breathing room air at sea level. The A-a gradient will be elevated if there is a block in diffusion, 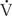 /
/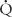 imbalance, or shunt. Note that in the other causes of hypoxemia (low inspired oxygen tension or hypoventilation), the alveolar pO2 and arterial pO2 will both be equally depressed, resulting in a normal A-a gradient.
imbalance, or shunt. Note that in the other causes of hypoxemia (low inspired oxygen tension or hypoventilation), the alveolar pO2 and arterial pO2 will both be equally depressed, resulting in a normal A-a gradient. 
Another way to assess gas exchange is with the diffusing capacity for carbon monoxide (DLCO), which is an integrative measurement that describes the transfer of oxygen from the alveolus into the red blood cell.12 In the single-breath technique for measurement of DLCO, the patient exhales completely to residual volume and inhales to total lung capacity a gas mixture of 0.3% carbon monoxide and an inert gas (usually helium or methane). The subject holds his or her breath for 10 sec, during which CO diffuses into the blood. The uptake of CO (in ml/min) is divided by the partial pressure gradient for CO (between alveolus and pulmonary capillary) to calculate DLCO (ml/mmHg/min). Alveolar ventilation is calculated from the inspired and expired concentrations of the inert gas. This is then used to calculate a dilutional factor for the inspired CO concentration and to normalize the DLCO according to lung volume in which the CO is diluted (DLCO/VA in ml/mmHg/min/L). It is frequently also adjusted for the patient’s hemoglobin.
Diseases that decrease the surface area for diffusion (emphysema, pulmonary emboli, resection of lung tissue) or diseases that increase the thickness of the alveolar-capillary membrane (fibrosis, pulmonary edema, proteinosis) decrease the diffusing capacity of the lung, but this decrease is not specific as to the cause. Increased DLCO is much less common but can be seen in patients with alveolar hemorrhage (hemoglobin in the airspace appears to make uptake of CO very high), polycythemia, or during exercise (via recruitment of more pulmonary capillaries). This test may be useful in evaluating patients with diffuse lung diseases or assessment of patients with pulmonary vascular obstruction.
LUNG VOLUME
 STRUCTURES/PHYSIOLOGY
STRUCTURES/PHYSIOLOGY
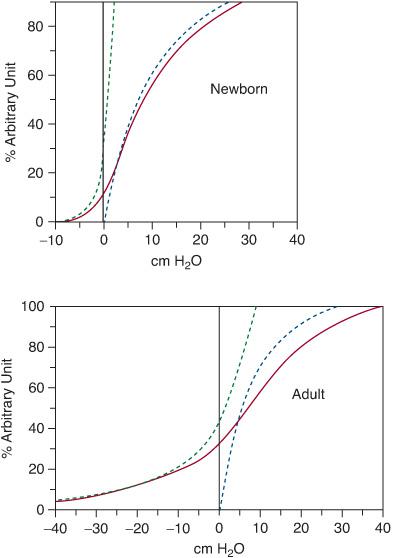
The chest wall, like the lung, has compliance (a measured change in volume for a given change in pressure). The lung volume can be plotted against the transpulmonary pressure (Fig. 503-2). At resting lung volume, called functional residual capacity (FRC), the natural tendency of the chest wall is to spring outwards away from the lung. The lung, in contrast, tends to collapse inwards. It is the balance of these two tendencies that determines FRC and is responsible for the maintenance of a negative intrathoracic pressure.
Note that the respiratory system curve (lung + chest wall) is the arithmetic sum of the chest wall curve and the lung curve. The lung volume at which the chest wall curve and the lung curves are equidistant from the vertical axis (transrespiratory system pressure = 0) is FRC. This relationship is important in situations where the chest wall may have increased compliance (infancy), or decreased compliance (adults with neuromuscular diseases). In infants, the rib insertions are more horizontal, and the rib cage itself is more compliant than in adults. Because of this, the resting lung volume (FRC), which is passively determined by the recoil between the lung and chest wall, is lower than in adults.
Infants, therefore, actively maintain their FRC utilizing expiratory braking and glottic closure to increase the lung volume at end-expiration, and also contraction of the diaphragm during exhalation, retarding expiration. Without this active maintenance of FRC, infants would be very prone to hypoxemia at rest due to low lung volume.
Plethysmography, Dilutional Techniques
Disease states that affect lung growth would be expected to alter lung volume in addition to airway caliber. These diseases include pulmonary hypoplasia due to severe oligohydramnios or space occupying lesions (eg, diaphragmatic hernia or cystic adenomatoid malformation), bronchopulmonary dysplasia, as well as conditions that alter the growth of the rib cage (thoracic dystrophies, radiation). The volume of gas in the lung can be measured utilizing two techniques: dilution and plethysmography.
Dilutional techniques utilize the principle of conservation of mass.13,14 The subject breathes through a mouthpiece connected to a closed system into which a known concentration of an inert and nonabsorbed marker gas (usually Helium) is added. After several minutes allowing for equilibration, the final concentration of the marker gas is measured, allowing calculation of the volume added to the system (the patient’s lung volume). True lung volume may be underestimated in the presence of significant obstruction, as any obstructed portions of the lung will not participate in the gas mixing and dilution.
Body plethysmography utilizes the principle of Boyle’s law, that is, in a closed system, pressure and volume change inversely when temperature is constant.13 With the subject sitting in a fixed volume chamber (“body box”) and breathing on a mouthpiece, a shutter is closed in the inspiratory limb of the breathing circuit. The subject makes small panting maneuvers, resulting in small changes in the volume of the lung and corresponding inverse volume changes in the box. Pressures in the box and at the mouth are measured, and this allows for calculation of the lung volume at which the panting efforts began. The subject usually begins the maneuvers at the end of a breath, and this “resting” lung volume is termed functional residual capacity (FRC). In the presence of significant obstruction, lung volume may be overestimated by plethysmography as one assumption of plethysmography (ie, that there is no flow during the occlusion, and alveolar pressure and mouth pressure equilibrate) may not be met.
A lung capacity is the sum of two or more lung volumes; in the case of FRC, it is the sum of residual volume (RV, the amount of gas remaining in the lung after a maximal exhalation) and expiratory reserve volume (ERV, the amount of gas exhaled from resting lung volume until the lung is empty). In combination with spirometry, other lung volumes and capacities (eg, total lung capacity) can be calculated (Fig. 503-3).
The pattern of lung volumes can also assist in diagnosis. Typically, patients with obstructive diseases will have an increased RV, especially as a fraction of total lung capacity (RV/TLC). The TLC may be normal or elevated. In contrast, low lung volumes are the hallmark of restrictive lung disease, and these patients will have a reduced TLC and RV.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


