Variables
Traditional (N = 100)
Early (N = 23)
P value
Age (years)
31 ± 6
30 ± 6
0.67
Parity
2.1 ± 1.7
1.9 ± 1.5
0.64
Hx of gestational HTN
43 %
30 %
0.56
Twin pregnancy
13 %
26 %
0.009
LVEF at diagnosis
31 ± 12 %
30 ± 12 %
0.72
LVEF at last F/U
46 ± 14 %
44 ± 16 %
0.54
Duration of F/U (months)
6 ± 7
7 ± 9
0.52
Mortality
9 %
13 %
0.7
Clinical Manifestations
Onset of PPCM can be masked and missed as features of normal pregnancy mimic those of mild heart failure.
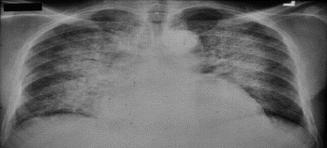
These patients present with dyspnea, dizziness, chest pain, cough, neck vein distension, fatigue, and peripheral edema [33].
Left ventricular dysfunction causes dilatation of left ventricle in turn causing arrhythmias and embolic events.
They also can have other features of heart failure – hypoxia, jugular venous distension, S3 and S4 gallop, hepatomegaly, and rales [32].
They have tachycardia, but blood pressure is normal or decreased.
Features of pregnancy like edema, tachycardia, JV distension, and S3 can be normal also.. Closer look at cardiac profile is necessary in order not to miss the diagnosis of heart failure [14].
Diagnosis
PPCM is a diagnosis of exclusion. Hence, other causes of heart failure are to be excluded such as pulmonary embolism, sepsis, myocardial infarction, valvular disease, severe preeclampsia, and other forms of cardiomyopathy [15].
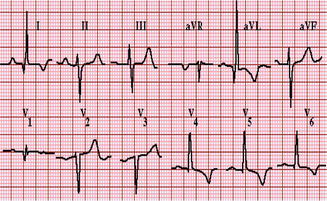
Electrocardiogram may be normal or shows changes of left ventricular hypertrophy, ST-T wave abnormalities, dysrhythmias, Q waves in the anteroseptal precordial leads, and prolonged PR and QRS intervals [34].
Laboratory tests – complete blood picture, blood urea, serum creatinine, serum electrolytes, and serum troponin levels (to rule out myocardial infarction, but may be raised in acute phase of PPCM). Levels of B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide can help in confirming the diagnosis [3].
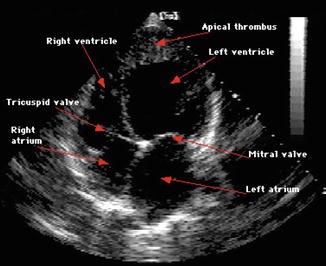
Echocardiography and Doppler imaging – it is the most validated and practical tool for establishing the diagnosis. Evaluate ventricular function, valvular structure and function, pathological pericardial changes, and mechanical complications. Features suggestive of PPCM are decreased ejection fraction, global dilatation, and thinned out cardiac walls [9].
Diagnostic Criteria for Peripartum Cardiomyopathy
All four of the following:
Classic
1.
Development of cardiac failure in the last month of pregnancy or within 5 months postpartum
2.
No identifiable cause for the cardiac failure
3.
No recognizable heart disease before the last month of pregnancy
Additional
1.
Strict echocardiographic indication of left ventricular dysfunction:
(a)
Ejection fraction <45 %
And/or:
(b)
Fractional shortening <30 %
(c)
End-diastolic dimension >2.7 cm/m2
Differential Diagnosis
Idiopathic dilated cardiomyopathy – seen at a younger age. It occurs postpartum (78–93 %). Higher incidence of myocarditis is seen in PPCM. PPCM leads to rapid worsening of clinical course and poor outcome, but heart size returns to normal in more number of patients soon after delivery [17].
Occult valvular heart disease can be ruled out by transthoracic echocardiography. Finding of normal systolic function rules out PPCM [41].
Ischemic heart disease is uncommon, but women with type 1 diabetes mellitus should have noninvasive assessment of coronary ischemia.
Pulmonary thromboembolism.
Severe eclampsia.
Pneumonia.
Management
Compensated heart failure:
The goal is to improve hemodynamic status and minimize signs and symptoms and to reduce preload and afterload and increasing cardiac inotropy [39, 40].
For preload reduction, nitrates are used which are safe in pregnancy and lactation.
Restriction of dietary sodium is helpful.
Loop diuretics are used in management of signs and symptoms of preload reduction, but it causes decrease in blood supply to uterus and fetus [41].
Exercise such as walking can be advised once stable.
During the intrapartum period, hydralazine, digoxin, nitrates, and diuretics can be used.
Angiotensin-converting enzyme inhibitors are contraindicated in pregnancy [40].
Beta-adrenergic antagonist (carvedilol and metoprolol) has been approved and improves the survival in PPCM. Carvedilol can be continued in the postpartum period in whom symptoms of heart failure persists and have left ventricular compromise more than 2 weeks after therapy [42].
Digoxin can be used in women whose ejection fraction is <40 % and who have signs and symptoms of heart failure [3].
Guidelines for Management of Compensated Heart Failure
Nonpharmaceutical Therapies [8]
Low sodium diet: limit of 2 g sodium per dayFluid restriction: 2 L/dayLight daily activity: if tolerated (e.g., walking)
Oral Pharmaceutical Therapies [8]
Antepartum Management of Peripartum Cardiomyopathy
Beta-blocker
Carvedilol (starting dosage, 3.125 mg twice a day; target dosage, 25 mg twice a day)
Extended-release metoprolol (starting dosage, 0.125 mg daily; target dosage, 0.25 mg daily)
Vasodilator
Hydralazine (starting dosage, 10 mg 3 times a day; target dosage, 40 mg 3 times a day)
Digoxin (starting dosage, 0.125 mg daily; target dosage, 0.25 mg daily). Monitor serum levels.
Thiazide diuretic (with caution)
Hydrochlorothiazide (12.5–50 mg daily)
May also consider loop diuretic with caution
Low molecular weight heparin if ejection fraction <35 %
Postpartum Management of Peripartum Cardiomyopathy
Angiotensin-converting enzyme (ACE) inhibitor
Captopril (starting dosage, 6.25–12.5 mg 3 times a day; target dosage, 25–50 mg 3 times a day)
Enalapril (starting dosage, 1.25–2.5 mg 2 times a day; target dosage, 10 mg 2 times a day)
Ramipril (starting dosage, 1.25–2.5 mg 2 times a day; target dosage, 5 mg 2 times a day)
Lisinopril (starting dosage, 2.5–5 mg daily; target dosage, 25–40 mg daily)
Angiotensin receptor blocker (if ACE inhibitor is not tolerated)
Candesartan (starting dosage, 2 mg daily; target dosage, 32 mg daily)
Valsartan (starting dosage, 40 mg twice a day; target dosage, 160 mg twice a day)
Consider nitrates or hydralazine if woman is intolerant to ACE inhibitor and angiotensin receptor blocker.
Loop diuretic
Furosemide intravenously or by mouth. Dosing considerations should be made on the basis of creatinine clearance.
Glomerular filtration rate >60 mL/min per 1.73 m2: furosemide 20–40 mg every12–24 h
Glomerular filtration rate <60 mL/min per 1.73 m2: furosemide 20–80 mg every 12–24 h
Vasodilator
Hydralazine (starting dosage, 37.5 mg 3 or 4 times a day; target dosage, 40 mg 3 times a day)
Isosorbide dinitrate (starting dosage, 20 mg 3 times a day; target dosage, 40 mg 3 times a day)
Aldosterone antagonist
Spironolactone (starting dosage, 12.5 mg daily; target dosage, 25–50 mg daily)
Eplerenone (starting dosage, 12.5 mg daily; target dosage, 25–50 mg daily)
Warfarin if ejection fraction is <35 %
Decompensated Heart Failure [28]
Establish airway, breathing, and circulation. Pregnancy results in third spacing of intravascular volume which can result in suboptimal airway [41].
ST segment monitoring with cardiac monitor.
Blood pressure monitoring with noninvasive blood pressure cuffs until cardiac catheters are placed [42].
Venous and arterial access.
In acute heart failure, administer intravenous positive inotropic agents such as milrinone and dobutamine. These agents facilitate diuresis, improve cardiac performance, and preserve end-organ function [40].
If systolic blood pressure is less than 90 mmHg, dobutamine is preferred over milrinone. Nitroglycerin and nitroprusside also may be used. Nitroprusside can cause toxic effects of thiocyanate which can be harmful to the fetus [40].
Warfarin is given in postpartum period and heparin or low molecular weight heparin is given in pregnancy in patients with ejection fraction <35 % as it causes left ventricular thrombus and continued until left ventricular function becomes normal on echocardiography.
If medical therapy fails, intra-aortic balloon pump or left ventricular assist device may be used [19].
In persistent pulmonary edema with hypoxemia, extracorporeal membrane oxygenation can be done [36, 43].
Heart transplantation is a practical therapeutic option for women with PPCM who have advanced heart failure and signs and symptoms unresponsive to medical therapies [38].
Summary for Management of Decompensated Heart Failure [21]
AirwayIntubate promptly upon distress for increased work of breathing to prevent complications with difficult airway later in treatment.BreathingProvide supplemental oxygen.Maintain continuous pulse oximetry to monitor SaO2.Measure arterial blood gases (if available) every 4–6 h until breathing is stable.CirculationStart cardiac and blood pressure monitoring.Insert arterial catheter for accurate blood pressure monitoring and blood sampling.Obtain central venous access with central venous pressure monitoring.In antepartum women, obtain fetal monitoring.Pharmacological management of acute heart failure in peripartum cardiomyopathy Intravenous loop diuretic (caution is advised in antepartum women)Furosemide: dosing considerations should be made on the basis of creatinine clearance.Glomerular filtration rate <60 mL/min per 1.73 m2Furosemide 20–40 mg intravenously every 12–24 hGlomerular filtration rate >60 mL/min per 1.73 m2Furosemide 20–80 mg intravenous every12–24 hIn severe fluid overload, consider furosemide infusion or ultrafiltration.VasodilatorNitroglycerin infusion 5–10 μg/min, titrate to clinical status and blood pressure.Nitroprusside 0.1–5 μg/kg/min is used with caution in antepartum women.Positive inotropic agentsMilrinone 0.125–0.5 μg/kg/minDobutamine 2.5–10 μg/kg/minAvoid beta-blockers in the acute phase, as they can decrease perfusion.Heparin sodium, alone or with oral warfarin (Coumadin) therapyMonitor oxygenation with arterial blood gases every 4–6 h until patient’s condition is stable.Consider endomyocardial biopsy; if proven as viral myocarditis, consider immunosuppressive medications (e.g., azathioprine, corticosteroids).Every effort should be made to devise an oral regimen that can maintain symptomatic improvement and reduce the subsequent risk of worsening clinical status.If no improvement clinically:Consider cardiac magnetic resonance imaging.Perform endomyocardial biopsy to detect viral myocarditis (if not previously completed).Assist devices:Intra-aortic balloon pump [19]Left ventricular assist devicesExtracorporeal membrane oxygenationTransplantationIf a woman remains refractory to therapy, consult your institution’s guidelines for bromocriptine or cabergoline administration for suppression of prolactin production [11].
Management During Pregnancy
Welfare of the fetus should always be considered along with that of the mother.
Patients with severe forms of heart failure will require ICCU management, with monitoring of arterial blood pressure (ABP), central venous pressure (CVP), and sometimes pulmonary artery catheter (PAC).
Coordinated management with specialist’s team is essential.
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers are contraindicated in pregnancy because these can cause birth defects, while remaining the main treatment option for postpartum women with heart failure [40].
The teratogenic effects occur particularly in the second and third trimester, characterized by fetal hypotension, pulmonary hypoplasia, oligohydramnios, anuria, and renal tubular dysplasia [44].
Digoxin, loop diuretics, sodium restriction, and drugs which reduce afterload such as hydralazine and nitrates have been proven to be safe.
Amlodipine has been found to improve survival in nonischemic cardiomyopathy patients.
Beta-blockers have been used in pregnant women with hypertension without any known adverse effects on the fetus, and patients taking these agents prior to diagnosis can continue to use them safely.
Management during Postpartum Period
Treatment is identical as for nonpregnant women with dilated cardiomyopathy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree