9 | Peripartal and Puerperal Infections |
Infections During Childbed
Infections during childbed are relatively common. This is due to the large wound surfaces in the uterus (the site of the placenta), the wounds caused by episiotomy and cesarean section, and the lactating breasts. Nevertheless, the body is surprisingly well equipped to cope with these risks, and the best defense is mainly provided by the high blood flow in these regions.
A dangerous situation arises only when highly pathogenic bacteria invade the open uterus. Here, group A streptococci are particularly dreaded. They are the only bacteria that quickly cause fulminant infections, overwhelming the body in a very short time. Women carrying the undetected pathogen in the vagina at the time of delivery are especially endangered. Transmission of pathogens by the obstetrician or personnel is possible, but this is more the exception.
Far more common are infections due to Staphylococcus aureus. This pathogen is normally found on the patient’s skin and may occur in the vagina as well. It is the primary pathogen of wound infection, abscess-forming infections, and above all, puerperal mastitis. Sepsis due to Staphylococcus aureus takes a slower course than that caused by group A streptococci, thus giving the body more time to develop symptoms and warning signs that make one think of an infection.
Another large group of pathogens consists of aerobic intestinal bacteria, such as Escherichia coli, Klebsiella, and many more. They become dangerous when large numbers are introduced into the uterus, tissue, or abdominal cavity, when local conditions favor their multiplication, or when the body’s defenses are weakened. The same applies also to anaerobic bacteria. More than 20 years ago, anaerobes occasionally caused severe infections; until that time, they were hardly known to cause diseases and were therefore not considered when deciding on the therapy.
Infections during childbed include:
 puerperal sepsis
puerperal sepsis
 endometritis
endometritis
 peritonitis (ascending infection, intestinal injury)
peritonitis (ascending infection, intestinal injury)
 infection of the episiotomy wound
infection of the episiotomy wound
 infection of the suprapubic incision
infection of the suprapubic incision
 abscess-forming infection between the intestine and the genitals after intestinal injury (suture error, tear)
abscess-forming infection between the intestine and the genitals after intestinal injury (suture error, tear)
 ascending Chlamydia infection
ascending Chlamydia infection
 urinary tract infection
urinary tract infection
 puerperal mastitis.
puerperal mastitis.
Ascending Infections after Delivery 
Promoting Factors
The genital tract is especially susceptible to bacterial infections immediately after delivery, and so are abdominal wounds. The following factors promote such infections: the altered and slightly suppressed immune system during pregnancy, the wide opening of the uterine cavity—making it easier for pathogens to be introduced in large numbers from the vagina by manipulation during delivery—combined with tissue trauma while giving birth.
The fact that severe infections are relatively rare after delivery is partly explained by the heavy blood flow to the genitals at that time, thus providing plenty of humoral and cellular defenses.
Factors that promote infection are listed below. No single factor is responsible for the occurrence of an infection; it is more likely that several factors have to come together. The presence of one or more of these factors should prompt the obstetrician to pay special attention.
Risks of infection:
 pathogenic bacteria (group A streptococci, Staphylococcus aureus, and others)
pathogenic bacteria (group A streptococci, Staphylococcus aureus, and others)
 high numbers of other bacteria (e. g., bacterial vaginosis)
high numbers of other bacteria (e. g., bacterial vaginosis)
 prolonged labor combined with many examinations
prolonged labor combined with many examinations
 injuries (tears of cervix and vagina)
injuries (tears of cervix and vagina)
 episiotomy
episiotomy
 vaginal delivery including surgical intervention (vacuum, forceps, manual detachment of placenta)
vaginal delivery including surgical intervention (vacuum, forceps, manual detachment of placenta)
 cesarean section
cesarean section
 premature rupture of fetal membranes
premature rupture of fetal membranes
 diabetes mellitus
diabetes mellitus
 immune suppression
immune suppression
 anemia.
anemia.
Puerperal Sepsis (see also p. 156)
Until the time of Semmelweiss (1818-1865), the primary pathogens involved in the lethal course of infections after delivery have been group A streptococci. During the worst puerperal fever epidemic, one in every four mothers died of this infection. In 1847, Semmelweiss published his results on the cause of puerperal fever, but they were ignored for many years—as so often is the case in the history of medicine.
The pathogen’s port of entrance is usually the uterus, but it may also be the episiotomy wound or the suprapubic incision. In the vast majority of patients, the pathogen resides in the vagina at the time of delivery. After delivery, it rapidly spreads from here over the entire uterus and into the blood, and thus to all organs of the body.
Pathogens. Streptococcus pyogenes, streptococci of serogroup A.
Epidemiology. Unfortunately, no data are available in Germany on the frequency of puerperal sepsis or infections caused by group A streptococci during pregnancy and childbed. Only deaths during puerperal sepsis are notifiable. Probably, not all of the postpartum deaths caused by group A streptococci are officially recorded because the pathogen is not yet known at the time of death, or because samples for bacteriology have not been collected—or only after antibiotic treatment.
The frequency of group A streptococcal infection during childbed is mostly unknown, since patients with fever are far more often treated with antibiotics than swabs are taken for microbiology. The media only report on rare spectacular cases with legal battles in the courts. As a result, the public seems to believe that puerperal infection due to group A streptococci are a rare event. This is not the case. Fortunately, a lethal outcome of puerperal sepsis is a rare event.
Based on official death statistics, figures from our own department, a large survey in German clinics, and a dissertation, I have estimated that there are at least 200 infections due to group A streptococci, about 10-20 cases of puerperal sepsis, and 0.5-1 cases of death per 100000 pregnancies.
According to one survey, group A streptococci were detected in 39 out of 97 patients with puerperal sepsis. Of the 39 patients infected, 13 died. Of 35 patients in whom other bacteria had been detected, five patients died, and of 23 patients without any bacteria detected, or without any information available, only one patient died.
Clinical picture. One of the most typical signs of a beginning puerperal sepsis is that the patient is very ill in the absence of any recognizable cause. Symptoms like fever and increased pulse rate are not very typical, since these are also present with many other infections.
More characteristic are respiratory problems, diffuse pain, in most patients the absence of guarding (due to the stretched abdominal wall), diarrhea, hypothermia, leukopenia, and the absence of any cause for the poor general health of the patient. Fever may be absent, especially if the infection appeared suddenly and took an unfavorable course.
Pain in the lower abdomen or in the region of the symphysis should not be taken for symphysiolysis, unless infection has been excluded.
A sore throat may indicate the beginning of an infection caused by group A streptococci. Sore throats or flulike infections in the patient’s surroundings should increase awareness regarding a possible risk.
A full-blown sepsis is always associated with massive respiratory insufficiency, since inflammatory damage to the endothelium makes gas exchange hardly possible.
Unfortunately, the uterine pain described in textbooks is not a reliable sign, although it should be taken seriously if present.
The full picture of puerperal sepsis is fortunately very rare. At an advanced stage of sepsis, lethality is still around 20 % even today. The endotoxins quickly cause disseminated intravascular coagulopathy (DIC), thus leading to multiorgan failures.
A common complication is fasciitis associated with tissue necrosis (Fig. 9.1). Here, tissues die because of inflammatory damage to the endothelium. This may lead to partial or complete amputation of limbs.
Differential diagnosis. The above-mentioned condition should be distinguished from the very rare case of necrotic fasciitis. This is a progressive, severe, necrotic inflammation of deep tissue layers after surgical intervention or manipulation. Initially, the skin area shows only a mild redness. A typical pathogen is not known. Various pathogens seem to be involved (synergism), particularly anaerobes. Unknown immunological processes may play an important role.
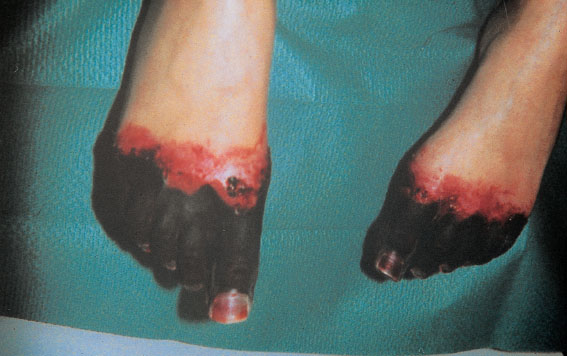
Fig. 9.1 Necrosis of both forefeet in a 26-year-old patient with severe puerperal sepsis.
Therapeutic approach. High doses of broad-spectrum antibiotics and surgical abrasion of the necrotic tissue, and high doses of cortisone, if necessary.
Diagnosis. If only few laboratory tests are carried out, the dangerous course of sepsis cannot be recognized in time. Just taking the temperature and ordering a general blood count are therefore totally inadequate for the early recognition of sepsis. CRP is the only reliable laboratory parameter, and the test is meanwhile available in every country. An increase by a factor of 30-100, associated with seemingly normal or decreasing leukocyte values, indicates a dangerous situation, namely, sepsis and septic shock. The decrease in thrombocytes occurs relatively late and is therefore not a good warning sign:
 clinical signs of disease, poor condition of the patient
clinical signs of disease, poor condition of the patient
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


