8 | Infections During Pregnancy |
General Introduction
Infections during pregnancy are especially feared because they may not only endanger the mother but also her child. Some infections take a more severe course during pregnancy, or even occur only during this time or during and after delivery. In addition, pregnancy is an extremely sensitive phase in the life of a woman, and any infections and their sequelae should be taken seriously.
Pathogens may infect the child:
 by means of the blood (hematogenous infection, only in case of primary infection)
by means of the blood (hematogenous infection, only in case of primary infection)
 by ascending from the vagina through the fetal membranes (mostly bacteria)
by ascending from the vagina through the fetal membranes (mostly bacteria)
 by passing through the placenta (transplacental infection) at the end of the pregnancy, especially during labor (e. g., HIV)
by passing through the placenta (transplacental infection) at the end of the pregnancy, especially during labor (e. g., HIV)
 during passage of the child through the birth canal (hepatitis B virus, cytomegalovirus, HSV, HPV, gonococci, chlamydiae, group B streptococci, E. coli, etc.).
during passage of the child through the birth canal (hepatitis B virus, cytomegalovirus, HSV, HPV, gonococci, chlamydiae, group B streptococci, E. coli, etc.).
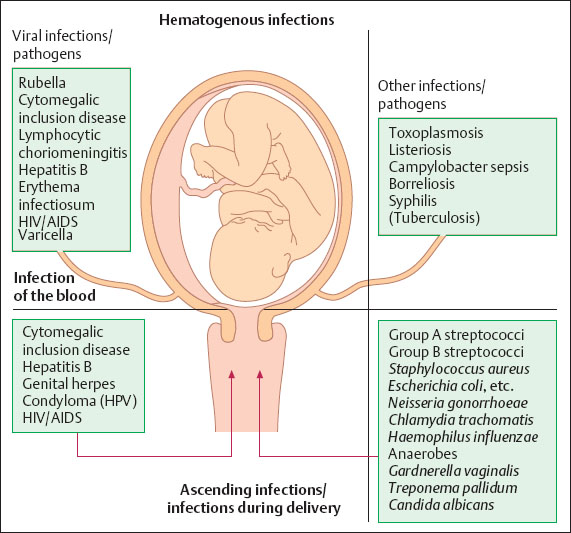
Fig. 8.1 Diagram illustrating the most common hematogenous and ascending infections during pregnancy and during delivery.
During pregnancy the mother has a higher risk of infections (malaria, puerperal sepsis, catheter infections) and other diseases.
The following complications of infections may occur in particular:
 direct damage to the child by infection in utero (embryopathy, fetopathy)
direct damage to the child by infection in utero (embryopathy, fetopathy)
 indirect damage to the child by premature delivery
indirect damage to the child by premature delivery
 infection of the child during birth
infection of the child during birth
 exacerbation of an infection of the mother
exacerbation of an infection of the mother
 reactivation of latent infections of the mother
reactivation of latent infections of the mother
 ascending infections of the mother (endometritis, sepsis)
ascending infections of the mother (endometritis, sepsis)
 death of the child
death of the child
 death of the mother.
death of the mother.
Viral infections during pregnancy, especially during the first trimester, pose the most serious danger to the child. Bacterial infections are particularly threatening during birth, but so are some viral infections, such as varicella (chickenpox), cytomegalic inclusion disease, hepatitis B, and herpes simplex (Fig. 8.1).
Viruses normally destroy the cells in which they have multiplied. They are therefore especially feared during the embryonic phase as they may cause irreparable embryopathies. Among the known viral infections, a high percentage of damage is done by the rubella virus; but also the virus causing erythema infectiosum and the LCM virus threaten the life of the child (see Fig. 8.2).
Bacterial infections endanger the child by inducing severe inflammatory responses during the fetal period.
Treatment options. Any care for a pregnant woman aims at saving her and her child from damage due to infections. However, this is only achieved when the infection can be recognized and when therapeutic agents are available.
This does not apply to all infections. There are a number of infections that may run their course without producing symptoms, and there are pathogens (viruses) for which we do not have any effective drugs.
Another problem during pregnancy is the reactivation of latent infections; this holds true especially for herpes viruses (HSV, CMV, EBV, VZV), papillomaviruses, and also other pathogens. Here, there is hardly any risk of hematogenous infection as the pathogen is inactivated by antibodies in the blood. A dangerous situation exists only when there is direct contact with pathogens excreted into the birth canal or when pathogens pass through the placental barrier.
The distinction between primary infection and reactivation occasionally creates problems. Diagnostic serology relies only rarely on a significant rise in titer (> 2 titer steps) in case of primary infection; it is often based only on the detection of IgM antibodies. This is problematic in case of chronic persistent infections, such as toxoplasmosis, cytomegalic inclusion disease, and other herpes virus infections, since IgM antibodies may be produced over many years.
By determining the strength of antigen–antibody binding (avidity), the start of an infection can be narrowed down more closely. Alternatively, this is done by means of a western blot, since the number of visible bands increases with the duration of the infection, reflecting the fact that antibodies are produced against an increasing number of pathogen structures.
Viral Infections
General Remarks 
Forms of infection, frequency, and risks for the child. Viral infections are common, also during pregnancy. Although all viral infections pose a risk theoretically, only a few pathogens surpass the 1% threshold of posing a risk for the child.
The overview in Fig. 8.2 lists different viral infections during pregnancy, their frequencies, their risks of damage, as well as the periods of highest risk.
Estimated values had to be used for some of the infections listed, either because data simply do not exist or because documented data on frequencies and risks are not available despite various studies performed.
Due to the limited therapeutic options for viral infections, only the following consequences are available at present:
 Termination of the pregnancy. This should only be performed—with proper indication and at the parents’ requests—if the risk of damage to the child is significantly increased. If the mother is endangered, termination is possible at any time under German law.
Termination of the pregnancy. This should only be performed—with proper indication and at the parents’ requests—if the risk of damage to the child is significantly increased. If the mother is endangered, termination is possible at any time under German law.
 Administration of immunoglobulins to avoid hematogenous spread of the virus. This measure is only worthwhile if immunoglobulins are administered early (one to four days after the initial contact with the virus).
Administration of immunoglobulins to avoid hematogenous spread of the virus. This measure is only worthwhile if immunoglobulins are administered early (one to four days after the initial contact with the virus).
 Administration of virostatics. Aciclovir is still administered with some reservation, although virustatics are now standard procedure if the mother is infected with HIV.
Administration of virostatics. Aciclovir is still administered with some reservation, although virustatics are now standard procedure if the mother is infected with HIV.
 Serological exclusion of certain infections that pose an increased risk often provides sufficient reassurance to the patient.
Serological exclusion of certain infections that pose an increased risk often provides sufficient reassurance to the patient.
 Informing the patient about the actual, and usually minor, risk of the current viral infection.
Informing the patient about the actual, and usually minor, risk of the current viral infection.
Rubella (German Measles) 
Infection with the rubella virus is the most feared complication during early pregnancy, since this virus—unlike any other virus—causes irreversible damage to the child in a high percentage of cases. For historical aspects, see Table 8.1.
Pathogen. The rubella virus belongs to the to-gaviruses; only one serotype is known. Its nucleic acid is single-stranded RNA, and its sensitive envelope contains hemagglutinin. Stability of the virus outside the cell is low. Replication of the virus in cell cultures does not induce a distinct cytopathic effect.
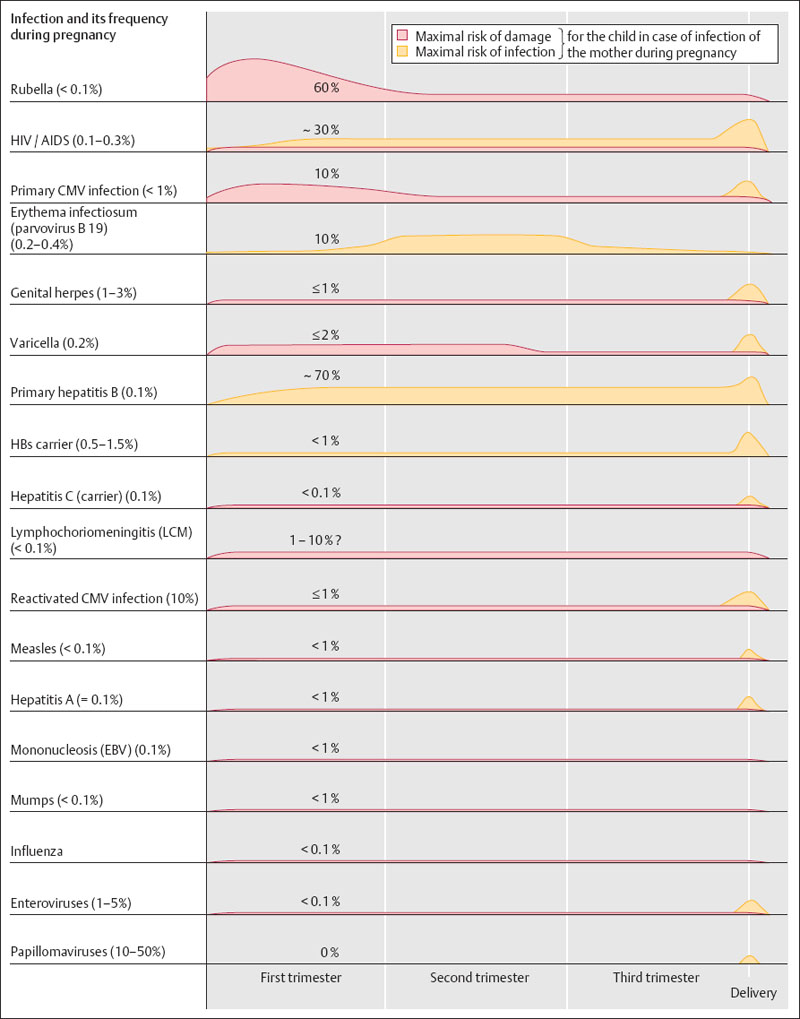
Fig. 8.2 Viral infections during pregnancy. In addition to the estimated frequencies of various infections during pregnancy, the diagram illustrates the risks of damage and infection for the child. Although the frequency of infection cannot always be separated from the frequency of damage, an attempt was made to illustrate by color-coding the different risk types of specific infections over the course of the pregnancy.
Table 8.1 Rubella
Year | Important historical facts |
|---|---|
1941 | Gregg (Australian ophthalmologist): discovery of cataracts after a rubella epidemic Gregg: triad of cataract, deafness, and heart defect |
1962 | Isolation of the rubella virus |
1964 | Rubella epidemic in the United States 250 000 pregnancies affected 13 410 cases of fetal death or death of the newborn |
1966 | Development of rubella vaccine |
1975 | Vaccination on a wide scale |
Frequency. Rubella infections have become rare because of vaccination. Over 90% of adults possess antibodies against the rubella virus. During recent years, about five rubella-induced embryopathies per year have been reported in Germany.
Transmission. Droplet infection, or transplacental infection in case of viremia during pregnancy.
Incubation time. Two (or three) weeks.
Clinical picture. This is a mild childhood disease, with 50% of infections taking an uneventful course without exanthema and with mild respiratory symptoms, at the most.
Typical symptoms include temporary, small-spotted exanthema, swelling of the lymph nodes behind the ears and on the neck, and arthralgia (more common in adults) (Fig. 8.3).
Risks for the child. Rubella infection during the first weeks of pregnancy causes a high percentage of embryopathies. Depending on the time of infection, it affects the eyes (cataract), the heart (malformations), the inner ear (deafness), and possibly the central nervous system (mental retardation, which is often recognized only much later) (Fig. 8.4).
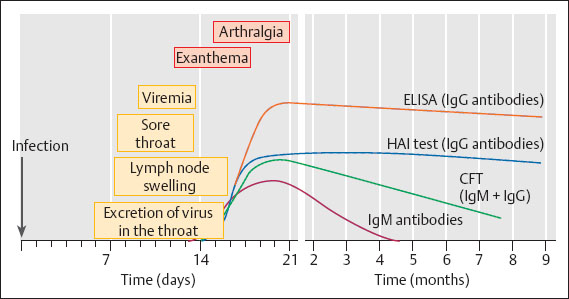
Fig. 8.3 Rubella infection. The diagram illustrates the period of infectiousness (viral excretion in the throat of contact persons, viremia during pregnancy), various clinical symptoms, and the time course of various antibody classes as detected by various methods (laboratory tests).
If there is clear evidence of rubella infection during the first 14 weeks, it is now common practice in Germany to terminate the pregnancy because the risk of damage is higher than 10%, and even higher than 50% during the first few weeks.
If there is the urgent desire to have a child, the risk may be assessed by means of prenatal diagnostics (see p. 37) as the child is not always infected.
Diagnosis (Table 8.2):
Serology:
 Hemagglutination inhibition (HAI) assay. This is the standard test for determining permanent immunity. Two samples of blood are required for detecting a fresh infection: the first one as early as possible, and the second one eight to 10 days after the appearance of the exanthema. Evidence for a fresh rubella infection is either the very first occurrence of antibodies in the second blood sample, or a titer rise between the first and the second blood sample by at least a factor of four.
Hemagglutination inhibition (HAI) assay. This is the standard test for determining permanent immunity. Two samples of blood are required for detecting a fresh infection: the first one as early as possible, and the second one eight to 10 days after the appearance of the exanthema. Evidence for a fresh rubella infection is either the very first occurrence of antibodies in the second blood sample, or a titer rise between the first and the second blood sample by at least a factor of four.
 ELISA. It yields high titers and is more suitable for the detection of IgM antibodies (it may yield false positive results).
ELISA. It yields high titers and is more suitable for the detection of IgM antibodies (it may yield false positive results).
 Hemolysis-in-gel test. This test is carried out for confirmation when the titer in the HAI assay is lower than 1:32 because the HAI assay is not absolutely reliable in the lower range.
Hemolysis-in-gel test. This test is carried out for confirmation when the titer in the HAI assay is lower than 1:32 because the HAI assay is not absolutely reliable in the lower range.
 Complement fixation test (CFT). It plays only a role in diagnosing a fresh rubella infection. High titers (1:80 and higher) raise suspicion, but they are no proof.
Complement fixation test (CFT). It plays only a role in diagnosing a fresh rubella infection. High titers (1:80 and higher) raise suspicion, but they are no proof.
 Detection of rubella-specific IgM antibodies. This test is always required when samples for serology are collected only after exanthema has developed and the antibody titer is already high. In this case, only the detection of specific IgM antibodies can prove that the exanthema was caused by a rubella infection.
Detection of rubella-specific IgM antibodies. This test is always required when samples for serology are collected only after exanthema has developed and the antibody titer is already high. In this case, only the detection of specific IgM antibodies can prove that the exanthema was caused by a rubella infection.
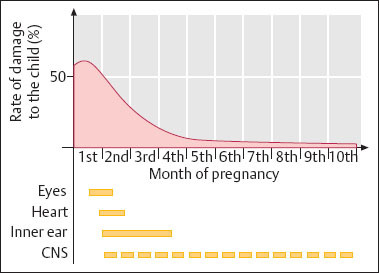
Fig. 8.4 Rubella infection during pregnancy. Level of damage and types of damage during the course of the pregnancy.
Pathogen detection:
 Detection of the virus in cultures. This is expensive and does not play a role in diagnostics.
Detection of the virus in cultures. This is expensive and does not play a role in diagnostics.
 Polymerase chain reaction (PCR). This method is used for the detection or exclusion of the virus, particularly in the amniotic fluid.
Polymerase chain reaction (PCR). This method is used for the detection or exclusion of the virus, particularly in the amniotic fluid.
Prenatal diagnosis. If there is the desire to have a child and the child’s risk of infection is low, infection of the child may be excluded with increasing success in special laboratories. This can be either by amniocentesis for the detection of rubella virus in the amniotic fluid by PCR, or—from the 22nd week of pregnancy on—by puncture of the umbilical cord for the detection of IgM antibodies.
Diagnosis of congenital rubella infection:
 persistence of rubella-specific antibody titer in the newborn: no titer fall six to eight weeks after birth (Fig. 8.5)
persistence of rubella-specific antibody titer in the newborn: no titer fall six to eight weeks after birth (Fig. 8.5)
 detection of rubella-specific IgM antibodies in the cord blood, or later in the newborn
detection of rubella-specific IgM antibodies in the cord blood, or later in the newborn
 detection of rubella virus from a throat swab. Rubella virus may be excreted over many months, sometimes over one to two years.
detection of rubella virus from a throat swab. Rubella virus may be excreted over many months, sometimes over one to two years.
Prophylaxis prior to a pregnancy. The immune status (rubella titer) is determined by means of the HAI assay (> 90% of women of childbearing age possess antibodies). A titer of more than 1:16 is reliable and indicates sufficient protection. A titer of 1:8 is unreliable (due to the nature of the method). Detection of rubella-specific antibodies in the hemolysis-in-gel test or by ELISA indicates protection. To be on the safe side, rubella vaccination is recommended. If the titer check does not yield a titer rise after eight to 12 weeks, no further measures are necessary as one can assume that there is sufficient immunity.
If the rubella titer is negative (lower than 1: 8) and the hemolysis-in-gel test is negative as well, vaccination is necessary. A titer check is required after eight to 12 weeks. If there is no measurable titer, vaccination should be repeated.
Table 8.2 Problems with rubella diagnosis during pregnancy
Diagnosis/Problems | Findings/Approach |
|---|---|
Antibody titer prior to pregnancy | No risk or minimal risk |
First titer determination during pregnancy | Possible risk |
— HAI titer 1:32 to 1:256 — HAI titer < 1:16 — HAI titer > 1:256 | Immunity is assumed |
Titer monitoring or further tests | — If the HAI titer is <1:16, monitoring is required during weeks 16–20 of pregnancy to exclude an asymptomatic infection during the first trimester — If the IgM antibody test is positive, further tests are required to confirm that rubella IgM antibodies are actually present, since false positive results are common with IgM antibody tests. Consultation with the laboratory is essential prior to discussing the risk with the patient |
Establishing the patient’s history | — Questioning about symptoms, vaccination, contact with exanthematous persons — Possibly reinfection: very low risk, if at all (no cases of damage are known) — False positive ELISA–IgM test, e. g., wrong test or cross-reaction in case of EBV infection — IgM antibody persistence (very rare) |
Recommendations: prenatal diagnostics in unclear cases and involvement of a special laboratory
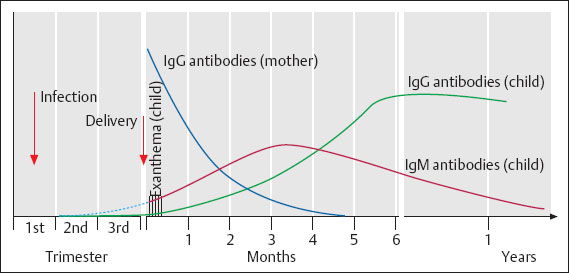
Fig. 8.5 Congenital rubella infection. Time course of antibody production by the child.
If the titer remains negative, no further measures are required, as one may assume that very low amounts of antibodies do exist, thus preventing the live vaccine from working. Nevertheless, a check-up during pregnancy is recommended (16th week of pregnancy).
Vaccination against rubella is exclusively done for building up immunity to prevent rubella infection during the planned pregnancy. It is carried out by subcutaneous injection of a live vaccine into the upper arm.
In very rare cases, there are mild side effects resembling a rubella infection (mild flulike symptoms, exanthema, arthralgia).
Antibody production after vaccination is slightly slower than after natural rubella infection. The outcome of the vaccination should therefore not be checked before eight weeks, preferably after 12 weeks. For documentation of the rubella titer, it is essential to check the titer after each vaccination.
Only in rare cases does vaccination fail to induce a measurable titer of rubella-specific antibodies. The reason for this may be that immunity already exists, although at sucha low level that it cannot be verified by means of the HAI assay. On the other hand, the vaccine—a heat-sensitive live vaccine—may have been inactivated to such an extent that the remaining amount of viable viruses is no longer sufficient to trigger an immune response.
There are also individuals who have an increased infection threshold. In particularly stubborn cases, vaccination may be repeated with a double dose to be on the safe side.
Vaccination should never be performed during pregnancy, and a new pregnancy should not start earlier than eight weeks after vaccination.
However, if vaccination has been carried out by mistake during an existing pregnancy, or if a pregnancy did start within the waiting period of eight weeks, this is of no consequence. There is no reason at all to terminate the pregnancy, for the risk from the attenuated virus in the vaccine is extremely low.
In over 800 well-documented cases in which vaccination occurred during early pregnancy, there has not been a single verified case of damage due to the vaccine virus. The warning against becoming pregnant should therefore be viewed as a precautionary measure only.
Prophylaxis during pregnancy:
 Determination of immune status, if this has not been done earlier.
Determination of immune status, if this has not been done earlier.
 Seronegative women without rubella exposure should have a titer check between the 14th and 16th weeks of pregnancy to make sure that there has been no rubella infection in the meantime.
Seronegative women without rubella exposure should have a titer check between the 14th and 16th weeks of pregnancy to make sure that there has been no rubella infection in the meantime.
 Seronegative women with recent rubella exposure (one to three days ago) should receive rubella-specific hyperimmune globulin (15 mL) as soon as possible. A titer check should follow after three weeks, and then again after six weeks, in order to confirm that a rubella infection did not take place (Table 8.3). The antibodies administered may yield a temporary titer of 1:8 to 1:16, at the most.
Seronegative women with recent rubella exposure (one to three days ago) should receive rubella-specific hyperimmune globulin (15 mL) as soon as possible. A titer check should follow after three weeks, and then again after six weeks, in order to confirm that a rubella infection did not take place (Table 8.3). The antibodies administered may yield a temporary titer of 1:8 to 1:16, at the most.
If a persistent antibody titer develops, an infection did take place. It is then essential to discuss with the patient the issue of prenatal diagnostics—and, possibly, termination of the pregnancy. However, timely administration of antibodies can reduce the risk by a factor of three.
Erythema Infectiosum (Fifth Disease) 
Erythema infectiosum is the fifth exanthematous childhood disease after rubella, measles, varicella, and scarlet fever. The pathogen, parvovirus B19, has been identified only in 1983 as the cause of erythema infectiosum. This disease has been regarded for a long time as a harmless infection that needed to be differentiated from rubella by serological means. Today, this infection is feared during pregnancy, since the child may die because of anemia following the state of fetal hydrops.
Table 8.3 Effects of timely administration of 15 mL rubella hyperimmune serum
|
Pathogen. Parvovirus B19 is a member of the family Parvoviridae. It is a relatively stable virus without an envelope. Its genome consists of linear single-stranded DNA.
Frequency. Endemic infection is higher than originally assumed. Antibodies against parvovirus B19 are present in 50–70% of adults. The frequency during pregnancy is estimated to be one in 400.
Transmission. Droplet infection; infection caused by banked blood and plasma products is also possible, though rare.
Pathogenesis. The B19 virus is transferred by the blood via the placenta to the fetus. The infection affects the bone marrow, thus leading to inhibition of erythropoiesis. In addition to this inhibition, the infection leads to hemolysis in the fetus; as in adults, this may cause hemolytic anemia and aplastic crisis. Finally, generalized fetal hydrops develops. The virus probably also infects other organs. Its involvement in chronic polyarthritis and Schönlein–Henoch purpura has been suggested.
Clinical picture. The course is asymptomatic in almost one third of the infections. Flulike symptoms with fever, headache, malaise, and nausea occur during the prodromal stage, followed by a maculopapulous erythema with a tendency to confluence. The erythema affects arms, legs, and trunk symmetrically but usually spares the palms and soles. Mild pain in the joints, especially in the small joints, myalgia, and lymphadenopathy may also be present. These may persist for weeks and months (in about 20% of cases). The seasonal peak of infection is during winter and spring.
Risk groups for serious infection are pregnant women, patients with congenital or acquired anemia, and immunocompromised persons.
Complications. The frequency of developing severe anemia with fetal hydrops in the event of erythema infectiosum infection during pregnancy is reported as lying between 4–17.5%. The highest risk is between the 14th and 24th weeks of pregnancy, since this is the time of the highest density of P antigen on fetal erythrocytes to which the B19 virus binds. Erythrocyte transfusion is only rarely required after the 26th week of pregnancy.
Diagnosis:
 Indication. Serodiagnostics should be performed if a pregnant woman develops exanthema, if erythema infectiosum is present in the woman’s surroundings, and in the event of fetal hydrops.
Indication. Serodiagnostics should be performed if a pregnant woman develops exanthema, if erythema infectiosum is present in the woman’s surroundings, and in the event of fetal hydrops.
 Serology. Enzyme or fluorescence tests for the determination of IgG and IgM antibodies by means of recombinant antigens. Further tests include immunoblot tests for the determination of IgG and IgM antibodies.
Serology. Enzyme or fluorescence tests for the determination of IgG and IgM antibodies by means of recombinant antigens. Further tests include immunoblot tests for the determination of IgG and IgM antibodies.
 Pathogen detection. Growth in culture is not possible. Detection of viral DNA by means of PCR (especially in the amniotic fluid, blood, serum, and other body fluids).
Pathogen detection. Growth in culture is not possible. Detection of viral DNA by means of PCR (especially in the amniotic fluid, blood, serum, and other body fluids).
 Distinctive features of laboratory parameters. Reticulocytopenia, low hemoglobin levels, often neutropenia with lymphocytopenia and thrombocytopenia, occasionally eosinophilia.
Distinctive features of laboratory parameters. Reticulocytopenia, low hemoglobin levels, often neutropenia with lymphocytopenia and thrombocytopenia, occasionally eosinophilia.
Therapy. Treatment is only symptomatic and includes erythrocyte transfusion in the event of severe anemia. Inhibition of viral replication is not possible.
Approach in case of exposure to erythema infectiosum. Infections usually occur as an epidemic disease in nurseries or schools:
 Determination of the immune status of the pregnant woman. If there are no antibodies, she should stay away from the source of infection, and serology should be repeated after three and six weeks because asymptomatic infections do occur.
Determination of the immune status of the pregnant woman. If there are no antibodies, she should stay away from the source of infection, and serology should be repeated after three and six weeks because asymptomatic infections do occur.
 In the case of infection during pregnancy (disease, or only detection of IgM antibodies), the fetus should be closely monitored (once a week) by ultrasound for eight to 10 weeks.
In the case of infection during pregnancy (disease, or only detection of IgM antibodies), the fetus should be closely monitored (once a week) by ultrasound for eight to 10 weeks.
 When the first signs of hydrops appear, cordocentesis (percutaneous umbilical blood sampling) and amniocentesis should be performed for diagnosing the infection of the fetus, followed by intrauterine erythrocyte transfusion. Despite an increased survival rate of the children treated this way, this approach is partly controversial because of theoretical immunological risks. Termination of the pregnancy is not justified, since no damages have been observed so far in the surviving children. The child either dies in utero or is born healthy.
When the first signs of hydrops appear, cordocentesis (percutaneous umbilical blood sampling) and amniocentesis should be performed for diagnosing the infection of the fetus, followed by intrauterine erythrocyte transfusion. Despite an increased survival rate of the children treated this way, this approach is partly controversial because of theoretical immunological risks. Termination of the pregnancy is not justified, since no damages have been observed so far in the surviving children. The child either dies in utero or is born healthy.
 If a child is born with fetal hydrops, antibodies against parvovirus B19 are determined in the mother; if present, the amniotic fluid (which may be stored frozen) is examined in a special laboratory for the presence of B19 DNA.
If a child is born with fetal hydrops, antibodies against parvovirus B19 are determined in the mother; if present, the amniotic fluid (which may be stored frozen) is examined in a special laboratory for the presence of B19 DNA.
Prophylaxis. An active vaccine is in the making. Passive vaccination with standard immune globulin is possible. However, there are hardly any indications for this.
HIV Infection (AIDS) 
Hardly any other infection has triggered such a quick and intensive research as this disease, which was first diagnosed more than 20 years ago. Due to the rapid development of new antiviral compounds, the prognosis and life expectancy for individuals infected by HIV have much improved, although neither a vaccine nor a cure have been found. In gynecology, HIV now plays only a role in the care of infected pregnant women. The care of HIV-positive patients has now been so well established that severe genital infections or malignant diseases because of HIV infection hardly ever occur today.
History and frequency. This infection posed a new problem for medicine. It occurred at the beginning of the 1980s and has affected to a considerable degree the work of gynecologists and obstetricians.
The infection has probably been transmitted from animals to humans some 50 years ago. Only through massive dissemination of the infection by special risk groups (homosexuals, drug addicts) has it become so widespread that it became visible as an epidemic disease. In Africa and other third world countries, transmission of the infection is predominantly heterosexual, and the women in these countries become as frequently infected as men. The main reason for the alarmingly high prevalence in these countries (up to 20% of the population) is poverty, the lack of information and understanding, and the occurrence of other genital infections that promote transmission.
Pathogen. The human immunodeficiency virus (HIV) belongs to the group of lentiviruses, which have been known for some time and cause chronic diseases of the central nervous system in animals (sheep). It is a retrovirus (Retroviridae), i. e., it possesses RNA as genetic material, which must first be transcribed in the cell into DNA. For this purpose, the virus carries its own enzyme, the reverse transcriptase.
The virus has a complex structure (Fig. 1.1, p. 3) with a lipid-containing envelope, and it is therefore very sensitive to alcohol and environmental factors.
The virus is extremely variable and changes its envelope continuously. None of the isolates are identical, not even those from the same patient. So far, we distinguish two types: HIV-1 and HIV-2; the latter is still rare and largely confined to West Africa. The pathogenicity of HIV-2 seems to be lower than that of HIV-1.
So far, the variability of the virus has frustrated any attempts to develop an effective vaccine. The ability of the virus to develop drug resistance is also very high, and new compounds need to be designed continuously. For the same reason, combinations of compounds are more effective than monotherapy.
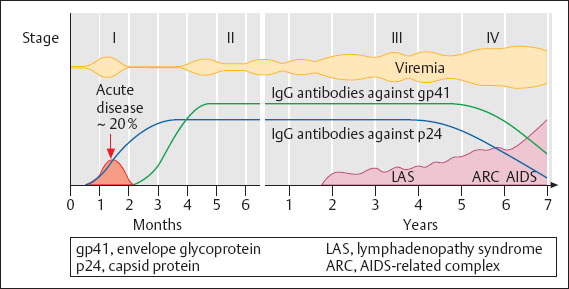
Fig. 8.6 HIV infection. Diagram illustrating the infectiousness (viremia) and the production of antibodies during the clinical course of HIV infection without therapy.
Transmission. The risk of transmission depends on the viral load of blood and tissues, and it is enhanced by local inflammations because of the increased presence of leukocytes. The risk of transmission rises also with the advance of symptoms.
 Anal intercourse. The likelihood of injuring the single-layered intestinal epithelium, the presence of HIV target cells in the mucosa, and the higher viral load in the semen than in the cervix increase the risk.
Anal intercourse. The likelihood of injuring the single-layered intestinal epithelium, the presence of HIV target cells in the mucosa, and the higher viral load in the semen than in the cervix increase the risk.
 Sexual contact. Inflammation in the genital region increases, the risk because an increase in leukocytes leads to increased excretion of the virus.
Sexual contact. Inflammation in the genital region increases, the risk because an increase in leukocytes leads to increased excretion of the virus.
 Through the placenta (depending on the viral load, membrane permeability, and uterine contractions).
Through the placenta (depending on the viral load, membrane permeability, and uterine contractions).
 During delivery (70–80% of child infections).
During delivery (70–80% of child infections).
 Contact with blood (injuries, contaminated needles, open wounds) (the risk is 1:100 to 1:1000).
Contact with blood (injuries, contaminated needles, open wounds) (the risk is 1:100 to 1:1000).
 Banked blood (the risk is about 1:106).
Banked blood (the risk is about 1:106).
 Breast-feeding. Though there is little experience, the virus can be detected in the mother’s milk (the risk of transmission is about 10%).
Breast-feeding. Though there is little experience, the virus can be detected in the mother’s milk (the risk of transmission is about 10%).
 So far, no transmission has been observed through social contacts. The risk is extremely low, although a causal connection is difficult to recognize because the infectiousness of the virus is low and the latency period is long (years).
So far, no transmission has been observed through social contacts. The risk is extremely low, although a causal connection is difficult to recognize because the infectiousness of the virus is low and the latency period is long (years).
 No transmission through immunoglobulin preparations.
No transmission through immunoglobulin preparations.
The risk of infection during sexual intercourse is increased by having contact with intravenous drug users, bisexuals, partners from areas where the disease is endemic, partners with infections that are usually sexually transmitted, partners with multiple applications of blood products, and promiscuity with unknown partners.
Incubation time:
 acute disease: two to four weeks
acute disease: two to four weeks
 antibody production (Fig. 8.6): three to 12 weeks, in extreme cases up to two years (measurable amounts of antibodies)
antibody production (Fig. 8.6): three to 12 weeks, in extreme cases up to two years (measurable amounts of antibodies)
 complete clinical picture of AIDS after eight months to 20 years.
complete clinical picture of AIDS after eight months to 20 years.
Pathogenesis. This is a very complex process involving many cell types including those of the immune system, and it ends with the breakdown of cellular immunity. This disease develops slowly over many years. The virus infects cells carrying the main receptor (CD4) on their surface, especially T4 cells, and cells of the central nervous system. The viral particle is taken up by the cell and undergoes uncoating. A DNA copy is then produced by the viral transcriptase and covalently integrated into the cell’s genome.
Using the viral DNA as template, RNA copies are transcribed and appear in the cytoplasm where they are assembled into viral particles. These are finally budding from the cell membrane that provides them with an envelope into which virus-specific proteins are integrated.
Due to the virus-specific antigens on their surface, T4 lymphocytes are recognized and eliminated by the body’s defense system, thus causing a gradual decline in T4 cells. Infected cells in the brain are destroyed as well. In addition to the increased susceptibility to opportunistic pathogens, cerebral defects associated with changes in personality may occur relatively early in about 40% of the affected individuals. Finally, extreme wasting and death occur.
The virus itself is extremely variable due to errors made by the reverse transcriptase. As a result, none of the isolates are identical. This explains why the HIV virus repeatedly dodges the body’s defenses, thus leading to progression of the disease.
Clinical picture. Here we must clearly distinguish between an asymptomatic virus infection and the full-blown disease. Thanks to increasingly more effective drugs, severe disease is becoming rare in industrialized countries.
The clinical course of the infection has been subdivided into four stages, although it is not yet known whether only 40–60% of the infected individuals reach stage IV or whether all of them fall ill after an extended period of time (> 10–20 years). New drugs have considerably improved the prognosis, with many of the infected persons remaining at stage II for a long time.
Stage I. Acute disease, with about 20% of the infected individuals showing flulike symptoms similar to those of mononucleosis, usually two to three weeks after transmission.
Stage II. Latent infection without symptoms.
Stage III. Lymphadenopathy.
Stage IV. Complete clinical picture of AIDS, with either opportunistic infections (Pneumocystis carinii, Toxoplasma gondii, cryptococci, mycobacteria), virus infections (CMV, HPV, herpesviruses [Fig. 8.7]), changes in the central nervous system with encephalitis and atrophy, or malignant diseases. The gastrointestinal tract, the lungs, and the central nervous system are especially affected.
All four stages are infectious, although infectiousness increases during the progression of the disease.
The disease varies considerably: it may run a very quick course, or it may be very mild and without any subjective impairment of the patient. Much has changed to the better with the therapeutic possibilities available today.
Risks for gynecological patients
Thanks to the increasingly more effective drugs and the reduction in viral load, the risks posed by AIDS patients to others have become much lower. Even the severe genital infections and increased rates of malignant disease initially observed have almost completely disappeared in industrialized countries.
Apart from that, other infections may accelerate the course of the disease, and genital infections pose an increased risk of infecting the sexual partner. Today, HIV-positive women can only receive normal gynecological care if they undergo regular examination by physicians specializing in HIV infection.
Consistent use of condoms with both HIV-negative and HIV-positive partners is recommended. Uptake of other HIV mutants from a positive partner may accelerate the course of the disease.
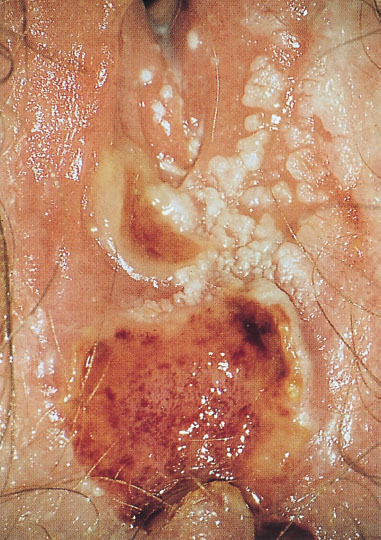
Fig. 8.7 Terminal stage of AIDS in a 21-year-old patient with severe recurrent genital herpes and condylomas.
Diagnosis:
Serology:
 Enzyme immunoassay. Indirect or competitive ELISA is used as a screening procedure (specific for HIV-1 and HIV-2).
Enzyme immunoassay. Indirect or competitive ELISA is used as a screening procedure (specific for HIV-1 and HIV-2).
 Fluorescence test (some cross-reaction between HIV-1 and HIV-2).
Fluorescence test (some cross-reaction between HIV-1 and HIV-2).
 Western blot (p. 34). Based on the appearance of different antibodies against individual viral components, it can provide information on the duration of an existing infection.
Western blot (p. 34). Based on the appearance of different antibodies against individual viral components, it can provide information on the duration of an existing infection.
Detection of pathogen:
 Cultures from blood (heparinized), semen, or cervical secretion. So far, this very expensive procedure is only used for research purposes, since only few virus particles per mL of blood are present. In contrast to infections with hepatitis B virus, where there may be up to 1012 virus particles per mL, there are perhaps only 104 /mL in case of HIV infection.
Cultures from blood (heparinized), semen, or cervical secretion. So far, this very expensive procedure is only used for research purposes, since only few virus particles per mL of blood are present. In contrast to infections with hepatitis B virus, where there may be up to 1012 virus particles per mL, there are perhaps only 104 /mL in case of HIV infection.
 DNA hybridization by Southern blot technique.
DNA hybridization by Southern blot technique.
 PCR (determination of viral load).
PCR (determination of viral load).
Diagnosis of HIV infection is established by serological tests. A very sensitive, but not absolutely specific, ELISA test is used as a screening procedure; it yields more positive results than HIV infections are actually present. For this reason, every positive ELISA has to be confirmed by a western blot.
Table 8.4 Prophylaxis and treatment of HIV infection during pregnancy
Prophylaxis | Treatment |
|---|---|
Mother without symptoms | Mother with symptoms |
In view of the far-reaching implications of the diagnosis and in order to eliminate any human error, the diagnostic tests should be repeated with a second serum sample. If this sample is also positive in all tests, the diagnosis is established.
Determination of viral load. This is carried out by means of PCR on a blood sample. The viral load is now regarded as a measure for infectiousness and prognosis.
Determination of sensitivity. The rapid development of drug resistance by the HIV virus requires combination treatment. Nevertheless, in special cases—above all if the antiretroviral therapy is unsuccessful—determination of sensitivity to the drugs used for the particular patient may become necessary. For economic reasons, this will be restricted to special cases.
HIV and Pregnancy
Now that severe genital infections have become rare thanks to improved HIV therapy, pregnancy has become the most common reason why gynecologists see HIV-positive women.
The pregnancy itself does not have a negative influence on the course of the HIV infection. There is also no increase in the rate of premature delivery. Infection of the child is considered the main risk during pregnancy. While initially relatively high at 50%, it is now below 5% with prophylaxis. The initially high rates of transmission were due to more advanced stages of infection with high viral loads and to insufficient prophylactic measures.
Today, the care for HIV-positive pregnant women can definitely be provided in a gynecological practice. Assessment of the stage (diagnostic procedures) and recommendations for treatment and prophylaxis should be carried out by specialists or in medical centers, or at least in consultation with them.
Results of studies indicate that about 35% of child infections occur during the last weeks of pregnancy (after the 32nd week), and about 65% occur during delivery. The increasing permeability between mother and child and the contractions during labor promote transmission of the virus.
The risk for the child depends on the stage of the disease in the mother. Declining titers of p24-specific antibodies correlate with an increase of virus particles in the blood and cervical secretion and, hence, with an increase in the viral load. The viral load is determined today by means of PCR on blood samples. Inflammation, uterine contractions, and premature delivery further increase the risk of transmission.
With the increase in experience and as the result of further studies, generally accepted recommendations for the care of HIV-positive pregnant women have been established. The aim is to keep both risks as low as possible: the risk of transmitting the virus to the child and also the risk of damaging the child by means of drugs.
Treatment during pregnancy. When deciding on a therapy, one has to choose between health issues of the mother and those of the unborn child. With the establishment of increasingly highly active antiviral compounds (see p. 47), a new problem has been created because the undesired effects of these compounds on the fetus are largely unknown.
Antiretroviral therapy is recommended for all pregnant women, since it has been observed that, even with a load of 1000 HIV-RNA copies per μL of blood, the rate of transmission can be lowered from 9.8% to 1%. Today, antiretroviral combinations (Table 8.4) are recommended, as they should lower the development of resistances.
Resistance screening during pregnancy is controversial. In general, such a test is recommended in case of acute infection, increase in viral load during therapy, or viral persistence in the blood.
The second approach to reducing HIV transmission is cesarean section in the event of uterine inertia.
These days, prophylaxis with zidovudine (AZT) (5 ×100 mg) or better Combivir from the 32nd week on and continuing peripartal is recommended (see Table 8.4)—together with a generous decision toward early primary cesarean section (in the 38th week). Precautionary measures are required prior to delivery. Breast-feeding is discouraged as transmissions have been reported.
Approach if a pregnant women is already undergoing antiviral treatment:
 If the pregnancy is discovered after the first trimester, treatment should be continued.
If the pregnancy is discovered after the first trimester, treatment should be continued.
 If the pregnancy is discovered during the first trimester, the treatment may be discontinued and then continued after the end of the first trimester. At the very least, compounds that carry an increased risk for the child should be discontinued (e. g., efavirenz or delavirdine).
If the pregnancy is discovered during the first trimester, the treatment may be discontinued and then continued after the end of the first trimester. At the very least, compounds that carry an increased risk for the child should be discontinued (e. g., efavirenz or delavirdine).
If the antiretroviral therapy is only started after delivery, the newborn is also treated for six weeks. There have been no studies on the treatment of newborns from mothers who did not receive prophylactic treatment. Starting AZT administration 48 hours after birth is thought to be moderately effective.
Other potential problems with HIV during pregnancy. The effect of methadone is reduced by protease inhibitors (e. g., nevirapine), though not by indinavir.
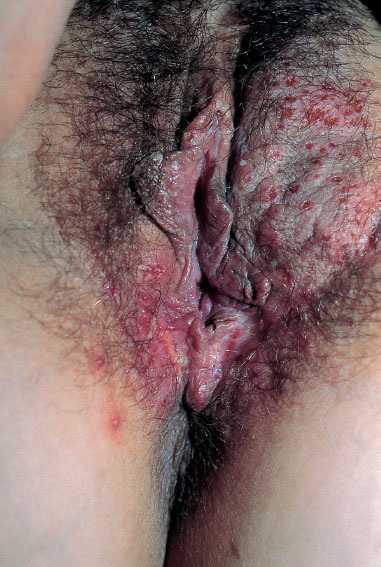
Fig. 8.8 Primary genital herpes in a 33-year-old patient during the 21st week of pregnancy.
Genital Herpes 
Genital herpes during pregnancy is a dreaded situation, particularly at the time of delivery, because it may cause serious disease in the newborn. In Central Europe though, the fear is greater than the actual risk. With about one in 25000 births, neonatal herpes is much less common here than in the United States. There also seem to be differences regarding the viral types, since both types of HSV now occur with similar frequencies in Central Europe, whereas type 2 is still predominant in the United States with 70%.
In principle, one needs to distinguish during pregnancy between the rather rare form of primary genital herpes, which poses a high risk for the child (about 30–50%) at the time of delivery, and the more common form of recurrent genital herpes, which has a much lower risk for the newborn—especially when sufficient amounts of antibodies are present.
Hence, only the children of seronegative mothers are in danger. These children may be infected due to a possible primary infection of the mother just before delivery or, because they do not have any protection provided by the mother, after birth by hospital personnel or visitors (herpes labialis).
Primary Genital Herpes
Primary genital herpes is always accompanied by clinical symptoms (Fig. 8.8). However, the lesions are not always interpreted correctly, and this may become dangerous in the peripartal period.
The intensity of symptoms depends in part on the serostatus of the patient. Except during the time of birth, even primary genital herpes seems to pose a very low risk for the child.
Early during pregnancy, the route of infection is probably hematogenous, although this is a rather rare event. Only very few isolated cases have been described. It is more likely that such cases result in the death of the fetus rather than in any damage to the child, since a slightly increased rate of abortion has been reported.
A congenital herpes simplex syndrome is not known.
The main risk for the child consists of infection during birth through the infected birth canal. Here, the risk for the newborn of contracting the disease is up to 50%. After rupture of the fetal membranes, an ascending infection in utero is also possible, and even cesarean section (e. g., four hours later) may not prevent the infection of the child.
Severe neonatal herpes infection is altogether a rare event. According to US figures, it is now observed at a frequency of only one in 7500 births. In Europe, it seems to be even much less frequent with one in 25000 births. Since effective treatment of herpes with aciclovir is possible today, the rare event of primary genital herpes is no longer that dangerous. However, as with all infections, it is important to initiate treatment or prophylaxis in time.
If the mother has primary genital herpes, the risk of infection for the child is:
 < 2% during the first trimester
< 2% during the first trimester
 about 10% during the third trimester
about 10% during the third trimester
 about 50% at the time of birth.
about 50% at the time of birth.
Recurrent Genital Herpes
Much more frequent than primary genital herpes infection is recurrent genital herpes. As it is often asymptomatic, it may not be noticed by the patient. The excretion of herpesviruses can only be detected by examining cultures from vaginal and cervical swabs, or by PCR.
Virus excretion during pregnancy has been detected in over 10% of pregnant women known to have recurrent genital herpes.
The endemic genital infection with herpes simplex virus (HSV-2 and HSV-1) is up to 20–30% in Germany. One may therefore assume that about 2% of all pregnant women excrete herpes simplex virus at some point during the pregnancy.
According to these figures and to studies carried out by Yeager (1984), 0.3% of pregnant women excrete virus into the birth canal at the time of delivery.
Whether or not an infection of the child actually takes place depends on the amount of virus particles excreted and on the antibody titer of the mother.
Yeager was able to demonstrate that infection of the child occurred only in those patients in which the mother showed a very low antibody titer in the serum. According to Brown, the child becomes infected in 5%, 1, 2% after cesarean section and 7, 7% after vaginal delivery, the child becomes infected only in 5–10% of women with florid recurrent genital herpes at the time of delivery.
If the mother has recurrent genital herpes, the risk of infection for the child is:
 < 0.1% during the first trimester
< 0.1% during the first trimester
 < 1% during the third trimester
< 1% during the third trimester
 < 5% at the time of birth.
< 5% at the time of birth.
Treatment during pregnancy. Teratogenicity of aciclovir at normal dosages has not been demonstrated in animal experiments. Experience with aciclovir treatment of herpes infections in pregnant women has been documented in the international registry of aciclovir use during pregnancy. Worldwide, 1060 pregnant women with herpes infection have so far been treated with aciclovir and evaluated, according to data provided by the original manufacturer (June 30, 1997). According to these data, there is no increased risk of malformation after taking aciclovir during pregnancy.
Nevertheless, there is a relative contraindication for treating herpes infection during pregnancy with aciclovir, i. e., the risks and benefits must be carefully considered and accurate diagnosis is imperative. Treatment should only be initiated in the following cases:
 primary genital herpes during pregnancy
primary genital herpes during pregnancy
 avoidance of cesarean section by prophylactic administration of aciclovir during delivery
avoidance of cesarean section by prophylactic administration of aciclovir during delivery
 severe herpes zoster during pregnancy.
severe herpes zoster during pregnancy.
To be on the safe side, aciclovir should not be prescribed during early pregnancy (1st to 14th weeks) if possible, since not enough patients have been observed so far to exclude completely any risk.
Approach if genital herpes is suspected during pregnancy up to two weeks before delivery:
 Confirmation of the diagnosis: isolation of the virus through culture (duration: two to three days), detection of antigen in the fluorescence test (one to two hours), ELISA (five to six hours) or better PCR.
Confirmation of the diagnosis: isolation of the virus through culture (duration: two to three days), detection of antigen in the fluorescence test (one to two hours), ELISA (five to six hours) or better PCR.
 Blood samples for determination of antibodies against genital herpes: to distinguish between primary and recurrent genital herpes and, at the same time, to determine the antibody titer.
Blood samples for determination of antibodies against genital herpes: to distinguish between primary and recurrent genital herpes and, at the same time, to determine the antibody titer.
 If the serological test is negative, it should be repeated after four weeks (prior to birth, at the latest) to know whether the child will have protection.
If the serological test is negative, it should be repeated after four weeks (prior to birth, at the latest) to know whether the child will have protection.
 In case of primary genital herpes: oral treatment with aciclovir (5 × 200 mg) for five days, close monitoring of the child after birth.
In case of primary genital herpes: oral treatment with aciclovir (5 × 200 mg) for five days, close monitoring of the child after birth.
 In case of recurrent genital herpes: no aciclovir treatment, reassurance of the patient, close monitoring of the child after birth.
In case of recurrent genital herpes: no aciclovir treatment, reassurance of the patient, close monitoring of the child after birth.
Approach in case of genital herpes at the time of delivery:
 If clinically clear, extensive herpes lesions are present (above all, during primary infection) and the immune status is unknown: cesarean section makes only sense if the rupture of the fetal membranes happened less than four hours ago. Aciclovir treatment of the mother, prophylactic aciclovir treatment of the newborn.
If clinically clear, extensive herpes lesions are present (above all, during primary infection) and the immune status is unknown: cesarean section makes only sense if the rupture of the fetal membranes happened less than four hours ago. Aciclovir treatment of the mother, prophylactic aciclovir treatment of the newborn.
 If lesions are known to be due to recurrent genital herpes and the antibody titer is high: vaginal delivery is possible under aciclovir treatment of the mother. Close follow-up of the child is essential, with administration of aciclovir to the newborn as soon as the slightest symptoms occur.
If lesions are known to be due to recurrent genital herpes and the antibody titer is high: vaginal delivery is possible under aciclovir treatment of the mother. Close follow-up of the child is essential, with administration of aciclovir to the newborn as soon as the slightest symptoms occur.
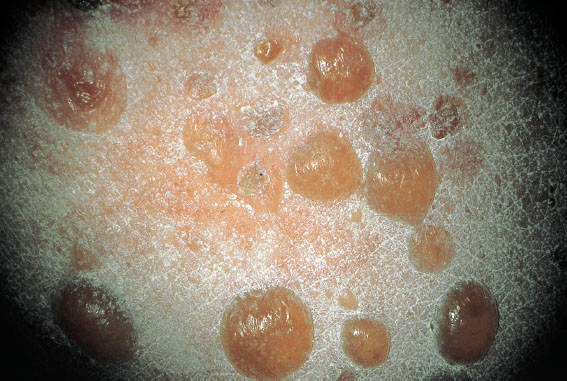
Fig. 8.9 Herpes gestationis in a 30-year-old patient during the 34th week of pregnancy.
Prophylaxis for children after birth if the mother has a florid infection:
 swab of the nasopharynx
swab of the nasopharynx
 immune globulins if the titer of the mother is low
immune globulins if the titer of the mother is low
 possibly aciclovir treatment.
possibly aciclovir treatment.
Herpes Gestationis
This disease should be correctly called pemphigoid gestationis. The clinical picture of blistering skin (Fig. 8.9), occurring in various regions of the body, is so different from lesions caused by herpes simplex virus that it cannot be mistaken. It is probably an autoimmune disease, and the vesicobullous skin lesions may also be absent. The diagnosis used so far, which is based on the detection of C3 deposition along the basement membrane in a skin biopsy, is increasingly being replaced by the detection of antibodies against BP180 autoantigen in the serum. Treatment consists of 0.3–0.5 mg of prednisolone/kg body weight.
Bullous Pemphigoid
(Differential diagnosis: genital herpes, candidiasis, and folliculitis)
This is a rare mild immune disease with blisters, and it is not easily recognized. It may also start on the vulva (Fig. 8.10). Diagnosis is established by means of biopsy and detection of immune complexes in the biopsy or serum. Treatment consists of administration of prednisone or azathioprine.
Cytomegalic Inclusion Disease (CID) __ 
The cytomegalovirus (CMV) belongs to the pathogens most commonly transmitted to newborns. This is because, in 10% of pregnant women with florid CMV infection, the virus is excreted in the urine and in the cervical secretion. Fortunately, the course of peripartal infections is usually asymptomatic or benign, with damages to the child being rare. Infection of the fetus, however, may be a serious disease with grave consequences. In such cases, the only possible option is termination of the pregnancy. So far, there is no effective, well-tolerated therapy available for this virus.
Cytomegalic inclusion disease is a sexually transmitted infection.
Frequency. Infection with the cytomegalovirus is one of the most common florid infections during pregnancy. Antibodies against CMV are present in 50–60% of adults, with the virus still remaining in the body. Suppression of the immune system during pregnancy causes reactivation of the virus in 20% of women carrying CMV.
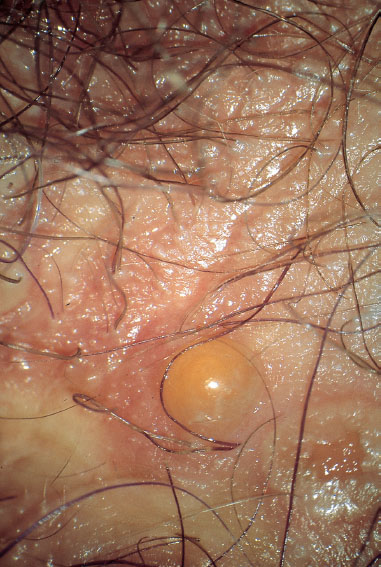
Fig. 8.10 Pemphigoid of the vulva in a 45-year-old patient.
One should therefore expect to find a florid CMV infection in about 10% of all pregnancies. About 10% of the children of these mothers become infected during birth, corresponding to about 1% of all newborns. In Germany, slightly more that 20 congenital infections are reported every year.
Pathogen. The cytomegalovirus belongs to the group of herpesviruses. It is a relatively large DNA virus with a lipid envelope, which makes it extremely unstable. Various subtypes have been described.
Clinical picture. The symptoms of cytomegalic inclusion disease are uncharacteristic; they include fatigue, weakness, swelling of the lymph nodes, and occasionally also mild fever. In immunocompetent persons, the disease usually takes a mild course. In immunocompromised persons—and this includes the fetus—the virus may cause serious damage (hepatitis, myocarditis, encephalitis, blindness). Apart from the unborn child, this group includes patients undergoing immunosuppression (e. g., after kidney transplantation) and especially people with AIDS. Reactivation of the virus poses a threat to AIDS patients.
As with other herpesvirus infections, the CMV is usually not eliminated after the infection but persists in the lymphocytes and in the kidneys. Due to the depressed immune system during pregnancy, the virus is often reactivated. As during primary infection, specific IgM antibodies are detected here as well. In addition, CMV is excreted in the urine and often also in the cervical secretion.
Risk for the child. During primary CMV infection of the mother, transmission to the child is by the hematogenous route, and infection may occur anytime during the pregnancy. Fetal damage is likely to be more severe if the infection occurs early during the pregnancy.
In case of primary infection of the mother during the first trimester, the rate of fetal infection is estimated at 10–20%. Transmission is probably more frequent during late pregnancy, though the damages are less severe.
Up to 90% of children with congenital cytomegalic inclusion disease (CID) exhibit sequelae.
As with other pathogens, transmission of the cytomegalovirus to the child occurs less often during early pregnancy and more frequently during late pregnancy. If there is evidence of fetal infection, the risk of damage is about 20%. Early during pregnancy, this may trigger a discussion about terminating the pregnancy.
The risk posed by reactivation of a CMV infection during pregnancy is far below 1%.
Based on the serological finding, it is a major problem to discern whether the detection of IgM antibodies during the first test indicates a primary infection or reactivation of the virus. This is why tests—or, at least, the collection of serum samples—before the pregnancy are helpful.
Studies using more recent methods indicate that congenital CMV infection in cases where the mother already possessed antibodies to CMV before she became pregnant may not be the result of viral reactivation in the mother, but rather that of reinfection with another CMV type during her pregnancy.
A typical sign for congenital CMV infection is hepatosplenomegaly, which can be discovered already in utero by means of ultrasound, and also after birth.
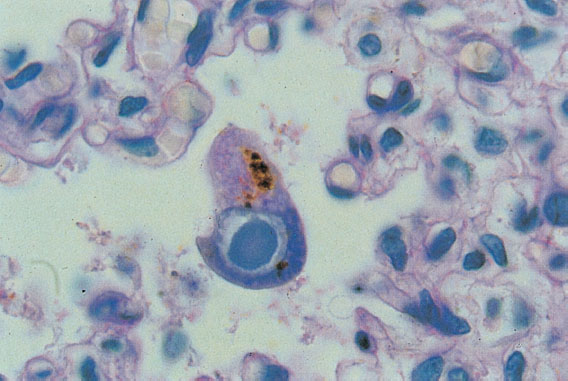
Fig. 8.11 Cytomegalovirus infection. The child died in the 30th week of pregnancy. The typical owl-eye cells found in the lungs support the diagnosis. (Micrograph by courtesy of Prof. Dr N. Böhm, Department of Pathology, University of Freiburg, Germany.)
Primary CMV Infection During Pregnancy 
Diagnosis:
 seroconversion from negative to positive (fluorescence test, ELISA)
seroconversion from negative to positive (fluorescence test, ELISA)
 rise in the IgG antibody titer by a factor of four. Possible clues:
rise in the IgG antibody titer by a factor of four. Possible clues:
 high IgM antibody titer (may also be high in the event of viral reactivation)
high IgM antibody titer (may also be high in the event of viral reactivation)
 high numbers of CMV particles excreted in the urine; however, the only clear evidence for primary CMV infection is seroconversion from negative to positive, indicating the first appearance of CMV-specific antibodies.
high numbers of CMV particles excreted in the urine; however, the only clear evidence for primary CMV infection is seroconversion from negative to positive, indicating the first appearance of CMV-specific antibodies.
Frequency during pregnancy. Less than 1%, perhaps 0.1–0.3 %.
Congenital CMV Infection and Disease
This is a rare but serious disease of the newborn due to hematogenous transmission of the virus. In the acute stage, the child exhibits hepatosplenomegaly, petechial bleedings due to thrombocytopenia, increased transaminases, massive CMV excretion in the urine, pronounced inflammatory reactions with typical giant cells in the placenta (which may weigh more than 1000 g). Persistent cerebral damages (microcephaly) are common.
The infection may already take its course in utero during the second and third trimesters. Hepatosplenomegaly and ascites can be detected by ultrasound. Cases of fetal hydrops have also been described. This may lead to the death of the unborn child, in which case the histological examination reveals the typical owl-eye cells (Fig. 8.11) in various organs.
Premature cesarean section in such patients does not have any advantage, only disadvantages.
If the infection takes place earlier during pregnancy, it may have already healed by the time of birth—or premature birth, which is more often the case—and only the lasting damages are found (e. g., microcephaly and cerebral retardation). The prognosis of these children is altogether moderate to poor.
Frequency. About 25 congenital CMV infections per year have been reported during recent years in Germany. One may therefore expect at least 100 CMV-damaged children per year. Since there are no measures available apart from terminating an early pregnancy, this disease is considered tragic.
Prophylaxis. Pregnant women should avoid risk areas where they may be infected with the CMV virus, especially if they did not develop antibodies against it. Such areas include dialysis units, transplant units, and pediatricwards. When dealing with children (day care, kindergarten), direct contact with urine should be avoided. There is no effective vaccine available at present.
Approach if primary CMV infection is suspected or confirmed during the first trimester. Intrauterine infection of the child occurs only in about 20% of these pregnancies. The infection can be recognized (or excluded) by the presence (or absence) of CMV in the amniotic fluid into which the virus is excreted by the kidneys. In the past, detection of the virus in the amniotic fluid required culture methods; today, the virus is detected by means of PCR.
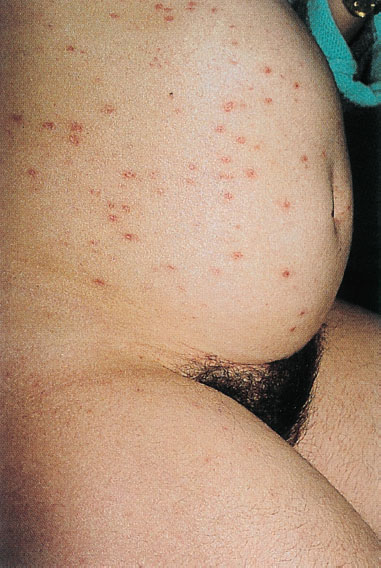
Fig. 8.12 Varicella in a 34-year-old patient during the 32nd week of pregnancy. The child was born healthy.
Cord puncture for the detection of IgM antibodies in the fetus is possible from the 20th to 22nd weeks of pregnancy onward, but it is difficult and not necessary.
If the PCR test is negative, and possibly also the test for CMV-specific IgM antibodies, the mother may be encouraged to carry on with the pregnancy, since the residual risk is extremely low.
Reactivation of CMV Infection During Pregnancy
Risk for the child. In 20% of pregnant women with a previous CMV infection, reactivation of the latent CMV infection may occur during pregnancy, or the latent infection is still florid. Close to 50% of pregnant women carry the CMV, and almost 10% of them exhibit a florid CMV infection with detectable IgM antibodies and with CMV excreted in the urine.
About 10% of the children of these women become infected with the virus during birth. This means that about 1% of all children become infected with CMV during birth. Clinical signs of CMV infection develop in only 10% of the infected children, which corresponds to one in 1000 newborns. Here, the late sequelae are much less common and severe than with congenital CMV infection due to primary infection of the mother.
The signs for reactivated CMV infection during pregnancy include:
 detection of CMV-specific IgM antibodies in the serum of the mother
detection of CMV-specific IgM antibodies in the serum of the mother
 no or only a slight rise in the already high titer of IgG antibodies
no or only a slight rise in the already high titer of IgG antibodies
 no or only minor CMV excretion in the urine
no or only minor CMV excretion in the urine
 absence of clinical symptoms
absence of clinical symptoms
 detection of CMV-specific IgM antibodies with known IgG antibody titer before the pregnancy.
detection of CMV-specific IgM antibodies with known IgG antibody titer before the pregnancy.
Persistence of CMV-specific IgM Antibodies and the Desire to Have Children
If antibodies against CMV are present already before the start of the pregnancy, they provide a relatively high level of protection against congenital infection by hematogenous transmission. There remains a potential risk for the child, since recent data show that the mother may become reinfected with another CMV strain.
In view of the very low risk, pregnancy is not a contradiction. Further serological checks should not be carried out because they do not provide new information and have no implications. Exceptions are manifestation of the disease in the mother and unusual ultrasonic findings in the child.
Varicella (Chickenpox) 
Varicella does not seem to be a harmless disease after all. It takes a severe course in 16% of the patients, and complications occur in 6% of these patients. As varicella is very contagious, most of the infections take place at the preschool age. Varicella during pregnancy (Fig. 8.12) is rather rare, since 90–95% of adults have already developed antibodies against the varicella–zoster virus. Like every virus infection, varicella is feared during pregnancy.
At the start of the pregnancy, the virus is transmitted to the fetus in about 20% of all cases, and toward the end of pregnancy in 80% of cases. However, the risk of damage to the child is low and exists only in the first half, being about 2% until the 20th week of pregnancy.
Frequency. The incidence of varicella infection during pregnancy is reported to be 0.1–0.5 %.
Pathogen. The varicella–zoster virus (VZV) belongs to the group of herpesviruses, all of which have a tendency to persist in the host.
Clinical picture. This is an exanthematous disease with symptoms that are not always characteristic enough to rely on the patient’s history and the clinical appearance. The coexistence of fine vesicles, nodules, and crusts (starry sky distribution) associated with itching is typical. Depending on the stage, the vesicles may be so discreet that they can be recognized only under the colposcope (Fig. 8.13), and the exanthema as such may be assessed incorrectly. Here, negative serological tests with subsequent determination of the titer, or detection of IgM antibodies, bring clarification.
Congenital Varicella Syndrome
Fetal damage is rare; it is about 2% until the 20th week of pregnancy. So far, there has been experience with 1739 patients (Enders, 1988).
Termination of the pregnancy is not generally justified, since the rate of damage caused by florid varicella infection during pregnancy is low, even in the first trimester. Only if there is evidence that the fetus has been damaged, termination of the pregnancy should be discussed with the parents (Table 8.5).
Perinatal Varicella of the Mother
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Absence of clinical rubella symptoms
Absence of clinical rubella symptoms Reduction in the rate of infection
Reduction in the rate of infection Diminished virus replication in the throat
Diminished virus replication in the throat Reduction/suppression of viremia
Reduction/suppression of viremia Low antibody response (titer level)
Low antibody response (titer level) Reduced risk of damage to the child
Reduced risk of damage to the child Seronegative women with rubella contact several days ago (_5 days): no administration of immune globulin because it is already too late; antibody monitoring, but no sooner than at least three weeks after the presumed contact.
Seronegative women with rubella contact several days ago (_5 days): no administration of immune globulin because it is already too late; antibody monitoring, but no sooner than at least three weeks after the presumed contact. Women with unknown immune status and rubella contact a short time ago (one to three days): Collection of blood samples for antibody determination, administration of immune globulin. If it turns out that antibodies are present, no further measures are required. If no antibodies are present, titer checks after three and six weeks.
Women with unknown immune status and rubella contact a short time ago (one to three days): Collection of blood samples for antibody determination, administration of immune globulin. If it turns out that antibodies are present, no further measures are required. If no antibodies are present, titer checks after three and six weeks. Women with unknown immune status and rubella contact six to 14 days ago: no administration of immune globulin, because it is already too late. If negative, titer check after three weeks; if positive, no further measures because immunity already exists.
Women with unknown immune status and rubella contact six to 14 days ago: no administration of immune globulin, because it is already too late. If negative, titer check after three weeks; if positive, no further measures because immunity already exists. Women with unknown immune status and rubella contact more than 14 days ago: if antibody titer is high, determination of rubella-specific IgM antibodies, titer check after eight days (titer rise is very rapid within a few days.)
Women with unknown immune status and rubella contact more than 14 days ago: if antibody titer is high, determination of rubella-specific IgM antibodies, titer check after eight days (titer rise is very rapid within a few days.)