Pediatric Transplantation
Patrick J. Healey
Solid organ transplantation is now a routine component of management for organ failure of the kidney, liver, heart, lung, and intestine in children. Improved patient selection, surgical technique, postoperative care, organ preservation, and immunosuppression management has led to tremendous improvements in both graft and patient survival. This chapter addresses general issues confronted by all solid organ transplantation. Renal, liver, multivisceral, and heart transplantation are discussed in subsequent chapters. Hematopoietic stem cell transplantation (bone marrow transplantation) is discussed in Chapter 133.
REFERRAL FOR TRANSPLANT EVALUATION
Children with progressive organ damage leading to likely end-stage organ failure should be referred for possible transplantation. The timing and role of transplant depends on the availability of other clinical management alternatives, the risk of death from progressive end-stage organ failure, and the likelihood of receiving a donor organ for transplant.
Children referred for transplant must undergo a thorough evaluation that strives to achieve two objectives: (1) to determine whether organ transplantation is the appropriate treatment for the child being evaluated, and (2) to identify and evaluate any additional medical, surgical, anatomic, or social considerations that may contraindicate transplantation, or otherwise decrease the likelihood of a successful outcome following transplantation, in the short and long term. A complete understanding of the natural history of the underlying disease, as well as any associated complications or manifestations of the primary disease, is essential.
The results of all of the diagnostic tests, imaging studies, and specialty consultations are then reviewed in a multidisciplinary conference to determine the appropriateness of transplantation, and to place the child on the national waiting list for transplant. A specific care plan is developed for any medical, social, nutritional, or other conditions identified during the evaluation.
ORGAN TRANSPLANTATION WAITING LISTS
Organ allocation policies are guided by the principles of equity and justice—providing donor organs to those at the greatest risk of death from their organ failure. The policies ensure that all candidates waiting for a donor organ have an equitable opportunity to receive a donor organ and, at the same time, avoiding futile transplants. These policies assure the best outcomes for donor organs, recognizing that these organs are a truly scarce resource.
The national transplant program in the United States is administered by the United Network for Organ Sharing (UNOS). Members of UNOS include transplant centers and organ procurement organizations that must comply with existing policy governing organ donation and transplantation. UNOS maintains a database in which all patients awaiting organ transplantation at a transplant center and all donors managed by organ procurement organizations are registered. A match run is performed to allocate organs from a deceased donor to candidates waiting, in a specific sequence, according to defined criteria, such as clinical urgency, ABO blood type, and distance from donor hospital to transplant center, to name a few.
There are currently more than 100,000 patients awaiting organ transplantation on the UNOS waiting list; the approximately 2000 children make up a small percentage of the total number of candidates on the list (Table 128-1). There is significant variation in relative percentage of children on the list according to organ type and age. Children make up approximately 1% of candidates awaiting kidney transplant and nearly 75% of those awaiting intestine transplant. Roughly 7% of the children waiting are less than a year old, 27% are ages 1 to 5, 20% ages 6 to 10, and 46% are ages 11 to 17.1 This reflects the difference in disease conditions leading to organ failure between children and adults.
UNOS organ allocation policy incorporates factors of recipient urgency based on severity of clinical disease, donor conditions that may impact outcomes following transplantation, and distance between donor and recipient centers based on differing acceptable ischemia times for the different organs.
Table 128-1. Transplant Waiting List
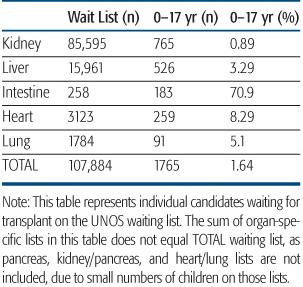
Data from www.optn.transplant.hrsa.gov, August 2010.
DONOR CONSIDERATIONS
Children may receive a donor organ from a live donor or a deceased donor. The relative frequency of live donor versus deceased donor varies according to organ type, with greater than 50% of children who undergo kidney transplantation receiving a living donor graft. All donors undergo a thorough medical evaluation to assess organ function and to identify any conditions or diseases that could potentially be passed on to the recipient with the transplant organ. In addition to organ-specific data, all donors must have accurate determination of ABO blood type; height and weight; and viral serologies, including hepatitis A, hepatitis B, hepatitis C, human immunodeficiency virus (HIV), and cytomegalovirus (CMV). Epstein-Barr virus (EBV) serologies have more recently been required because of the recognized risk for recipient posttransplant lymphoproliferative disease (PTLD) associated with EBV infection.
Selection of an appropriate donor for a specific recipient will depend on the availability of a potential living donor, the clinical urgency of the transplant, donor–recipient size mismatch, donor organ function, ABO, and expected graft ischemia times.
The majority of pediatric kidney transplant recipients receive an adult-sized renal allograft with good outcomes, whereas heart and intestine recipients must receive an appropriately size-matched organ from a pediatric donor. Liver and lung recipients may receive a reduced-size lobar or segmental graft from an adult-sized donor in the event of a lack of available pediatric donor of equal size, although this option does require the technical expertise to create such a donor organ graft, and may therefore not be universally available.
IMMUNOSUPPRESSION
Advances in understanding of the immune response to the transplant organ have led to the development of more potent immunosuppressive agents. The allograft response involves antigen-presenting cells, T cells, B cells, and NK cells, which together mount an antigen-specific amplified response. Available immunosuppressive agents target various steps in this response.
 CALCINEURIN INHIBITORS
CALCINEURIN INHIBITORS
Tacrolimus (FK 506, Prograf) and cyclosporine (Neoral, Sandimmune) act by inhibiting calcineurin phosphatase activity and thereby inhibiting activation of the transcription factor NFAT (nuclear factor of activated T cells), which in turn inhibits T-cell activation. Tacrolimus has less pronounced side effects than cyclosporine and has therefore largely supplanted cyclosporine as the preferred immunosuppressive agent for pediatric transplantation. It is effective for treatment of both acute and chronic rejection, and maintenance prophylactic immunosuppression. It is dosed orally, twice daily. Monitoring of blood levels is essential to assure adequate efficacy and to avoid toxicity. Target levels are reduced over the first year following transplantation if rejection does not ensue. Doses need to be adjusted for renal disease, or in cases of posttransplantation lympoproliferative disease (see below). Levels can be altered by ingestion of certain foods and medications. Both agents bind variably to enteric feeding tubes so that when administered through a tube, dosing is unreliable. Common side effects include hyperglycemia, hypertension, headache, renal disease, and hyperlipidemia.
 CORTICOSTEROIDS
CORTICOSTEROIDS
Corticosteroid therapy is used for induction of immunosuppression, with dosing often beginning in the operating room. Long-term steroid therapy is avoided when possible so that now some protocols minimize steroid usage. Adverse effects include hypertension, hyperglycemia, psychosis, cataract, decreased bone density, and aseptic necrosis of the femoral head.
 MYCOPHENOLATE MOFETIL (CELLCEPT)
MYCOPHENOLATE MOFETIL (CELLCEPT)
Mycophenolate mofetil (MMF) is converted in the liver by ester hydrolysis to mycophenolic acid, which is a potent selective inhibitor of inosine monophosphate dehydrogenase, and thereby inhibits synthesis of the purine nucleotide guanosine. T and B lymphocytes are dependent for proliferation on de novo purine synthesis, whereas other cell types can utilize alternate salvage pathways. Thus, this agent selectively inhibits T- and B-cell proliferation and antibody formation. It is an effective treatment for acute rejection, and in some cases is being used for maintenance therapy. Common adverse effects include gastrointestinal symptoms of diarrhea, vomiting, and cramping.
 SIROLIMUS (RAPAMYCIN, RAPAMUNE)
SIROLIMUS (RAPAMYCIN, RAPAMUNE)
Sirolimus is a macrolide that binds to the TOR (target of rapamycin) receptor, an intracellular regulator of protein kinases, and thus decreases Interleukin-2 production and B-cell and T-cell activation and proliferation. It is generally used as second line treatment for rejection in combination with tacroliums or mycophenolate.
 MONOCLONAL ANTI-IL-2Rα ANTIBODIES
MONOCLONAL ANTI-IL-2Rα ANTIBODIES
Basiliximab (Simulect) is a chimeric mouse–human antibody, and daclizumab is a monoclonal antibody. Both block the IL-2 receptor alpha on the T cell. Binding to T cells prevents replication and activation of B cells. These agents are usually given at the time of transplant or within hours of transplantation and are highly effective at reducing acute rejection.
 ORTHOCLONE OKT3
ORTHOCLONE OKT3
Orthoclone OKT3 is a mouse antibody against the CD3 antigen of human T cells. Orthoclone OKT3 is used for treatment of acute rejection that is steroid resistant or sometimes for induction. Administration is accompanied by a cytokine release syndrome associated with flulike symptoms that typically last for several hours after the first infusions. Premedication can ameliorate these effects. Use of Orthoclone OKT3 is associated with increased risk for bacterial and viral sepsis, aseptic meningitis, and development of a PTLD.
 POLYCLONAL ANTI-T-CELL ANTIBODIES
POLYCLONAL ANTI-T-CELL ANTIBODIES
Antithymocyte or antilymphocyte globulin (Thymoglobulin, Atgam) is a horse- or rabbit-derived antibody against human T cells. Anti-IL-2Rα antibodies have largely replaced these agents for induction therapy because they do not cause cytokine-release syndrome and may improve the development of tolerance.
TRANSPLANT IMMUNOSUPPRESSION STRATEGY
 INDUCTION
INDUCTION
Induction immunosuppression is given at the time of transplant to rapidly create a state of immunologic unresponsiveness of the recipient to donor antigens. Induction agents may be monoclonal or polyclonal. The most commonly used monoclonal agents include daclizumab and basiliximab, humanized and chimeric forms of the antibody, which bind CD25, the receptor for interleukin-2 on the activated T cell.2,3 The polyclonal antibody thymoglobulin causes lymphocyte depletion, although the mechanism of action of these agents is thought to be due to actions other than lymphocyte depletion alone. They are typically given for a short course early after transplant. Monitoring the T-cell subsets and dosing based on CD3+ may be helpful in guiding therapy.4
 MAINTENANCE
MAINTENANCE
Following transplant, a protocol-based maintenance immunosuppression is initiated that differs among organ transplant types. Most pediatric protocols include a 2- or 3-drug combination with different sites of immunosuppressive activity. This strategy allows for lower individual drug doses, thereby reducing toxicity.
The calcineurin inhibitor tacrolimus is most frequently used in all transplant types, combined with other immunosuppressive agents.
REJECTION
Allograft rejection is defined as organ injury caused by the recipient’s immune response. The primary stimulus for this immune response is the donor human leukocyte antigen (HLA). Donor antigens are processed by recipient antigen-presenting cells, the macrophages and dendritic cells, and presented to the T-cell. Binding the T-cell receptor initiates an antigen-specific lymphocyte response that is amplified rapidly and results in the clonal expansion of T cells, cytotoxic T cells, and B cells in addition to a more generalized inflammatory response. These cells target the endothelium and epithelium of the donor graft. The risk for rejection is highest during the first 6 months to 1 year following transplant, but late rejection presenting after more than 1 year can also occur, and speaks to the fundamental importance of self/non-self immune recognition.
 ACUTE REJECTION
ACUTE REJECTION
Histologically, acute cellular rejection is characterized by a lymphocytic infiltrate into the graft, endothelialitis, and subepithelial infiltrate, such as lymphocytic cholangitis in the case of liver transplant rejection. The rejection infiltrates are graded according to severity, which incorporates the extent of infiltrate and the degree of underlying organ injury (see http://tpis.upmc.com/TPIShome). Arterial vascular endothelial involvement is termed vascular rejection, and is a severe form of rejection, as it may also directly impact the overall perfusion of the transplant organ graft. Organ function is typically compromised by the rejection infiltrate. Definitive diagnosis of rejection is made by biopsy of the allograft. The initial treatment of acute cellular rejection typically includes a steroid pulse, given intravenously over a few days, along with an increase in maintenance immunosuppression. Appropriate response is evaluated differently for each organ type, using functional measures or laboratory results. Rejection refractory to steroid bolus therapy, may require an anti-lymphocyte therapy, such as Orthoclone OKT3 or antithymocyte globulin, and often also includes the addition of another agent for maintenance immunosuppression. The impact of an acute rejection episode on long-term graft survival varies by organ type, but generally a single episode does not have significant negative impact.
 CHRONIC REJECTION
CHRONIC REJECTION
Chronic rejection results from a long-standing, noncellular immune response against the allograft, and may result in an obliterative fibrotic injury with little likelihood of recovery. Bronchiolitis obliterans in lung transplantation, ductopenic rejection in liver transplantation, and chronic interstitial fibrosis in kidney transplantation are some of the pathologic findings characteristic of chronic rejection. The development of chronic rejection is often associated with declining organ function, progressing to end-stage organ failure, and is the most common indication for retransplantation. Nonadherence with the prescribed immunosuppression regimen is frequently seen in patients who develop chronic rejection.
INFECTION
 INFECTION TYPE AND RISK
INFECTION TYPE AND RISK
The pretransplant evaluation should identify a history of prior infections, determine the immunization status, and include viral serologies to anticipate any potential risk for acquiring or reactivating a viral infection following transplant. Serologic studies to determine preformed antibody against CMV, EBV, hepatitis B and C, HIV, and the herpes viruses varicella zoster and HSV are critical. Transplant recipients at highest risk for CMV or EBV disease, including PTLD, are those with negative IgG serologic titers against these viral agents.
The risk for bacterial, fungal, viral, or opportunistic infection is increased in the pediatric transplant recipient, with the specific type of infection largely determined by the type of organ transplanted and the period of time following transplant. Early infections, within the first month following transplant, are often bacterial or fungal, being related to conditions or complications of the underlying organ disease, the transplant procedure itself, or of the interventions required to provide care during this period of critical illness.5 For example, bacteremia or intra-abdominal infection with gram-negative organisms or organisms of Enterococcus can be seen in the child undergoing liver transplant for primary sclerosing cholangitis during this early time period. Complications related to poor organ function or technical complications of surgery, for example, bile leak related to ischemia of the bile ducts, also present during this time period.
Intermediate infections occur after the first month until 6 months after transplant and reflect opportunistic infections related to immunosuppression, newly acquired or reactivation of viral infections, or donor-transmitted infections. CMV infection, either primary infection acquired via the donor graft or reactivation, is at highest risk during this time period, and is frequently seen in the first few months after antiviral prophylaxis has been discontinued. The overall condition of the recipient has generally improved at this point in time as transplant organ function has recovered to normal, nutritional status has improved, and postsurgical recovery is complete. The development of infections during this time period is greatly influenced by the amount of immunosuppression administered, and increases with treatment of rejection.6 EBV infection and the risk for PTLD increase dramatically during this intermediate time period.
Late infections occur 6 months following transplantation and generally reflect an uncorrected anatomic or functional abnormality of the graft, such as biliary stricture, or are related to the degree of ongoing immunosuppression. It is during this time period that organ-specific differences in maintenance of immunosuppression strategies may be reflected by different rates of viral or opportunistic infection. For example, most pediatric liver recipients are receiving minimal immunosuppression because of the low risk for rejection this long after transplantation, whereas heart and lung recipients are typically maintained on higher levels of immunosuppression. In the child receiving minimal immunosuppression, the incidence of infection is not significantly different from that seen in the healthy population.
 PREVENTION OF INFECTION
PREVENTION OF INFECTION
In addition to identification of specific patient risk for posttransplant infection, transplant infection management includes strategies for prophylaxis and surveillance of the most common or serious infections. Specific regimens for prophylaxis vary by type of transplant and transplant center. Antibiotic prophylaxis is given during the perioperative time period to prevent wound- or procedure-associated bacterial infection. Viral prophylaxis against CMV varies in choice of agent and in duration, but typical regimens include ganciclovir for the first 3 months post transplant. Longer duration of prophylaxis and the use of intravenous immunoglobulin vary according to specific center preference and to type of transplant. Surveillance for CMV viremia using quantitative polymerase chain reaction (PCR) or pp65 antigenemia assay is becoming more common to inform prophylaxis strategies, in addition to its use in evaluation for clinical disease in pediatric transplant recipients. Similarly, quantitative EBV-PCR surveillance is being increasingly incorporated into posttransplant management; however, there is considerable variation in its use by different centers, and in the interpretation of the results in clinical management. Anti-fungal prophylaxis with clotrimazole or nystatin is typically used for the first 3 months. The combination trimethoprim and sulfamethoxazole is used to prevent Pneumocystis jeroveci pneumonia for at least 6 to 12 months, recognizing the highest risk for disease occurring during the first year.
POSTTRANSPLANT LYMPHOPROLIFERATIVE DISORDER
Improved survival following solid organ transplantation has been achieved in part by the advent of more effective immunosuppression. Unfortunately, this has resulted in an increased incidence of posttransplant lymphoproliferative disease (PTLD). PTLD is a serious and life-threatening complication of immunosuppression that encompasses a broad spectrum of clinical conditions resulting from an abnormal immune response, varying from a benign lymphoproliferative response to the development of lymphoid malignancy with death.7 The large majority of cases of PTLD are associated with primary Epstein-Barr virus (EBV) infection, acquired after transplant, and most occur within the first 2 years following transplantation.
Children are at increased risk for EBV-associated PTLD in comparison to adults because they are often EBV seronegative at the time of transplant, and may receive an allograft from a seropositive donor. They may also receive blood transfusions that increase the EBV exposure risk. In addition, children may acquire primary EBV infection, mononucleosis, in the community just as most nontransplant children do prior to adult age. Primary cytomegalovirus (CMV) infection, which may also be acquired via the graft, is also a risk factor for PTLD. Current transplant immunosuppression strategies directed at T-cell suppression interfere with the normal surveillance and response to the primary EBV infection. When PTLD presents early after transplant in the EBV-naïve recipient, it is likely that the graft is the primary agent of infection, and also that immunosuppression has a role in the development of PTLD. The prevailing view is that PTLD occurring early after transplant is a result of overimmunosuppression, whereas that occurring several years after transplant in the setting of low-dose baseline immunosuppression, represents more of a primary malignant transformation in the immune response. These late-onset cases of PTLD are less likely to be EBV related, and may require more directed lymphoma type therapy.
 EPIDEMIOLOGY
EPIDEMIOLOGY
The incidence of posttransplant lymphoproliferative disease (PTLD) varies across the different organ transplant types and reflects the differing patient and donor characteristics, age at transplant, and immunosuppression strategies used. In general, reported rates of PTLD are higher in thoracic transplant recipients: lung 10% to 20%, and heart 10%,8 although rates are still significant in kidney transplant recipients (3–10%),9 and liver recipients (5–10%).10 The organ-specific immunosuppression strategies used reflect the differing risks of rejection, the effectiveness of available therapies for rejection, and the ability to support the recipient with graft dysfunction due to rejection all vary by type of transplant.
 CLINICAL FEATURES AND DIAGNOSIS
CLINICAL FEATURES AND DIAGNOSIS
The clinical presentation of PTLD can vary from a nonspecific viral illness to a malignant lymphoma. Symptoms may include fever, malaise, weight loss, sore throat, lymphadenopathy, diarrhea or gastrointestinal bleeding, headache, and allograft dysfunction. Physical examination may reveal tonsillar enlargement, asymmetry, ulceration, lymphadenopathy, splenomegaly, and/or graft tenderness and enlargement. The transplant graft is often involved in the presentation of PTLD. Identification of EBV viremia by PCR in the clinical setting of graft dysfunction, or nonspecific viral illness strongly suggests the diagnosis of PTLD. The diagnostic evaluation for PTLD should include a complete blood count (CBC) with differential, electrolytes, lactate dehydrogenase, calcium, and uric acid. Assessment of liver function and renal function should be included, as well as measurement of EBV serologies and PCR. Imaging studies should include CT scan of chest, abdomen, and pelvis specifically looking for nodal or extra-nodal disease, and nodular or infiltrative changes in the allograft. Mesenteric adenopathy or bowel wall thickening are additional gastrointestinal manifestations of PTLD. A biopsy of any suspicious lesions should be obtained. In the absence of other specific radiographic findings, endoscopy with biopsy may be able to demonstrate non-bulky PTLD or mucosal ulceration. A transplant graft biopsy should also be done to rule out involvement with PTLD.
 PATHOLOGY
PATHOLOGY
The pathologic evaluation of PTLD will also reflect the range of clinical disease presentation. In 2001, the World Health Organization (WHO) published a classification of PTLD that has been broadly adopted in the description and evaluation of PTLD.11 Characteristics of the proliferating mononuclear infiltrate, preservation or destruction of the underlying tissue architecture, and degree of lymphocytic differentiation are the fundamental aspects of the WHO classification system. A polymorphic infiltrate can be seen in benign lymphoproliferation and also in the majority of EBV-associated PTLD. A monomorphic infiltrate may appear more malignant with transformed lymphocytes resembling a large B-cell lymphoma. The tissue is also evaluated for presence of EBV using an EBER (Epstein-Barr early RNA) stain to identify infected lymphocytes. Determination of lymphocyte expression of CD-20 within the mass or infiltrate is being increasingly incorporated into the diagnostic algorithm for PTLD. In addition to more specifically characterizing the infiltrate as involving activated B cells, CD-20-positive PTLD may show improved response to treatment that includes anti-CD-20 monoclonal antibody, although this work is ongoing at this time.
 TREATMENT
TREATMENT
The primary treatment for posttransplant lymphoproliferative disease (PTLD) is reduction of immunosuppression, which will allow the recipient immune system to recover some of its antiviral effectiveness against the EBV infection. Ganciclovir is also widely used in the initial treatment of PTLD, although its efficacy is not clearly demonstrated. A clinical response and/or a decline in EBV-PCR copy number should occur within the first 2 to 3 weeks. PTLD refractory to reduction of immunosuppression or that presenting with fulminant disease may require systemic chemotherapy. A low-dose cytoxan and prednisone regimen has been shown to be effective in the initial treatment of refractory PTLD.12 The role of anti-CD-20 monoclonal antibody (rituximab) is currently under investigation. Children presenting with overt B-cell or Hodgkin-like lymphoma are treated with the appropriate lymphoma chemotherapy regimen as in the nontransplant patient.
The keys to successful management of PTLD include maintaining a vigilant approach to reducing immunosuppression as much as possible. Surveillance for EBV by PCR is being increasingly incorporated into the routine post-transplant management, particularly during the first few years. It remains to be seen if this will result in improved outcomes for PTLD, although early detection of disease is generally associated with improved outcome.
LONG-TERM MANAGEMENT OF THE SOLID ORGAN RECIPIENT
As patient survival continues to improve following solid organ transplantation, more children are able to return to age-appropriate “normal” activities and lifestyle. This return to improved general health is one of the basic goals of successful organ transplantation, and yet, when approached, is associated with anxiety and uncertainty. The realization that the successful treatment of organ failure with transplantation comes with a necessary commitment to active lifelong health maintenance can be a source of stress to the patient, family, and the primary care provider. The medical care plan, even in its simplest form, will consist of interval medical appointments, laboratory tests, imaging studies, and often a complicated medication regimen. Transplant medications may be unfamiliar or even intimidating to community care providers. Their use requires adherence to a specific dosing schedule and there are multiple drug interactions that require special considerations when prescribed. The result of the medication compliance is an ongoing state of immunosuppression, which while effective in preventing graft rejection, increases the long-term risk for certain infections and toxicities not seen in the immunocompetent non-transplant population.
Long-term posttransplant care is typically shared between the transplant center and the primary physician. The transplant center is responsible for providing ongoing care and assessment of graft function, and management of immunosuppression for the life of the allograft or until transition is made to an adult transplant center. The primary care includes provision of ongoing health maintenance, such as well-child care, assessment of growth and nutritional status, care of intercurrent illness, and maintenance of current immunizations. Changes in organ function or the development of chronic changes in the graft may be clinically asymptomatic, and only detected with active surveillance with laboratory tests. A general understanding of the potential toxicities of posttransplant medications and some of the associated drug interactions is important in the ongoing primary care of the pediatric recipient. Some of the more common complications of solid organ transplant. These include nephrotoxicity; cardiovascular morbidity; bone and metabolic toxicities; and gastrointestinal, hematopoietic, and neurotoxicity.
 RENAL COMPLICATIONS
RENAL COMPLICATIONS
The most common cause of nephrotoxicity is calcineurin inhibitor therapy. Tacrolimus and cyclosporine produce a dose-dependent reduction in renal blood flow and in glomerular filtration rate that are usually related to the blood level of the drug. In contrast, dose-independent chronic structural changes and/or interstitial fibrosis may develop as a result of exposure to aminoglycoside antibiotics, vancomycin, and the combination trimethoprim and sulfamethoxezole.13 Ganciclovir exposure may also contribute to interstitial change. It is now recognized that proteinuria may develop with sirolimus. An abnormal creatinine level may be seen in approximately 10% of pediatric heart or lung transplant recipients by 5 years post transplant. Some of these may progress to end-stage renal disease and require dialysis and transplantation.
 HYPERTENSION
HYPERTENSION
Hypertension is relatively common following transplantation, and had been typically seen in cyclosporine-based protocols. Although still a risk factor, tacrolimus is associated with a lower incidence of hypertension compared with cyclosporine.14 Steroids therapy also contributes to hypertension risk. In a multicenter study by the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS), hypertension was improved in patients receiving tacrolimus/MMF/prednisone compared with cyclosporine/MMF/prednisone.15 A hypertrophic cardiomyopathy associated with tacrolimus has been reported.16 Minimization protocols of steroids and calcineurin inhibitor decrease these adverse effects.
 METABOLIC COMPLICATIONS
METABOLIC COMPLICATIONS
The major metabolic toxicities that occur with increased frequency in the posttransplant child on long-term immunosuppression include diabetes mellitus, hyperlipidemia, and obesity. Cyclosporine- and tacrolimus-associated diabetes develops in approximately 2% to 4% of renal and heart transplant recipients, via a direct inhibitory effect on insulin secretion, as well as an effect on insulin utilization. The incidence is increased in lung transplant recipients up to 30%, reflecting the prevalence of cystic fibrosis in this group. Several immunosuppression agents may contribute to hyperlipidemia. Steroids are well known to contribute to hypercholesterolemia and elevated triglycerides and very low-density lipoprotein (VLDL). Sirolimus is also known to cause hyperlipidemia. In addition to the general increase in cardiovascular morbidity, hyperlipidemia and diabetes contribute to decreased graft survival. Obesity is associated with an increased risk of death, death due to cardiopulmonary disease, as well as increased risk for graft thrombosis and loss.
 BONE AND GROWTH COMPLICATIONS
BONE AND GROWTH COMPLICATIONS
Several factors, such as immobilization, metabolic bone disease, and immunosuppressive drugs, can compromise the quality of bone in children who have undergone solid organ transplantation. Decreased bone mineral density is reported in only a small proportion of pediatric transplant patients, and the relationship between low bone mineral density and fracture risk has not been established in children. Despite this, fractures, scoliosis, and joint and spinal degeneration are common in patients who received solid organ grafts as children. Avascular necrosis occurs in up to 1% of transplant recipients. Linear growth may be stunted. While the pediatric recipients may exhibit some catch up growth following transplantation, final height achieved depends to a large extent on age at transplant, duration of organ failure, and use of steroids in maintenance immunosuppression, but may be decreased due to premature epiphysial closure.
 GASTROINTESTINAL COMPLICATIONS
GASTROINTESTINAL COMPLICATIONS
Gastrointestinal toxicities of immunosuppression are common and may be nonspecific in nature, including nausea, vomiting, and abdominal pain. Mycophenolate mofetil is frequently associated with diarrhea and nausea, with toxicity higher in the youngest patients.17 These GI side effects, while general in nature, can have a significant impact on patient hydration status, and absorption of other medications and thereby affect graft function. Steroid-associated gastritis and gastroesophageal reflux also may require specific treatment.
 HEMATOLOGIC COMPLICATIONS
HEMATOLOGIC COMPLICATIONS
Bone marrow suppression may be a complication of specific immunosuppression agents, or of antiviral prophylaxis, and may impact any of the three cell lines. Neutropenia is commonly associated with azathioprine or mycopheno-late mofetil, or ganciclovir. Liver recipients who may have had chronic pretransplant hypersplenism may be particularly sensitive to the marrow suppression seen with MMF. Anemia is fairly common and has been reported in up to 30% of pediatric renal transplant recipients at 1 year post transplant.18 Causes of anemia include hemolysis associated with sulfur-containing antibiotics, calcineurin-associated hemolytic uremic syndrome, or infection, and frequent phlebotomy.
 NEUROLOGIC COMPLICATIONS
NEUROLOGIC COMPLICATIONS
Neurotoxicity is most frequently associated with calcineurin inhibitors and most commonly includes seizure or tremors. Headache may be directly related to medication toxicity or be secondary to posttransplant hypertension. Acute demyelinating disease and pseudotumor cerebri have also been reported and are associated with calcineurin inhibitors. Anxiety, restlessness, and depression may be related to steroid dose, and may be manifest during periods of rejection treatment with pulse steroids.
 INTERCURRENT ILLNESS MANAGEMENT
INTERCURRENT ILLNESS MANAGEMENT
Intercurrent illness that develops in the pediatric recipient after transplant can have an impact on the ability of the recipient to maintain therapeutic levels of immunosuppression and may trigger episodes of rejection A nonspecific viral illness with vomiting or diarrhea may quickly result in significant dehydration, and subtherapeutic levels of immunosuppression. The child may require intravenous fluids for hydration if unable to maintain adequate intake orally. Acute rejection episodes may be triggered by recent infection, as the increased nonspecific immune response to the illness may also include specific antidonor antibody or cellular response. Close communication with the transplant center during these times of illness is important and an assessment of graft function should be included either during the illness or shortly following illness, particularly if the recovery is protracted.
 DRUG INTERACTIONS
DRUG INTERACTIONS
An additional consideration when treating acute illness in the transplant recipient is an awareness of the many drug interactions that may have an impact on the serum levels of immunosuppression. Tacrolimus and cyclosporine are metabolized by the cytochrome p450 enzyme system, which also metabolizes many other medications. Concomitant intake of these p450 metabolized medications may cause an increase or a decrease in the metabolism of tacrolimus, depending on whether the second agent induces or inhibits enzyme activity. Drugs that may increase tacrolimus blood levels include calcium channel blockers; antifungal agents fluconazole and ketoconazole; and macrolide antibiotics erythromycin and clarithromycin. Metoclopromide and cimetidine can also result in increased levels of tacrolimus. Increased metabolism of tacrolimus resulting in decreased tacrolimus blood levels is seen with use of phenytoin, phenobarbital, and rifampin. Measuring the tacrolimus level at the time of initiating any of these agents and also measuring after starting a treatment course are important to assure therapeutic immunosuppression dosing and to avoid toxicity. Grapefruit juice also inhibits the p450 metabolism of tacrolimus and can be associated with very high blood levels of tacrolimus and cyclosporin.
 VACCINATIONS
VACCINATIONS
Children and adolescents being considered for solid organ transplantation should receive immunizations recommended for their age before the transplantation is performed. In general, vaccines will be more immunogenic before transplantation. Live-virus vaccines may be given until 1 month before transplantation but generally should not be given to patients receiving immunosuppressive medications after transplantation. Monovalent measles (or if not available, measles, mumps, rubella [MMR]) vaccine may be given before transplantation to patients as young as 6 months of age if transplantation is anticipated before 12 to 15 months of age. If possible, it is advisable to administer the HPV vaccine to pretransplant nonpregnant female transplant candidates between the ages of 9 and 26.
Children who have undergone transplantation should continue to receive the routine vaccinations as recommended for well-child care, including IPV, HiB, DTaP, Hep B, pneumococcal and meningococcal conjugate, and polysaccharide vaccines as indicated. Inactivated poliovirus should be used for protection against poliovirus. Usually, these are given at least 6 months following transplantation so that immunosuppression is minimized and vaccine response is improved. There are no established recommendations for the administration of the HPV vaccine posttransplant but the risk of neoplasias in transplant recipients with anogenital HPV is substantially increased, so this may soon be advised.
Use of live vaccines is somewhat controversial. MMR vaccine may be considered for susceptible solid organ transplant recipients in the event of an outbreak of measles, mumps, or rubella in the local community. If a transplant patient is exposed to measles, immunoglobulin should be given immediately after exposure. There is growing evidence that varicella vaccine may be safe, particularly in seronegative pediatric transplant recipients who are not in the early posttransplant phase and who are clinically stable, but case reports suggest that the vaccine is not necessarily safe for all posttransplant patients. Thus, the approach varies among transplantation centers. If exposed to varicella in a household member or with face-to-face indoor play, the transplant patient should be given varicella-immune globlulin and monitored closely, especially if fever occurs.
Serum antibody concentrations for measles, mumps, rubella, and varicella should be measured in all patients 1 year or more after transplantation. Household and close contacts of a solid organ recipient should receive MMR and varicella vaccines, if susceptible, to prevent transmission of wild-type virus to the immuno-suppressed child. Oral poliovirus vaccine is contraindicated for transplant recipients and their household contacts. Live-bacteria vaccines (eg, BCG and Ty21a S typhi) are contraindicated in patients receiving immunosuppressive medications after transplantation. Administration of influenza vaccinations are recommended for transplant patients but the protection obtained from vaccination appears to be decreased compared to the general population.19,20
REFERENCES
See references on DVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


