Patent Ductus Arteriosus and Ductus Venosus
Ronald I. Clyman
During fetal life, the ductus arteriosus diverts blood away from the fluid-filled lungs toward the descending aorta and placenta. In full-term infants, obliteration of the ductus arteriosus takes place after birth through a process of vasoconstriction and anatomic remodeling. Premature infants frequently fail to close their ductus arteriosus. The clinical consequences of a patent ductus arteriosus (PDA) are related to the degree of left-to-right shunt through the PDA with its associated change in blood flow to the lungs, kidneys, and intestine.
REGULATION OF DUCTUS ARTERIOSUS PATENCY
In the full-term infant, closure of the ductus arteriosus occurs in 2 phases: (1) functional closure of the lumen within the first hours after birth by smooth muscle constriction and (2) anatomic occlusion of the lumen over the next several days due to extensive neointimal thickening and loss of smooth muscle cells from the inner muscle media.
 BALANCE BETWEEN VASOCONSTRICTION AND VASORELAXATION
BALANCE BETWEEN VASOCONSTRICTION AND VASORELAXATION
Although the fetal ductus arteriosus was originally thought to be a relatively passive structure that constricted after birth, it is now clear that, even in utero, the ductus has an active tone. Ductus arteriosus patency, therefore, is determined by the balance between dilating and constricting forces. The factors known to play a prominent role in ductus arteriosus regulation involve those that promote constriction (oxygen, endothelin, calcium channels, catecholamines, and Rho kinase) and those that oppose it (intraluminal pressure, prostaglandins, nitric oxide, carbon monoxide, potassium channels, and cyclic adenosine monophosphate and cyclic guanosine monophosphate). The relative importance of each of these factors depends on the intrauterine and extrauterine environment, the degree of ductus maturation, and the genetic background and species being studied.
 IN UTERO REGULATION
IN UTERO REGULATION
The fetal ductus normally has a high level of intrinsic tone.1 The intrinsic tone is due to mechanisms that both depend on and are independent of extracellular calcium.1 The contractile proteins (smooth muscle myosin, calponin, and caldesmon) are more differentiated in the ductus than they are in adjacent fetal arteries.2-4 In addition, the fetal ductus arteriosus is more sensitive to the contractile effects of calcium than are the aorta and the pulmonary artery. Other potential factors contributing to ductus tone in utero include an increased Rho kinase activity in the ductus,6 endothelin-1,7 and catecholamines that circulate in high concentrations following birth.8-10
The factors that oppose ductus arteriosus constriction in utero are better understood. The elevated vascular pressure within the ductus lumen (due to the constricted pulmonary vascular bed) plays an important role in opposing ductus constriction.11 The fetal ductus also produces several vasodilators that maintain ductus patency. Vasodilator prostaglandins appear to be the dominant vasodilators that oppose ductus constriction in the later part of gestation.12 Inhibitors of prostaglandin synthesis constrict the fetal ductus both in vitro and in vivo.
Prostaglandin E2 is the most potent prostaglandin produced by the ductus and placenta,13,14 and appears to be the most important prostanoid to regulate ductus patency. Among blood vessels, the ductus is uniquely sensitive to this prostaglandin E2. It relaxes the ductus by interacting with several of the prostaglandin E receptors, activating adenylate cyclase, and reducing the ductus contactile protein response to calcium.15 Inhibitors of phosphodiesterase (the enzyme that degrades cyclic AMP) relax the ductus in utero.16 In addition, one of the prostaglandin E receptors (E3) also relaxes the ductus by opening KATP channels and hyperpolarizing the smooth muscle cells.15 Other factors that may contribute somewhat to maintenance of ductus patency in utero include nitric oxide and carbon monoxide, synthesized by the ductus.22,25
It is interesting to note that pharmacologic inhibition of prostaglandin synthesis by indomethacin in human pregnancy also is associated with an increased incidence of patent ductus arteriosus after birth.32 However, this appears to be due to indomethacin’s ability to produce ductus constriction in utero. In utero constriction produces ischemic hypoxia, increased nitric oxide production, and smooth muscle cell death within the ductus wall. These factors prevent the ductus from constricting after birth and make it resistant to the constrictive effects of postnatal indomethacin.33,34
 POSTNATAL REGULATION
POSTNATAL REGULATION
Several events promote ductus constriction in the full-term newborn following delivery: (1) an increase in arterial PO2, (2) a decrease in blood pressure within the ductus lumen (due to the postnatal decrease in pulmonary vascular resistance), (3) a decrease in circulating prostaglandin E2 (due to the loss of placental prostaglandin production and the increase in prostaglandin removal by the lung), and (4) a decrease in the number of prostaglandin E2 receptors in the ductus wall.15 Although the newborn ductus continues to be sensitive to the vasodilating effects of nitric oxide, it loses its ability to respond to prostaglandin E2.35,36 All of these factors promote ductus constriction after birth.
The postnatal increase in arterial PaO2 plays an important role in ductus constriction. Normoxic contraction occurs in the absence of the ductus endothelium37 and in the presence of inhibitors of prostaglandin, nitric oxide, and endothelin signaling. This suggests that these vasoactive substances are not essential for normoxic constriction. In most species, oxygen appears to constrict the ductus arteriosus through (1) a mechanism that involves smooth muscle depolarization and (2) a mechanism that is independent of changes in membrane potential.38
The unique oxygen sensors within the ductus wall are still not clearly elucidated and may vary by species. The mitochondrial electron transport chain may act as an oxygen sensor by generating reactive oxygen species that constrict the rabbit and human ductus.39,48
Elevated oxygen tensions can also increase the formation of the potent vasoconstrictor endothelin-1.52 However, the exact role of endothelin-1 in postnatal ductus closure is still unclear.
The postnatal increase in PaO2 not only constricts the ductus arteriosus but also has a profound modulatory effect on other vasoactive systems.56 Elevated oxygen tensions can increase the ductus’s contractile response to neural mediators57 and can decrease the formation of vasodilator prostaglandins.1 Although neural and hormonal factors contribute to ductus closure, they do not appear to mediate the oxygen-induced constriction itself.
 DEVELOPMENTAL REGULATION
DEVELOPMENTAL REGULATION
Gestational age has a marked effect on the rate of ductus closure after birth. In contrast with the full-term ductus, the premature ductus is less likely to constrict after birth due to several mechanisms. The intrinsic tone of the extremely immature ductus (< 70% of gestation) is decreased compared to the ductus at term,1 perhaps because of the presence of immature smooth muscle myosin isoforms, with a weaker contractile capacity,2,4,58,59 and decreased Rho kinase expression and activity.6,47 Calcium entry through L-type calcium channels appears to be impaired in the immature ductus (especially under hypoxic conditions).6,44 The potassium channels that promote ductus relaxation also change during gestation (switching from KCa channels, which are not regulated by oxygen tension, to Kv channels, (which can be inhibited by increased oxygen concentrations).42,60 Reduced expression and function of the putative oxygen-sensing Kv channels (Kv1.5 and Kv2.1) appear to contribute to ductus patency in the preterm rabbit.60
Premature infants have elevated circulating concentrations of prostaglandin E2, which may also play a significant role in maintaining ductus patency during the first days after birth. This is due to the decreased ability of the premature lung to clear circulating prostaglandin E2. In the preterm newborn, circulating concentrations of prostaglandin E2 can reach the pharmacologic range during episodes of bacteremia and necrotizing enterocolitis and are often associated with reopening of a previously constricted ductus arteriosus.62
In most mammalian species, the major factor that prevents the preterm ductus from constricting after birth is its increased sensitivity to the vasodilating effects of prostaglandin E2 and nitric oxide.63 As a result, inhibitors of prostaglandin production (eg, indomethacin, ibuprofen, and mefenamic acid) are usually effective agents in promoting ductus closure in the premature infant. It follows that drugs interfering with nitric oxide synthesis or function also could become useful adjuncts, especially in situations where indomethacin has proven to be ineffective.66
In some late gestation newborns, increased prostaglandin sensitivity may contribute to delayed ductus closure. Among infants of 37 or more weeks’ gestation, approximately 30% will close their PDA following indomethacin treatment and another 30% will develop partial ductus constriction.67
The factors responsible for the changes that occur with advancing gestation are currently unknown. Prenatal administration of vitamin A has been shown to increase both the intra-cellular calcium response and the contractile response of the preterm ductus to oxygen.68 However, vitamin A administration does not improve the rate of ductus closure in preterm infants.69 During normal fetal development, there is an increase in circulating cortisol near the end of gestation. Elevated cortisol concentrations in the fetus decrease the sensitivity of the ductus to the vasodilating effects of prostaglandin E270; consistent with these findings, prenatal administration of glucocorticoids significantly reduces the incidence of PDA in premature humans and animals.70-75 Postnatal glucocorticoid administration also reduces the incidence of PDA.76 However, postnatal glucocorticoid treatment also increases the incidence of several other neonatal morbidity.76,77
 GENETIC REGULATION
GENETIC REGULATION
Both species and genetic background play a significant role in determining the relative importance of ductus regulatory pathways. There is a marked species difference among several of these pathways. Several genes that play a crucial role in ductus maturation have been identified in mouse models. These include homeobox genes (Prx1 and Prx2)80 and genes that regulate myosin heavy chains,81 prostaglandin signaling (COX2 and EP4),18,82 gap junctions (connexin 43),83 and transcription (TFAP2B).84 Some of these genes have also been associated with an increased incidence of PDA in humans: MYH11,85ATR type 1,86,87 filamin 1,88TFAP2B,84TRAF1, PGI synthase, and HIF2 alpha (John Dagle, unpublished observations).
RELATIONSHIP BETWEEN VASOCONSTRICTION AND ANATOMIC CLOSURE
In full-term animals, loss of responsiveness to prostaglandin E2 shortly after birth prevents the ductus arteriosus from reopening once it has constricted,35,36 in part because of the decreased synthesis of prostaglandin E2 receptors in the ductus. Both the loss of vasodilator regulation and the anatomic events that lead to permanent closure appear to be controlled by the degree of ductus smooth muscle constriction. Experimental models that alter the ability of the ductus to constrict at term also prevent the normal histologic changes that occur after birth.11,18,28,89-91 Constriction produces ischemic hypoxia of the vessel wall.92 In the full-term newborn ductus, the ischemic hypoxia that accompanies constriction occurs even before luminal flow has been eliminated and depends on the presence of intramural vasa vasorum.93 With advancing gestation, the thickness of the ductus wall increases in size to a dimension that requires the presence of intramural vasa vasorum to provide nutrients to its outer half. These collapsible, intramural vasa vasorum provide the ductus with a unique mechanism for controlling the maximal diffusion distance for oxygen and nutrients across its wall. In the full-term newborn, ductus constriction obliterates vasa vasorum flow to the outer muscle media; this turns the entire thickness of the muscle media into a virtual avascular zone. The profound ischemic hypoxia that follows the compression of the vasa vasorum is followed by an inflammatory response with subsequent anatomic remodeling, leading to permanent closure.
In preterm infants, the ductus frequently remains open for many days after birth. Even when it does constrict, the premature ductus frequently fails to develop profound hypoxia and anatomic remodeling. The preterm infant seems to require a greater degree of ductal constriction than the full-term infant in order to develop a comparable degree of hypoxia. In contrast with the full-term ductus, the thin-walled preterm ductus does not depend on intramural vasa vasorum to provide oxygen and nutrients to its wall. The absence of intramural vasa vasorum leaves the preterm ductus without a mechanism to rapidly increase the diffusion distance across its wall during postnatal constriction. As long as there is any degree of luminal patency, the thin-walled preterm ductus fails to become profoundly hypoxic and fails to undergo anatomic remodeling. As a result, the preterm ductus requires that the lumen be completely obliterated before it can develop the same degree of hypoxia as found at term. If the preterm ductus develops the same degree of ischemia as the full-term ductus, then most of the anatomic changes seen at term will occur in the preterm ductus.1,66 However, if the premature ductus does not develop the degree of ischemic hypoxia needed to induce cell death and anatomic remodeling, it will continue to be responsive to vasodilators and be susceptible to vessel reopening.
In the full-term newborn, all of the ductus arteriosus prostaglandin E2 EP receptors are downregulated after birth; in contrast, in the preterm newborn, the dominant prostaglandin E2 receptor, E4, continues to be synthesized after birth, and the ductus continues to relax with prostaglandin stimulation. In addition, prostaglandin E2 production increases in the ductus wall after birth due to an increased expression of the COX-2 isoform.96 The premature newborn ductus also synthesizes increased amounts of other vasodilators, after birth, in response to the postnatal ductus wall hypoxia (eg, nitric oxide, tumor necrosis factor α, and interleukin-6).94,95 These new vasodilators produce a change in the balance of vasodilators that maintain ductus patency. Ductus patency becomes less dependent on prostaglandin generation and more dependent on other vasodilators during the first weeks after birth. In addition, there is a decrease in ductus adeno-sine 5′-triphosphate concentrations despite the continued ductus patency. The decreased adenosine 5′-triphosphate concentrations prevent the immature neonatal ductus from contracting as vigorously as the fetal ductus.97 These postnatal changes could explain why the effectiveness of indomethacin wanes with increasing postnatal age.98,99 In premature animals and humans, the combined use of a nitric oxide synthase inhibitor and indomethacin produces a much greater degree of ductus constriction than indomethacin alone.66,100 It follows that drugs that interfere with nitric oxide synthesis could become a useful adjunct, especially in situations where indomethacin has been found to be ineffective.
HEMODYNAMIC AND PULMONARY ALTERATIONS
Virtually all shunting through the ductus arteriosus in the preterm infant is left to right. The pathophysiologic features of a PDA depend both on the magnitude of the shunt and on the cardiac and pulmonary responses to the shunt. Preterm infants are capable of increasing their left ventricular output and maintaining their “effective” systemic blood flow even with left-to-right PDA shunts equal to 50% of left ventricular output. With shunts greater than 50% of left ventricular output, effective systemic blood flow falls despite a continued increase in left ventricular output. The increase in left ventricular output associated with a PDA is accomplished not by an increase in heart rate but by an increase in stroke volume.101 Stroke volume increases primarily as a result of the simultaneous decrease in after-load resistance on the heart and the increase in left ventricular preload. Despite the ability of the left ventricle to increase its output in the face of a left-to-right ductus shunt, blood flow distribution is significantly rearranged. This redistribution of systemic blood flow occurs even with small shunts. Blood flow to the skin, bone, and skeletal muscle is most likely to be affected by the left-to-right ductus shunt. The next most likely organs to be affected are the gastrointestinal tract, kidneys, and brain. These organs receive decreased blood flow due to a combination of decreased perfusion pressure (caused by a drop in diastolic pressure) and localized vasoconstriction. These organs may experience significant hypoperfusion before there are any signs of left ventricular compromise. Decreased organ perfusion can lead to a decrease in glomerular filtration rate and has been implicated in the development of necrotizing enterocolitis.
In the preterm lung, there is a delicate balance between PDA-induced increased fluid filtration and lung lymphatic fluid reabsorption. A wide-open PDA exposes the pulmonary microvasculature to systemic blood pressure and increased pulmonary blood flow. Early severe pulmonary hemorrhages frequently occur when a large PDA shunt produces a sudden increase in pulmonary blood flow.102 Because the premature infant has low plasma oncotic pressure and increased capillary permeability, any increase in pulmonary microvascular pressure can lead to an increase in interstitial and alveolar lung fluid. If a PDA persists for longer than 72 hours or if lymphatic drainage is impaired (as it is in the presence of pulmonary interstitial emphysema or fibrosis), the likelihood of edema increases dramatically. Infants with a persistent PDA usually develop pulmonary edema and alterations in pulmonary mechanics by 7 to 10 days after birth. The increased inspired oxygen and mean airway pressures required to overcome the hypoxemia consequent to low lung compliance may explain why a persistent PDA increases the risk of developing chronic lung disease.103 In these infants, improvement in lung compliance occurs following closure of the PDA.104
DIAGNOSIS
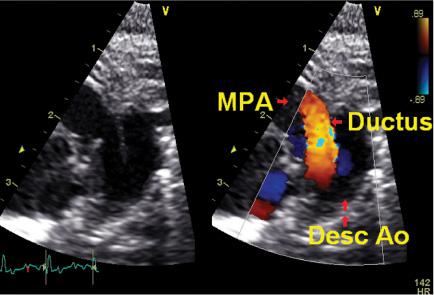
Two-dimensional echocardiography and color Doppler flow mapping are helpful in assessing the magnitude and direction of PDA shunting (see Fig. 55-1). Ductus diameter of 1.4 mm or greater, left atrial–to–aortic root (LA/Ao) ratio of 1.4 or greater, and reversal of forward blood flow in the descending aorta during diastole are signs consistent with a moderate-to-large PDA shunt.105
Although the magnitude of shunt flow plays a significant role in creating neonatal morbidity, equally important factors are the duration of exposure to the shunt and the infant’s ability to compensate for the shunt. For example, the same magnitude left-to-right PDA shunt may be clinically “silent” when present within the first 24 hours after delivery, whereas it may be associated with signs of congestive failure if it persists for 7 to 10 days.
Clinical signs of a PDA (systolic murmur, hyperdynamic precordial impulse, full pulses, widened pulse pressure, and/or worsening respiratory status) usually appear later than echocardiographic signs and are less sensitive in determining the degree of left-to-right shunt. Certain signs, such as continuous murmur or hyperactive left ventricular impulse, are relatively specific for a PDA but lack sensitivity; conversely, worsening respiratory status, while a sensitive indicator, is relatively nonspecific for a PDA. Tachycardia is not a useful or reliable indicator of a PDA in preterm infants. Infants with large left-to-right shunts may have evidence of cardiomegaly and increased pulmonary arterial markings on their chest x-rays; however, in general, the chest x-ray and electrocardiogram are not useful in diagnosing a PDA. Recently, elevated plasma concentrations of brain natriuretic peptide have been found to correlate with the presence of a moderate-sized left-to-right PDA shunt.106
INCIDENCE
Pulsed Doppler echocardiography assessments of full-term infants indicate that functional closure of the ductus arteriosus occurs in almost half of the infants by 24 hours, in 90% by 48 hours, and in all by 72 hours. Premature birth, lack of exposure to antenatal betamethasone, excessive fluid administration, severity of neonatal respiratory distress, and Caucasian race are independent risk factors that appear to delay the rate of ductus closure.107 Preterm infants of less than 28 weeks’ gestation have a 60% incidence of persistent PDA. On the other hand, infants of 30 or more weeks’ gestation have only an 11% incidence of PDA even in the presence of respiratory distress syndrome. Use of exogenous surfactants leads to earlier presentation of the PDA shunt because of lower pulmonary vascular resistance.
TREATMENT
Conservative measures like fluid restriction and diuretics can be used to treat the symptoms associated with a PDA. However, fluid restriction and diuretics frequently lead to electrolyte abnormalities, dehydration, and most importantly, caloric deprivation. Increasing positive end-expiratory pressure and hematocrit can increase pulmonary vascular resistance and decrease the amount of left-to-right shunt. These therapies usually put off, rather than prevent, the ultimate need for PDA closure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


