Disease related
Neuropathy secondary to uncontrolled diabetes mellitus
Tumor infiltration and nerve impingement
Gynecologic malignancy-specific pain syndromes
Lumbosacral plexopathy
Pelvic pain
Malignant psoas syndrome
Treatment related
Radiation-induced pain (e.g., proctitis, pain from fistulas, mucositis, esophagitis)
Postsurgical pain syndromes (e.g., following amputation, mastectomy, thoracotomy)
Post chemotherapy pain (e.g., chemotherapy-induced painful peripheral neuropathy, myalgia, and arthralgia)
Preexisting pain
Chronic and/or preexisting pain (e.g., fibromyalgia, low back pain, postherpetic neuralgia, osteoarthritis)
Psychosocial pain
Spiritual pain (e.g., pain deep within the soul)
Total pain (e.g., the suffering that encompasses all of a person’s physical, psychological, social, spiritual, and practical struggles)
Types of Pain
Pain can be separated into two categories by pathophysiology: nociceptive pain and neuropathic pain.
Nociceptive Pain
Somatic pain is often well localized, usually to the skin, bone, muscle, or other soft tissues. It is usually associated with tenderness or swelling and can be described as sharp, gnawing, and aching.
Visceral pain is vague and not well localized but may be referred to a distant structure. Visceral pain is caused by activation of pain receptors in the chest, abdomen, or pelvic areas via stretching, ischemia, inflammation, or invasion of an organ. Visceral pain is often described as deep, squeezing, aching, dull, or sickening.
Neuropathic Pain
Neuropathic pain is caused by damage or disease affecting any part of the nervous system, central or peripheral. Neuropathic pain has two components: an epicritic or sharp lancinating component and a protopathic or chronic burning component. As such, neuropathic pain is often described as burning, tingling, electrical, pinching, stabbing, sharp, shooting, or pins and needles.
Time Course of Pain
Another way to classify pain is by timing and duration. Pain can be either acute or chronic. Acute pain occurs after trauma via nociceptive activation at the site of tissue damage and typically lasts for hours to days while the injured tissue heals. Chronic pain exceeds the expected time frame for healing and is typically perpetuated by factors other than the cause of pain.
Assessing Pain
The first step in devising a therapy plan is to obtain a thorough pain history and perform a physical assessment in order to determine the proper pain syndrome and select the most appropriate analgesic agents. When standard pharmacologic therapies are ineffective or produce unacceptable, unmanageable adverse effects, interventional techniques are warranted. Clinicians must be aware of common concurrent emotional and physical symptoms as well as management techniques and available resources.
In order to formulate an effective therapeutic strategy, the different dimensions of pain need to be assessed, including the etiology of pain, the quality and intensity of pain, how pain affects daily activities and function, and barriers to pain management. An astute clinician must differentiate between the different causes and types of pain using history and physical and other available tools, including nonverbal cues and body language, radiologic imaging, and various pain scales. Pain is a complex and subjective syndrome. There are many tools to measure pain, including visual analog scales, verbal digital scales, numerical rating scales, or more complex pain questionnaires [6]. Other tools include the Brief Pain Inventory, Wisconsin Brief Pain Questionnaire, the Wong-Baker Faces Scale, and the Edmonton Symptom Assessment System (Fig. 42.1).


Fig. 42.1
Edmonton symptom assessment scale (ESAS) (Reproduced with permission from Elsayem A, Driver LC, Bruera E. The MD Anderson Palliative Care Handbook. Houston, TX: MD Anderson Cancer Center, 2002)
Managing Pain
Once pain has been thoroughly assessed, relief can be obtained through use of available analgesic agents appropriate for that specific pain syndrome.
Pharmacological and Nonpharmacological Treatment Options
Given the complexity of pain as a syndrome, no one treatment may effectively treat pain. The delicate balance of achieving relief yet minimizing side effects must be successfully met. Careful patient selection and a thorough assessment of pain should precede the decision to initiate a trial of opioids and/or nonopioid analgesics.
Opioids and Nonopioids for Pain Management and Addressing Their Side Effects
Opioids are commonly used in the treatment of cancer pain as recommended by the World Health Organization analgesic ladder for cancer pain [7]. Opioids have been shown to be slightly more effective in relieving pain and improving function in patients with various forms of chronic non-cancer pain as compared to placebo in a meta-analysis of 41 randomized trials [8]. There are low-potency and high-potency opioids as listed in Table 42.2. Guidelines such as those put forth by the World Health Organization (WHO), the National Comprehensive Cancer Network (NCCN), and American Cancer Society (ACS) exist as a systematic approach to guide treatment tailored to the individual patient and pain syndrome. Please refer to Fig. 42.2 for the WHO model. The most commonly used short-acting opioids are morphine, hydromorphone, oxycodone, oxymorphone, and fentanyl. Common side effects of opioids as well as their management are listed in Table 42.3.
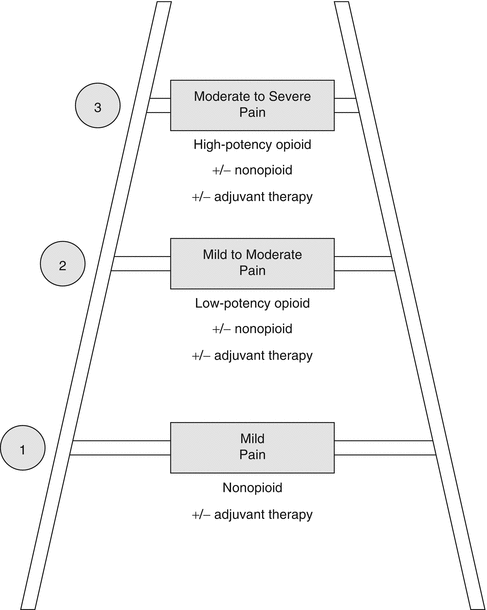
Table 42.2
Low-potency opioids and high-potency opioids
Low-potency opioids | High-potency opioids |
|---|---|
Tramadol | Morphine |
Codeine | Hydromorphone |
Oxycodone | |
Oxymorphone | |
Hydrocodone | |
Fentanyl | |
Methadone |

Fig. 42.2
World Health Organization (WHO) three-step ladder oral analgesic program for managing cancer pain
Table 42.3
Common side effects of opioids
Side effect | Cause | Management |
|---|---|---|
Sedation | Most commonly excessive dosing | Downward titration of dose to level of analgesia Add an adjuvant If refractory, may consider stimulant (i.e., methylphenidate) |
Tolerance | Reduction in effectiveness of central or peripheral opioid activity despite attempts at dose escalation | Reevaluate etiology of pain Rotation of opioids is usually necessary |
Nausea and vomiting Compounded by decreased GI peristalsis in advanced malignancy secondary to circulating inflammatory cytokines and mediators | Direct effect of decreasing gastrointestinal motility Indirect effect of constipation | Metoclopramide works via multiple mechanisms centrally and peripherally to antagonize opioid effects at the central chemoreceptor trigger zone and the GI tract May also consider corticosteroids and neuroleptics such as haloperidol In special cases, diphenhydramine, serotonin antagonists, prochlorperazine, or neurokinin-1 antagonist may help |
Constipation Watch for masquerading signs such as intractable nausea and vomiting, increased abdominal pain, delirium, anorexia, and/or overflow diarrhea | Directly caused by opioids | This may develop very slowly Thus, start a regular laxative regimen from the initiation and throughout the duration of opioid therapy Use a bowel stimulant (senna) and a softening agent (polyethylene glycol) For severe cases, osmotic laxatives and bowel lavages can be used Caution: Constipation may also be due to ileus, intestinal obstruction, or spinal cord compression. A simple abdominal x-ray may be helpful to delineate further etiology |
Cognitive impairment Hallucinations may occur | Rule out other causes first before implicating opioids Sepsis, leptomeningeal disease, brain metastases, metabolic abnormalities, chemotherapy, antifungal therapy, radiation, hepatic encephalopathy, psychotropic medications | If opioid-induced cognitive impairment is suspected, the first step is to lower the dose, which can be diagnostic Do not add medications to treat agitation or other symptoms without this step May also rotate opioids If ineffective, add haloperidol or another neuroleptic |
Urinary retention Relatively rare | More likely to occur in patients at extremes of ages or in conjunction with anticholinergic medications | Temporary catheterization Tolerance usually develops |
Myoclonus | Dose-dependent phenomenon related to opioid metabolites, more often those of morphine and meperidine Results from central motor excitability Usually a sign that a patient’s level of tolerance has been overwhelmed | Dose adjustment may stop symptom Sometimes, opioid rotation is required Temporary addition of benzodiazepine may be necessary |
Respiratory depression Rare occurrence in patient on chronic opioid therapy | Can occur in accidental overdose Can be due to the addition of another sedative agent, such as benzodiazepines | If respiratory function is not significantly impaired, temporary discontinuation and restarting at a lower dose is recommended If severe respiratory compromise has occurred, give naloxone in 40 mcg increments until response occurs. Be wary of acute opioid withdrawal |
Pruritus | Mechanism is not well understood Peripheral histamine release occurs Central action of mu-opioid receptors also contribute to phenomenon | H2 antagonists such as ranitidine may be beneficial However, centrally mediated pathways are more difficult to treat Opioid rotation may be necessary Naloxone reversal should be reserved for only severe cases |
Fentanyl is unique in that it is semisynthetic and highly lipophilic; its rapid onset and relatively short duration of action make it a good choice for control of acute pain and breakthrough pain. Methadone is a completely synthetic opioid agonist and an N-methyl-D-aspartate (NMDA) antagonist with unique pharmacodynamics and pharmacokinetic properties that make it a potent weapon against pain unrelieved by other potent opioids.
Much controversy exists over the use of opioids as it is not without risk. Side effects of opioids can include hyperalgesia, constipation, nausea and vomiting, somnolence, and opioid-induced neurotoxicity such as myoclonus, delirium, and hallucinations. Even more controversy surrounds the use of methadone given the possibility of prolonged QTc intervals; however, there is limited evidence regarding the efficacy or safety between methadone and placebo, other opioids, or other analgesics. Some studies have shown no prolongation of QTc interval in patients taking methadone in the palliative care setting [9, 10]. As such, caution must be used when deciding to use methadone, carefully weighing risks and benefits of the use of methadone in the palliative care setting. Methadone will be available in India in the future.
Nonopioids including acetaminophen, nonsteroidal anti-inflammatory medicines, and adjuvant therapies such as anticonvulsants, antidepressants, local and topical anesthetics, and corticosteroids have also been effective in pain relief. Nonopioids can be the primary treatment for mild pain or an adjuvant therapy to opioid therapy for moderate to severe pain [1]. Tables 42.4 and 42.5 list common adjuvant therapies
Table 42.4
Adjuvant therapies
Adjuvant | Use |
|---|---|
Acetaminophen | Headache Musculoskeletal pain |
Nonsteroidal anti-inflammatory drugs (NSAIDs) Ibuprofen Ketorolac | Musculoskeletal pain |
COX-2 inhibitors Celecoxib | Musculoskeletal pain |
Tricyclic antidepressants (TCAs) Nortriptyline Amitriptyline | Neuropathic pain syndromes |
Anticonvulsants Gabapentin Carbamazepine Pregabalin Lamotrigine | Neuropathic pain syndromes Postherpetic neuralgia Phantom pain Nerve plexopathies (brachial, lumbosacral) |
Lidocaine | Systemic administration can help with neuropathic pain and phantom pain with a predominance of central features |
Ketamine | NMDA receptor antagonist usually used in cases of extreme opioid tolerance |
Capsaicin | Topical cream form is used for neuropathic pain |
Table 42.5
Common side effects of opioids
Side effect | Cause | Management |
|---|---|---|
Sedation | Most commonly excessive dosing | Downward titration of dose to level of analgesia Add an adjuvant If refractory, may consider stimulant (i.e., methylphenidate) |
Tolerance | Reduction in effectiveness of central or peripheral opioid activity despite attempts at dose escalation | Reevaluate etiology of pain Rotation of opioids is usually necessary |
Nausea and vomiting Compounded by decreased GI peristalsis in advanced malignancy secondary to circulating inflammatory cytokines and mediators | Direct effect of decreasing gastrointestinal motility Indirect effect of constipation | Metoclopramide works via multiple mechanisms centrally and peripherally to antagonize opioid effects at the central chemoreceptor trigger zone and the GI tract May also consider corticosteroids and neuroleptics such as haloperidol In special cases, diphenhydramine, serotonin antagonists, prochlorperazine, or neurokinin-1 antagonist may help |
Constipation Watch for masquerading signs such as intractable nausea and vomiting, increased abdominal pain, delirium, anorexia, and/or overflow diarrhea | Directly caused by opioids | This may develop very slowly Thus, start a regular laxative regimen from the initiation and throughout the duration of opioid therapy Use a bowel stimulant (senna) and a softening agent (polyethylene glycol) For severe cases, osmotic laxatives and bowel lavages can be used Caution: Constipation may also be due to ileus, intestinal obstruction, or spinal cord compression. A simple abdominal x-ray may be helpful to delineate further etiology |
Cognitive impairment Hallucinations may occur | Rule out other causes first before implicating opioids Sepsis, leptomeningeal disease, brain metastases, metabolic abnormalities, chemotherapy, antifungal therapy, radiation, hepatic encephalopathy, psychotropic medications | If opioid-induced cognitive impairment is suspected, the first step is to lower the dose, which can be diagnostic Do not add medications to treat agitation or other symptoms without this step May also rotate opioids If ineffective, add haloperidol or another neuroleptic |
Urinary retention
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|