Pain Management and Sedation
Alan Farrow-Gillespie
“Although the world is full of suffering, it is also full of the overcoming of it.”
—Helen Keller, 1880–1968.
The word patient derives from the Latin word patiens, or “one who suffers.” The concept that pain is a necessary part of illness or injury is ingrained in human thought. Until the advent of anesthesia in the 1800s, few means of pain relief were available. As cultural, religious, political, and medical ideas have evolved, attitudes concerning pain relief have progressed as well. The modern view, most recently expressed in a landmark article by Brennan, Carr, and Cousins, is that “the unreasonable failure to treat pain is poor medicine, unethical practice, and is an abrogation of a fundamental human right.”1
Even as many advances in managing pain suffered by adults have been embraced, pain management for children has lagged behind. Early arguments suggesting that neonates have a reduced ability to experience pain or that pain is somehow enlightening and necessary for the maturing child have only slowly lost favor. To the child in pain, the rationalizations to deny treatment are of little comfort. Even the youngest neonates have shown a negative response to painful stimuli with signs of distress or withdrawal.2 Because of the plasticity of the central nervous system in the developing neonate, repeated pain may lead to abnormal reorganization and sensitization; however, analgesic intervention will reduce enhanced pain behavior in the infant, suggesting that analgesia can inhibit these changes.3,4
Almost 30 years ago, the first published reports of the undertreatment of pain in children appeared. Although numerous studies followed and education in pediatric pain management has been greatly expanded, the inadequate treatment of pain in children remains a problem throughout the world’s health care systems.
Every medical provider should aspire to provide appropriate pain management to the pediatric patient using all available options for treatment or prevention. An individualized pain management plan should be established with the goal of returning the child to normal activities such as moving, playing, bathing, and interacting with his or her surroundings. If appropriate, family and caregivers should be involved in formulating the plan. They should be educated about aspects of the plan and encouraged to be a part of the overall pain treatment strategy. The medical provider should perform an initial assessment, frequent reassessments, and an evaluation of the success of pain interventions. Relief of procedural pain (eg, from needle sticks) should be of consistent concern. Preoperative interventions such as epidural analgesia or regional anesthesia should be an integral part of the overall management of painful surgery. Ultimately, pediatric patients should be provided with a safe, comforting environment that minimizes the destructive effects of illness or injury.
PATHOPHYSIOLOGY
The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of damage.”5 Pain is an important signal for tissue damage; it elicits protective responses that are fundamental for survival. These responses become more intricate as we ascend the evolutionary scale from single-cell creatures to humans. 
In the human fetus, all mechanisms for the signal of a painful stimulus to arrive at the cerebral cortex are functional sometime between the 24th and 29th week of gestation. Multiple processes are required for the sensation of pain to be ultimately recognized. Transduction is the process by which a physical stimulus is converted into a neuronal signal by afferent nociceptors in the dermal layers or within a visceral organ. Transmission is the process by which this neuronal signal is transported from its origin to the brain. A-delta nerve fibers are fast-transmitting, myelinated fibers that transmit the initial “pricking” pain. C-fibers are the slower, unmyelinated fibers that transmit the secondary “burning” pain sensation. Modulation is the augmentation or inhibition of the painful signal at or above the level of the spinal cord. Recognition of the pain occurs in the cerebral cortex after modification of the signal in the lower and midbrain. This final cognition event identifies and localizes the pain and leads to a physiological and psychological response. The overall experience of pain in humans is determined by cognitive state, physical health, maturity, emotion, attitude, family, culture, environment, fear, and anxiety.
Animal studies suggest that full-term neonates (and possibly preterm infants) are more sensitive to noxious stimuli than older children. For instance, the newborn rat’s spinal cord is generally more excitable than the mature rats. The explanation may lie with the still-undefined differentiation and connectivity of A-delta fibers and C-fibers during early development, which cause low-intensity stimuli to activate pathways that later may be reserved only for high-intensity stimuli.6 Moreover, the size of the dorsal horn peripheral cutaneous receptive field decreases with age. The resultant field overlap increases the chance for skin stimulation to activate pain circuits.6 Because of inherent nervous system plasticity, repetitive exposure to painful stimuli in infants may affect behavior in adulthood.7 Newborn rats subjected to repetitive needle-stick pain develop a decrease in pain thresholds and hyperalgesia. As adults, these rats exhibit anxiety and defensive behavior, which are attenuated by opioid medications given at the time of the painful stimuli.7
Tissue damage stimulates the local release of algogenic substances, including H+, K+, hista-mine, bradykinin, serotonin, cytokines, prostaglandins, substance P, and growth factors. This “inflammatory soup” leads to peripheral sensitization of surrounding nociceptors, decreasing the threshold necessary for their activation and increasing the total noxious signal presented to the central nervous system. Repetitive A-delta fiber and C-fiber activation causes hyperexcit-ability of sensory neurons in the dorsal horn of the spinal cord, with exaggerated and prolonged responses to normal stimuli. This “central sensitization” at the level of the dorsal horn of the spinal cord results in the hyperalgesia (increased sensitivity to painful stimuli) and allodynia (pain induced by nonpainful stimuli) in areas surrounding the injury.
Acute pain from an interventional procedure or injury is time-limited, while chronic pain is usually defined as greater than 3 months’ duration. Neuropathic pain from nerve injury is often chronic, continuing after tissue damage has healed. It is characterized by allodynia, spontaneous pain, hyperalgesia, and sensory deficits. In children, untreated acute pain may lead to immunosuppression, decreased food intake, delayed ambulation, poor respiratory effort, anxiety, social withdrawal, avoidance of therapy, chronic pain behavior, and increased mortality.
PAIN ASSESSMENT
The experience of pain is ultimately cognitive and subjective, and the intensity of the sensation can be described only by the patient. Effective pain management requires an accurate initial assessment of a child’s pain and an ongoing reassessment to evaluate the response to treatment. The approaches to pain measurement most frequently noted in the medical literature are (1) self report, (2) observational/behavioral, and (3) physiological.
Interpreting distressed behavior in infants and nonverbal children is a challenge for the medical practitioner. Hunger, fear, sleeplessness, or unknown emotional factors may be misinterpreted as pain. Conversely, lack of movement and interaction may be misinterpreted as an absence of pain. The use of physiological and behavioral distress scales in conjunction with observation and experience can give the caregiver ongoing information to successfully treat pain in nonverbal infants and children. Examples of observational scales include the FLACC Scale (Face, Legs, Arms, Cry, Consolability) Scale (see Table 113-1), the CHEOPS (Children’s Hospital of Eastern Ontario Pain Scale), and the N-PASS (Neonatal Pain, Agitation, & Sedation Scale).
Table 113-1. FLACC: Nonverbal Pain Scale
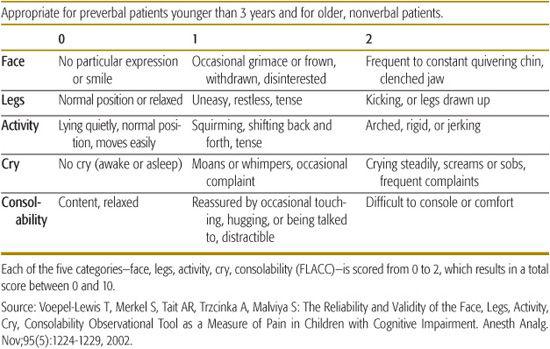
Sedated and brain-impaired children are also challenging to assess. Although substantial progress has been made in this area, no developmentally appropriate pain scale exists for most of these patients. Physiological indicators such as pupil size, facial expression, blood pressure, heart rate, and sweating may be used for assessment. Measuring stress-related chemicals, such as hormone levels, is not timely and is cumbersome to use in the clinical setting. Additional information may be obtained by employing the COMFORT Scale (a comfort/sedation scale, not a pain scale).
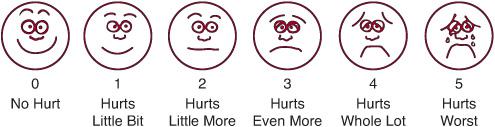
FIGURE 113-1. FACES Pain Scale. Which face shows how much hurt you have now? Ask the child to choose the face that best describes how he is feeling. FACES is recommended for children ages 3 years and older. Explain to the child that each face is for a person who feels happy because he has no pain or “hurt” (Face 0), to one who “hurts as much as you can imagine, although you don’t have to be crying to hurt this bad” (Face 5). (Source: Originally published by Whaley L, Wong D. Nursing Care of Infants and Children. 3d ed. St. Louis, MO: 1987: 1070. Copyrighted by the C. V. Mosby Company.)
In older children who can communicate, self-report assessment scales have been validated for different age groups. Initial assessment should include the character, location, quality, duration, frequency, and intensity of the pain. This information should be obtained from direct questioning of the child and should be supplemented with information from parents and caregivers. Pain should be assessed at regular intervals or after intervention. In the USA, the Joint Commission for Hospital Accreditation now requires documented, reoccurring pain assessments for hospitalized children.
Examples of self-report scales include the Faces Pain Scale, the Oucher Scale, and the Visual Analogue Scale. A 0-to-5 scale can be used for older children who can perform simple one-digit subtraction. Explain the 0-to-5 scale to the child using the descriptions from the Faces scale (see Fig. 113-1).
In 2005, the Pediatric Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (Ped-IMMPACT; www.immpact.org) commissioned a review of observational/behavioral pain measures and of self-report pain measures. The Ped-IMMPACT reviews, with comments, are included in eTables 113.18 and 113.2  .9
.9
NONPHARMACOLOGICAL INTERVENTIONS
Other than the time involved for implementation, there is little to lose in using nonpharmacological modalities for pain management. Alone or in conjunction with other modalities, these techniques can be quite effective (eTable 113.3). However, they should not replace pain medications if pain relief is inadequate. Information regarding the cause of a child’s pain should be given to the patient and family in a sensitive manner, and a thorough explanation of any nonpharmacological modalities to be used should precede treatment. Potentially effective modalities include positioning, soothing, touching, swaddling, warm or cold packs, oral sucrose, pacifiers, massage, physical therapy, distraction, play therapy, relaxation therapy, hypnotherapy, psychotherapy, biofeedback, transcutaneous electrical nerve stimulation (TENS) and acupuncture. Several of these are further discussed in Chapter 14.
PHARMACOLOGICAL INTERVENTIONS
Infants and small children metabolize and may react to medications differently than older children and adults. In neonates, total body water is a larger proportion of total body weight than in older children and adults. Immature hepatic enzyme systems and decreased glomerular filtration rates cause infants to metabolize and excrete drugs differently than older children and adults. Neonates develop higher levels of free, unbound drug because they have decreased levels of albumin and alpha1-glycoprotein than older children or adults. As a result, drug dosages and schedules may differ from that of older children and adults.
Initial drug choices should be based on an assessment of pain intensity, the nature of the pain, the patient’s age, available routes of administration, and urgency. Degree of pain will dictate the required potency of medication. Issues such as opioid tolerance and prior medication needs should also be considered. Pain management in patients with chronic illness or at the end of life is discussed in Chapter 126.
 ACETAMINOPHEN
ACETAMINOPHEN
Acetaminophen (or paracetamol as it is known in the United Kingdom) is one of the most common analgesic and antipyretics in use today. It is available in the United States without a prescription as an oral or rectal medication. An intravenous formulation of the pro-drug propacetamol is available in Europe. Acetaminophen has little anti-inflammatory action, and in contrast with non-steroidal anti-inflammatory drugs (NSAIDs), it does not cause gastrointestinal irritation or platelet inhibition. Although its mechanism of action is uncertain, acetaminophen has central and peripheral effects, possibly by inhibiting unique cyclooxygenase (COX) derivatives, as well as other less well characterized inflammatory-modulating pathways such as those involving endogenous cannabinoids. Acetaminophen is often formulated in combination with other drugs and may potentiate the effect of opioids.
The oral dose for acetaminophen is 10 to 15 mg/kg given every 6 hours, initially as a regularly scheduled medication and then as needed after 3 to 5 days. The maximum daily dose is 100 mg/kg for children, 80 mg/kg for infants, 60 mg/kg for term neonates, and 45 mg/kg for preterm neonates. Cytochrome P-450 oxidation of acetaminophen results in the hepatotoxic metabolite N-acetyl-p-benzocinonimine, and a risk of liver toxicity exists for single doses exceeding 150 mg/kg. The risk of hepatic damage is increased in the presence of fever, alcohol, dehydration, cholestasis, and other hepatotoxic medications.
Acetaminophen is an aniline derivative that in high doses can lead to the formation of met-hemoglobin and methemoglobinemia. Acute nephrotoxicity has been reported with massive overdoses of acetaminophen, but there is little evidence that chronic use with appropriate doses leads to analgesic nephropathy.
 NONSTEROIDAL ANTI-INFLAMMATORY DRUGS
NONSTEROIDAL ANTI-INFLAMMATORY DRUGS
NSAIDs are one of the most commonly used classes of drugs in the world. They are effective in treating fever, cutaneous and muscular pain, headache, dysmenorrhea, and arthritis pain. Prescription and over-the-counter NSAIDs approved by the US FDA are listed in eTable 113.3  and at http://www.fda.gov/cder/drug/infopage/cox2. Specific NSAIDs have been found to be as effective as opioids in certain types of pain.10 NSAIDs inhibit the enzyme cyclooxygenase (COX), thereby decreasing the production of prostaglandins. COX has two isoenzymes. Older NSAIDs are nonselective inhibitors of the COX enzyme with differing ratios of COX-1 and COX-2 inhibition. Inhibition of COX-1, a “housekeeping” enzyme important in the production of prostaglandins that regulate normal cell activity, is responsible for the gastrointestinal (GI) irritation, decreased platelet function, renal injury, and impairment of osteoblast activity associated with many NSAIDs. Selective inhibition of COX-2, which is responsible for the production of proinflammatory prostaglandins at the site of injury, appeared to be a more desirable therapeutic strategy to maximize analgesic and anti-inflammatory effects and minimize complications. Unfortunately, the well-publicized market recall of some of the first COX-2 selective inhibitors such as rofecoxib and valdecoxib following reports of severe adverse cardiovascular events has left celecoxib as the only selective COX-2 inhibitor available in the United States.
and at http://www.fda.gov/cder/drug/infopage/cox2. Specific NSAIDs have been found to be as effective as opioids in certain types of pain.10 NSAIDs inhibit the enzyme cyclooxygenase (COX), thereby decreasing the production of prostaglandins. COX has two isoenzymes. Older NSAIDs are nonselective inhibitors of the COX enzyme with differing ratios of COX-1 and COX-2 inhibition. Inhibition of COX-1, a “housekeeping” enzyme important in the production of prostaglandins that regulate normal cell activity, is responsible for the gastrointestinal (GI) irritation, decreased platelet function, renal injury, and impairment of osteoblast activity associated with many NSAIDs. Selective inhibition of COX-2, which is responsible for the production of proinflammatory prostaglandins at the site of injury, appeared to be a more desirable therapeutic strategy to maximize analgesic and anti-inflammatory effects and minimize complications. Unfortunately, the well-publicized market recall of some of the first COX-2 selective inhibitors such as rofecoxib and valdecoxib following reports of severe adverse cardiovascular events has left celecoxib as the only selective COX-2 inhibitor available in the United States.
In 2005, the FDA concluded that an increased risk of serious adverse cardiovascular events may be a class effect for NSAIDs (excluding aspirin) and announced warnings to highlight the potential increased risk of cardiovascular events and the risk of serious GI bleeding. The FDA also advised of renal and hepatic toxicity associated with NSAID usage. The nonselective NSAIDs aspirin, ibuprofen, naproxen, and ketoprofen remain available in the United States without a prescription and are often used in drug combinations. Many other NSAIDs with differing qualities are available through prescription. Aspirin has been associated with Reye’s syndrome, and its use in children has therefore declined. Indomethacin has unique qualities in certain headache syndromes such as hemicrania continua and is used to facilitate patent ductus arteriosus closure in neonates.
Ibuprofen is the most commonly used NSAID in children and is available for oral usage. It is effective to treat fever and mild pain and to diminish the need for opioids in more severe pain at doses of 5 to 10 mg/kg every 6 to 8 hours, not to exceed 40 mg/kg per day. Oral ketorolac is effective for moderate pain, and IV ketorolac is effective for moderate to severe pain as the only intravenous NSAID available in the United States. An IV dose of 30 mg provides analgesia comparable to 12 mg of morphine.10 It is given as a single intravenous dose of 1 mg/kg followed by 0.5 mg/kg every 6 hours for up to 5 days. Ketorolac should be used with caution in small children and is contraindicated in hypovolemic patients because of the risk of renal failure. Neither ibuprofen nor ketorolac is appropriate for patients with decreased renal or hepatic function, for patients with a history of gastric ulcers, for patients with coagulopathies or who are receiving anticoagulants, or for patients who are at risk for bleeding for other reasons.
 OPIOID MEDICATIONS
OPIOID MEDICATIONS
According to the earliest accounts from Mesopotamia, juice from the opium plant has been used for medicinal purposes since approximately 3500 bc. Opioid refers to all the compounds, naturally occurring or synthetic, that are related to opium. Opiates are compounds derived, directly or semisynthetically, from opium. Opioid drugs imitate the effect of naturally occurring endorphins by acting as opioid receptor agonists in the central and peripheral nervous system. Opioids are rapid and effective analgesics for moderate to severe pain and can be administered as an oral, transmucosal, rectal, intramuscular, intravenous, transdermal, epidural, or intrathecal preparation (see Table 113-2). Opioid drugs act at multiple opioid receptor sites (including mu, kappa, delta, and sigma) and are available as pure agonists, pure antagonists, or mixed agonist-antagonists. Mixed agonist-antagonist opioids were developed to minimize the side effects of the agonists, but unfortunately they have not demonstrated a practical advantage as analgesic medications.
In addition to analgesia, morphine-like drugs can cause respiratory depression, cardiovascular depression, nausea, vomiting, constipation, pruritus, urinary retention, dependence, pituitary dysfunction, and immunosuppression. Many of these side effects can be controlled with small doses of a mixed agonist-antagonist or with very small doses of an agonist opioid. Anti-histamines and laxatives can be quite effective to ameliorate the pruritus and constipation produced by opioids, respectively.
Many opioids can be titrated to analgesic effect at the bedside. The dose limit for opioids is not fixed and is defined to a great extent by side effects such as respiratory depression. Opioids should be dosed cautiously in infants, patients with impaired drug clearance, or patients with increased risk of respiratory or cardiovascular dysfunction. Opioids produce tolerance (the need for increasing doses to achieve the same level of analgesia), dependence (the need for continued dosing to prevent physical symptoms of withdrawal), and addiction (the compulsive use of medication, resulting in physical, psychological, and social dysfunction). Children are often underdosed, and higher doses should be planned in opioid-tolerant patients. The use of mixed agonist-antagonist opioids in patients receiving pure agonist could result in withdrawal.
Table 113-2. Commonly Used Opioid Medications
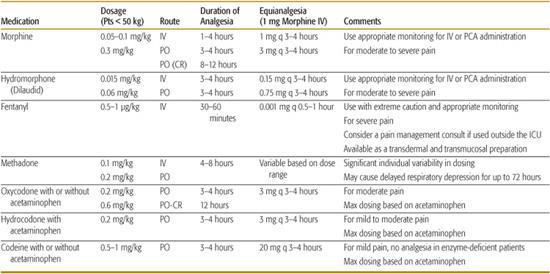
Scheduled dosing of oral opioids or continuous intravenous infusion tends to produce less variability in blood levels and more consistent analgesia. Patient-controlled analgesia (PCA), the administration of opioids with an infusion pump that the patient activates when the pain level becomes too high, can be used effectively in children as young as 5 or 6 and may lead to lower total drug usage. The inherent safety of PCAs is due to the inability of a somnolent or unconscious patient to “push the button”; parents or caregivers must be cautioned against assisting a child with PCA administration.
Except for methadone, the highest risk of respiratory depression occurs with the initial dose of an opioid and during the first 24 hours of therapy. If respiratory depression occurs, ventilation may need to be supported until the μ-receptor antagonist naloxone (0.1 mg/kg for children less than 20 kg or 2 mg/dose for children greater than 20 kg; in many instances, much lower doses are sufficient) can be given to restore ventilation. Multiple doses may be required to sustain ventilation, because the effects of the original opioid last longer than their reversal by naloxone. To avoid sudden and pronounced hemodynamic changes, naloxone may be titrated at 0.01 to 0.03 mg/kg per dose. Methadone reaches steady-state blood levels over 48 to 72 hours, and patients should be monitored for delayed respiratory depression. With IM, IV, or PCA administration of opioids, infants and children should be appropriately monitored for respiratory depression with pulse oximetry and other modalities. For children who have a history of apnea, who weigh less than 10 kg, or who are younger than 6 months of age, the initial opioid dose should be one quarter to one half of the recommended dose and titrated to analgesic effect. When switching from one opioid to another, it is reasonable practice to administer the newly introduced medication at a dose lower than the expected equianalgesic dose by as much as 50% to allow for incomplete cross-tolerance. Patients should be advanced from IV opioids to oral opioids as soon as possible, keeping in mind that insufficient oral dosing may prolong hospitalization.
Meperidine (Demerol), with its atropine-like structure, is no longer recommended, as it can cause tachycardia; its toxic metabolite, normeperidine, may produce tremors, muscle twitches, hyperactive reflexes, and convulsions. Severe, catastrophic reactions have occurred in patients who take monoamine oxidase inhibitors or who have untreated hyperthyroidism.11 Patients should be advanced from IV opioids to oral opioids as soon as possible, keeping in mind that insufficient oral dosing may prolong hospitalization. Codeine, a weak analgesic, is not recommended, as between 4% to 12% of patients lack the enzyme to convert it to its active form, morphine.12
 ADJUVANT AND NONCONVENTIONAL ANALGESICS
ADJUVANT AND NONCONVENTIONAL ANALGESICS
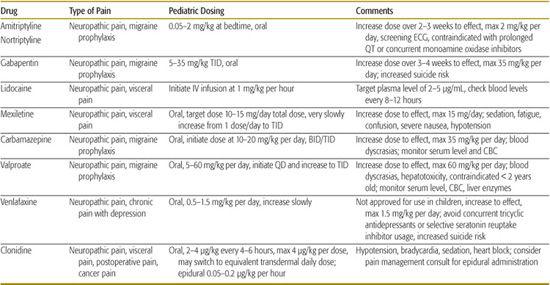
Most nociceptive pain (pain resulting from stimulation of nociceptors or pain receptors and transmitted over intact nerve fibers) is often well managed with the conventional analgesics discussed previously. However, neuropathic pain (pain resulting from injury to the nerve fibers) is typically described as burning, lancinating, or “pins and needles” and may require the addition of less conventional pain medications (see Table 113-3). The painful area is often subject to hyperalgesia or allodynia.
Many classes of drugs decrease neuropathic pain, including anticonvulsants, tricyclic anti-depressants (TCAs), selective serotonin/norepinephrine reuptake inhibitors (SSRI/SNRI), alpha-2 agonists, and capsaicin. Newer drugs such as pregabalin (a novel antiepileptic) and duloxetine (an SNRI) are effective for certain types of neuropathic pain but are not currently approved for use in children. Recently, the FDA has released warnings that SSRI/SNRIs can cause serotonin syndrome when used with triptans and that anticonvulsants, including valproate and gabapentin, are associated with suicidal ideation and behavior.
 LOCAL ANESTHETICS
LOCAL ANESTHETICS
Local anesthetics such as lidocaine and bupivacaine prevent depolarization and propagation of the nerve signal by blocking sodium channels along the nerve axon. They can be applied topically, by local injection, or by neuraxial infusion (in the vicinity of the spinal nerves). Although local anesthetics can be used safely in many situations, exceeding recommended doses can result in seizures, central nervous system depression, cardiac depression, arrhythmia, and death. Practitioners should be familiar with safe guidelines concerning drug concentration, tissue absorption, maximum dose, and administration route. Specific toxicities exist with certain local anesthetics such as prilocaine, which carries the risk of methemoglobinemia when used in neonates. Local anesthetics are some of the most successful analgesics for procedure-related pain. Some of the available are listed in eTable 113.4  .
.
Table 113-3. Adjuvant and Unconventional Analgesics
Lidocaine is the most commonly used local anesthetic for local infiltration, and doses should not exceed 5 mg/kg or 6 mg/kg with epinephrine-containing solutions. Bupivacaine is a long-acting local anesthetic for local infiltration, peripheral nerve block, or neuraxial administration. Doses should not exceed 2.5 mg/kg. Ropivacaine is another long-acting amide local anesthetic with a safer cardiac profile than bupivacaine. Doses should not exceed 2.5 to 3 mg/kg. Epinephrine has not been shown to prolong the effects of ropivacaine.
Table 113-4. Sedative Drugs, Dosing, and Side Effects
ADVANCED PAIN TECHNOLOGIES
Advanced pain technologies such as neuraxial application of medications through an epidural or intrathecal infusion, implantable pump systems, implantable nerve stimulators, and regional anesthesia catheter systems can produce significant anesthesia or analgesia when used in the appropriate patient population and by skilled practitioners. A pain management referral is recommended for evaluation of the patient for these modalities.
PROCEDURAL SEDATION
The goal of pediatric procedural sedation is to medicate children safely until they can tolerate an unpleasant procedure. One of the most important decisions is to recognize which patients require a higher level of care and to determine when referral to an anesthesiologist for general anesthesia is necessary.
The American Society of Anesthesiologists, the American College of Emergency Physicians, the American Academy of Pediatrics, and the American Academy of Pediatric Dentistry have published sedation policy guidelines.13-16 These guidelines address patient selection and preparation, equipment, monitoring, personnel, recovery, and discharge (see eTables 113.5, 113.6,13 and 113.714 ).
).
In 2006, the Joint Commission refined its sedation requirements to include a mandate that the licensed independent practitioner administering sedation medications be capable of rescuing the patient from one level of sedation deeper than the level anticipated. Other Joint Commission requirements include the following: the patient must have a history taken and a physical with a focus on the airway, reevaluation of the patient must occur immediately prior to the administration of sedation, the patient must be continuously monitored, and the practitioner must remain immediately available to intervene after induction. Discharge from the facility to the care of a responsible adult must follow pre-established discharge guidelines and expectations.
Patients who qualify for minimal to deep sedation should have no more than mild systemic disease that is well controlled (ASA Physical Status Classification I & II) (see Table 112-3). Children with anatomic airway abnormalities or problems are at increased risk and deserve special consideration. The child must be accompanied by a responsible adult, and it is preferable to have two chaperones for children transported in safety seats. The sedation facility must have monitors, equipment, medications, and personnel immediately available to treat all possible adversities, including airway/breathing problems, seizures, and cardiac arrest. A protocol for access to emergency services must be in place and does not override the sedation practitioner’s responsibility to provide initial emergency medical care. An on-site emergency kit must contain all age-appropriate drugs, equipment, and monitors to resuscitate and transport a non-breathing pediatric patient. Presedation documentation should include informed consent, detailed discharge instructions with any special considerations, and practitioner/facility contact information.
Fasting guidelines prior to sedation should be consistent with those required for general anesthesia and include 2 hours for clear liquids, 4 hours for breast milk, 6 hours for milk/formula and for light meals, and 8 hours for heavy or fatty meals. Documentation at the time of sedation includes a complete history and physical with special attention to the airway, patient contact information, and discharge prescriptions. Documentation during sedation includes a protocolized re-examination of the procedure plan (the so called time out), time-based drug dosages, level of consciousness, vital signs, oxygen saturation, and adverse events. After sedation, the patient must regain a level of consciousness that is safe for discharge with consideration for the length of effect of drugs used.
Discharge criteria include the following: satisfactory and stable cardiovascular function and airway patency, easy arousability with intact protective reflexes, age-appropriate speech, unaided ability to sit (if age appropriate), usually expected presedation responses for the young or disabled patient, and adequate hydration.14
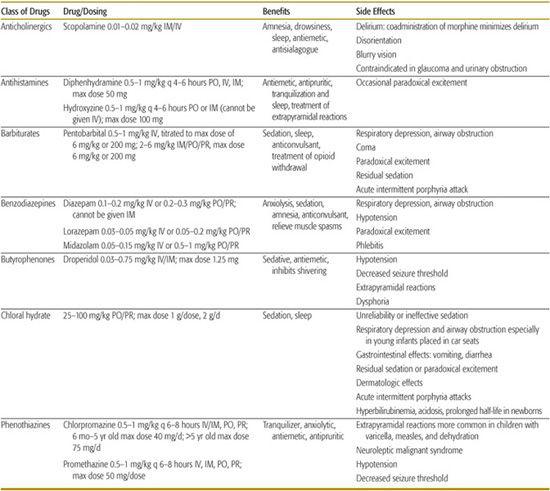
Medications for sedation and anxiolysis and sedation are numerous and cover many classes of drugs (see Table 113-417). Opioids and other respiratory-depressant drugs should be used with caution. Sedation should not be confused with analgesia, and strong analgesics should not be used to sedate a child for a nonpainful procedure. Adding a local anesthetic infiltration will often decrease the amount of sedation and analgesia required to perform a painful procedure.
Sedation can be performed safely by practitioners with special training. However, the potential for serious or fatal effect must be recognized, and the limits of experience and skill of the practitioner should not be exceeded.
REFERENCES
See references on DVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


