The concept was annunciated by Haberlandt, but steroid chemistry was not ready. The extraction and isolation of a few milligrams of the sex steroids required starting points measured in gallons of urine or thousands of pounds of organs. Edward Doisy processed 80,000 sow ovaries to produce 12 mg of estradiol.
Russell Marker
The supply problem was solved by a cantankerous iconoclast, Russell E. Marker, who completed his thesis, but not the course work, for his Ph.D. The following story is derived from Marker’s own words, in an autobiographical article and from a 2-hour interview for the oral history archives of the Chemical Heritage Foundation in Philadelphia.
3,
4Marker, born in 1902 in a one-room log cabin on a farm near Hagerstown, Maryland, received his bachelor’s degree in organic chemistry and his
master’s degree in colloidal chemistry from the University of Maryland. Although he had completed his work for a Ph.D., his supervisor, Morris S. Kharasch, announced that Marker still lacked some required chemistry courses. Considering the courses a waste of time, Marker said, “The hell with it,” and abruptly left.
After leaving the University of Maryland, Marker worked first in the laboratory of the Naval Powder Factory, then with the Ethyl Gasoline Corporation, where in 1926 he developed the system of octane rating of gasoline. Frank Whitmore, dean of Pennsylvania State College, now Pennsylvania State University, visited Marker at Ethyl. Impressed with his work, Whitmore said, “If you’re ever looking for a job, let me know.”
From 1927 to 1934, Marker worked at the Rockefeller Institute, publishing a total of 32 papers on configuration and optical rotation as a method of identifying compounds. He became interested in steroid chemistry, but he was told to continue with his work in optical technology. Instead, Marker called Dean Whitmore at Penn State.
In September 1935, Marker moved to Penn State at a reduced salary, from $4,400 per year at Rockefeller to $1,800, but with the freedom to pursue any field of research. His work was supported mainly by research grants from the Parke-Davis pharmaceutical company. At that time, it required the ovaries from 2,500 pregnant pigs to produce 1 mg of progesterone. Marker decided to pursue the goal of an abundant and inexpensive supply of progesterone, and for several years he concentrated on urine from pregnant animals. Then in 1939, Marker devised the method, called the Marker degradation, to convert a sapogenin molecule into a progestin.
Marker was convinced that the solution to the problem of obtaining large quantities of steroid hormones was to find plants in the family that includes the lily, the agave, and the yam that contained sufficient amounts of diosgenin, a plant steroid, a sapogenin, that could be used as a starting point for steroid hormone production. He discovered that a species of Trillium, known locally as Beth’s root, was collected in North Carolina for the preparation of Lydia Pinkham’s Compound, popular at the time to relieve menstrual discomfort. A principal ingredient in Beth’s root was diosgenin, but the rhizome was too small to provide sufficient amounts for commercial production.
Marker’s search for an appropriate plant took him to California, Arizona, and Texas. Spending his summer vacations in the Southwest and Mexico collecting sapogenin-containing plants, Marker’s laboratory analyzed more than 100,000 lbs of over 400 different species of plants. Marker discovered that the roots of the Dioscorea plant (a wild yam) were the richest source of sapogenins.
On a visit to Texas A & M University, Marker found a picture of a large Dioscorea (Dioscorea mexicana) in a book that he just happened to pick up and browse through while spending the night at the home of a retired botanist who was helping him collect diosgenin-containing plants. After returning to Pennsylvania, he traveled by train for 3 days to search for this Dioscorea in Mexico.
Marker first went to Mexico City in November 1941, but his effort was blocked by the lack of a plant-collecting permit from the Mexican government. He returned in January 1942, and the American Embassy arranged for a Mexican botanist who had a collecting permit to accompany Marker to Veracruz. Marker rented a truck with a driver, and when the botanist arrived at Marker’s hotel, he was accompanied by his girlfriend and her mother, who served as the girl’s chaperone. Marker was forced to take the entire group. They covered 80 miles the first day, staying overnight in Puebla. The next day, the drive to Tehuacan was a shorter trip, but the botanist insisted on a 2-day stay devoted to his own collection of specimens. Then next morning, the botanist refused to go any further, claiming that the natives had discovered Marker was American and wanted nothing to do with him. They turned around, managed to overcome a breakdown of the truck near Puebla, and made it back to Mexico City 5 days after starting, with nothing to show for the trip.
The next day, a Monday morning, Marker reported to the American Embassy and was advised to leave Mexico. It was just after Pearl Harbor and Mexico was being courted by Germany. The Embassy was concerned for the safety of Americans traveling in Mexico. Instead of returning home, Marker took an overnight bus to Puebla, arriving after midnight, and boarded a second bus that already held pigs and chickens in addition to a few passengers. He arrived in Orizaba the next morning, and fortunately there was a small hotel next to the bus terminal. Marker remembered that the botany book in which he first read a description of D. mexicana indicated that the plant, a wild yam vine that grows up trees in the mountains of southern Mexico, could be found along a stream that crossed the road between Orizaba and Cordoba. He climbed aboard the local bus to Cordoba, which he stopped and disembarked when the bus drove through a large stream crossing the road about 10 miles after leaving Orizaba. He found a small country store next to the road, owned by an Indian named Alberto Moreno.
Moreno did not speak English; Marker did not speak Spanish. But somehow, Marker conveyed his desire to obtain the Dioscorea that was known locally as “cabeza de negro,” black tubers. Moreno in turn somehow made Marker understand that he should return the next morning. And there in the store, the next morning, were two plants, each in a bag that Moreno placed on the roof of the next bus back to Orizaba. Each tuber was 9 to 12 in long and consisted of white material like a turnip; it was used by local Mexicans as soap and as a poison to catch fish. When Marker got off the bus in Orizaba, both bags were missing. A policeman was there, but it became apparent he was there to collect a fee for the return of the bags. Marker gave him what he had, a 10-dollar bill, but that only retrieved one bag, which he managed to smuggle back to Pennsylvania.
Marker used only a portion of the plant to isolate diosgenin. In February 1942, he took the remainder to the Parke-Davis chemists in Detroit. Demonstrating his process for obtaining diosgenin, Marker convinced the director of research, Oliver Kamm, that he was on to something, a source for raw
material that could provide for the commercial production of hormones. Unfortunately, they could not convince the president of Parke-Davis, nor could Marker convince anyone at several other companies.
Unable to obtain support from the pharmaceutical industry, Marker, drew on half of his life savings and returned to Mexico in October 1942. He arranged with Albert Moreno to collect the roots of the Mexican yam. Marker paid Mexican medical students to collect the yams. The students were arrested when farmers reported that their yams were being stolen, but not before Marker had enough to prepare a syrup.
Back in the United States with his syrup, Marker arranged to work in the New York laboratory of a friend, Norman Applezweig, an organic chemist involved in steroid research, in return for one-third of whatever progesterone his syrup could yield.
5 He isolated diosgenin and synthesized 3 kg of progesterone, the largest lot of progesterone ever produced. United States pharmaceutical companies still refused to back Marker, and even his university refused, despite Marker’s urging, to patent the process.
Before Marker left Mexico, he looked through the yellow pages in a Mexico City telephone directory and found something he recognized, a company called “Laboratorios Hormona,” owned by a lawyer who was a Hungarian immigrant, Emeric Somlo, and a German immigrant who had both a medical degree and a Ph.D. in chemistry, Frederick A. Lehman.
… when the phone rang. A distant voice asked in barely comprehensible Spanish if he {Frederick Lehman} spoke English.
“Yes, of course.”
“I found your company’s name in the telephone book, since I recognized two words, ‘Laboratories’ and ‘Hormones.’ I have something you may be interested in: a cheap source for progesterone.”
“Who are you?”
“I am Marker, a steroid chemist.”
6
Visiting the company, Marker met Lehman, the minority owner of Laboratorios Hormona, who had the good sense to see where this was going. From his reading of the literature, he knew who Marker was; he knew the value of steroids; and he was a businessman. Lehman called his partner who was visiting New York and convinced him to return as soon as possible. The three men agreed to form a Mexican company for the production of hormones, and Marker returned to the United States, leaving behind a list of equipment and chemicals to be ordered.
Marker returned to Mexico in spring 1943 to collect plants and to check on progress at Laboratorios Hormona. He just happened to mention to Lehmann that he had 2 kg of progesterone. As soon as Marker returned to Pennsylvania, he received a phone call from Somlo who said that if Marker still had those 2 kg of progesterone he sure would like to see it; could he meet him in New York? Over dinner at the Waldorf-Astoria, Somlo offered Marker 40% of their new company in exchange for the progesterone, with a share in future profits. Marker arranged for a friend to deliver the progesterone
to Somlo in New York. Somlo had a small company in New York called Chemical Specialties, and the progesterone used in the first studies leading to oral contraception was obtained from this Syntex subsidiary.
In December 1943, Marker resigned from Pennsylvania State College and went to Mexico where he collected the roots of D. mexicana—10 tons worth! Marker chopped them up with a machete, and left the pieces to dry in the sun across from Moreno’s store in a small structure for drying coffee. It took 2 months of work in an old pottery shed in Mexico City to prepare several pounds of progesterone, worth $160,000, with the help of several young women who had little education and spoke no English.
Somlo suggested calling their new company Synthesis, but Marker insisted on some link to Mexico, and the three partners formed Syntex (from synthesis and Mexico), incorporated in March 1944. Marker moved into a new four-room laboratory, and over the next year, produced over 30 kg of progesterone and 10 kg of dehydroepiandrosterone. The price of progesterone fell from $200 to $50 a gram.
During this time, Marker received expenses, but he was not given his share of the profits or the 40% share of stock due to him. In March 1945, Somlo claimed there were no profits, but then admitted that the profits had been paid to the two partners in Mexico as salaries. Failing to reach a settlement, Marker left Syntex in May 1945, took some of his young female workers with him, and started a new company in Texcoco, called Botanica-Mex. He changed to Dioscorea barbasco, which gave a greater yield of diosgenin, and the price of progesterone dropped to $10 a gram, and later to $5.
After I broke up with Lehmann and Somlo, I chose a place east of Mexico City (Texcoco), where labor and water were plentiful. I there repeated my simple procedure of converting diosgenin into progesterone. My workers were happy but one day they came to me and said, “We all live on this dry-lake bed, and we come from very far away. If you want us to go on working for you, we need bicycles.” “Sure,” said Marker, “I’ll buy them for you, and you will pay them back from your salary.” The workers, happy with this offer, and the image of a white man with promise, celebrated drunkenly one evening. Late at night they went to a nearby quarry where a great effigy of the Aztec rain god was still attached by its back to the bedrock (It wasn’t moved to the museum until 1964). They then began chiseling my name over Tláloc’s right eyebrow, but were interrupted by angry villagers and had to run away after having carved only the first two letters.
3
The volcanic stone monolith of Tláloc the rain god was carved in a horizontal position sometime in the period of 400 B.C. to 200 A.D. On April 16, 1964, the unfinished statue was detached and transported on a day’s journey to Mexico City, and placed in a vertical position at the road entrance to the Museo Nacional de Antropologia, an imposing 168 tons, 23 ft high. The initials “MA” can be easily discerned at the right edge of the headdress; Marker’s workers obviously intended to place his full name across the entire
width. The evening arrival of the rain god was greeted by a crowd of 25,000 people. Despite the fact that it was the dry season, a record rainfall fell on the day the statue arrived!
7Marker’s new company was allegedly harassed, legally and physically, by Syntex, and in 1946 it was sold to Gideon Richter, which moved it to Mexico City and renamed it Hormosynth. Eventually it came under the ownership of Organon of Holland, which still uses it under the name of Quimica Esteroides. By the 1960s, several pharmaceutical companies were benefiting from the root-gathering operations in Mexico, closely regulated by the Mexican government that imposed annual quotas, about 43,000 tons, to balance harvesting with the new annual growth. Mexican yams provided the starting material for the manufacture of oral contraceptives for about 15 years, giving way to other sources, such as soya beans, methods for total synthesis, or microbial fermentation.
8In 1949, Marker retired to Pennsylvania to devote the rest of his life to traveling, and in 1959 he began an association with a French silversmith who had emigrated to Mexico City, and then with his son, Pedro Leites. After 1970, Marker turned to collecting paintings by Mexican artists. The artwork and the replicas of antique works in silver were successful businesses that allowed him, in the 1980s, to endow scientific lectureships at both Pennsylvania State University and the University of Maryland. In 1970, the Mexican government honored Marker and awarded him the Order of the Aztec Eagle; staying true to his irascible nature, he declined. In 1984, Pennsylvania State University established the annual Marker Lectures in Science and, in 1987, the Russell and Mildred Marker Professorship of Natural Product Chemistry. In 1987, Marker was granted an honorary doctorate in science from the University of Maryland, the degree he failed to receive in 1926.
In 1990, Marker was planning on a quiet visit to Mexico to present a plaque made in his honor by Pennsylvania State University to Adolfina Moreno, the daughter of Alberto, the owner of the small country store whom Marker met in 1942. Mexican scientists and pharmaceutical people learned of the visit, and that summer a chartered busload of fifty people retraced Marker’s trip from Mexico City to Orizaba.
6 Marker rode in a car with Frederico Lehman’s son, Pedro, who had become a distinguished chemist. Meeting in an auditorium at the University of Veracruz, Marker was honored by speeches and an engraved silver tray. After lunch at a local brewery, nearly 100 people made their way to the bridge over the Mezcala River. Marker entered the living quarters behind the store now owned by Adolfina. She tearfully thanked him and pointed to a nearby photo, her marriage picture from 50 years ago, with Marker in the wedding group. At the age of 92, Russell Earl Marker died in Wernersville, Pennsylvania, in 1995, from complications after a broken hip.
The Race for Cortisone
When Marker left Syntex, he took his know-how with him. Fortunately for Syntex, there still was no patent on his discoveries. George Rosenkranz left his native Hungary to study chemistry in Switzerland under the renowned
steroid chemist Leopold Ruzicka, who was awarded the 1939 Nobel Prize in Chemistry.
9 On the day Pearl Harbor was attacked, Rosenkranz was in Havana waiting for a ship to Ecuador where the chair in organic chemistry awaited him at the University of Quito. The ship never showed. Rebuffed by the national university in Cuba, Rosenkranz took a job with a local pharmaceutical firm for $25 per week. Because of his success in developing new products, he was soon earning $1,000 per month and directing a research program with Ph.D. candidates from the university. He was also learning how to be a business man; for example, he organized the shark-fishing business in Cuba in order to produce vitamin A from shark liver oil.
10The Rosenkranz laboratory was following Marker’s published techniques and making small amounts of progesterone and testosterone from sarsaparilla roots imported from Mexico. The news of this activity led to an invitation from Syntex to take over for Marker, with an option of buying 15% of Syntex stock, although the company was currently practically bankrupt.
Rosenkranz’s task was complicated by Marker’s secretiveness. He found reagents labeled with code words; Marker’s workers identified solvents by their weight and smell. Rosenkranz gave up on reconstructing Marker’s process, and worked out his own commercial manufacture of progesterone and testosterone from Mexican yams, and soon Syntex was making large profits providing the sex hormones as raw material to other pharmaceutical companies. Rozenkranz now had a large active laboratory that attracted a young chemist, Carl Djerassi. These men knew each other, meeting and interacting with each other at the Laurentian Hormone Conference, the annual meeting organized and directed by Gregory Pincus.
The Djerassi family lived in Bulgaria for hundreds of years after escaping Spain during the Inquisition.
11 Carl Djerassi, the son of a Bulgarian physician, was born in Vienna, as was his physician mother. Djerassi, age 16, and his mother fled the Nazi Anschluss and emigrated to the United States in 1939. A Jewish refugee aid organization placed Djerassi with a family in Newark, New Jersey. With a scholarship to Tarkio College in Tarkio, Missouri, he was exposed to Middle America, where he earned his way giving talks to church groups about Bulgaria and Europe. His education was further supported by another scholarship from Kenyon College in Ohio, where he pursued chemistry. After a year working for CIBA, Djerassi received his graduate degree from the University of Wisconsin. Returning to CIBA and being somewhat unhappy, he responded to an invitation to visit Syntex. Rosenkranz proposed that Djerassi head a research group to concentrate on the synthesis of cortisone. Djerassi’s initial reaction was that “the location of Syntex in the chemical desert of Mexico made the offer seem ludicrous.”
12 But the 26-year-old Djerassi, impressed by Rosenkranz and excited by the challenge to develop a method to synthesize cortisone, accepted the position and moved to Mexico City in the fall of 1949.
Earlier in 1949, Philip S. Hench, a Mayo Clinic rheumatologist, showed a movie at a medical meeting documenting crippled arthritic patients before treatment and the same patients active, even dancing, after daily injections
with cortisone. Cortisone can be converted to the more active cortisol (also called hydrocortisone), the major product of the adrenal cortex. Cortisone is produced by hydroxylation, which converts the oxygen attached at the 11 position to a hydroxyl group by adding a hydrogen.
Hench had obtained the very expensive cortisone through a biochemist at the Mayo Foundation, Edward C. Kendall, the discoverer of the thyroid hormone, thyroxine, who had been working with Lewis H. Sarett at Merck & Company to determine the structures of compounds isolated from extracts of the adrenal cortex and from cattle bile; cortisone was known as Kendall’s Compound E. Hench reported good results in 14 patients; his movie received a standing ovation,
13 and in 1950, Hench and Kendall were awarded the Nobel Prize in Physiology or Medicine. It was recognized that continuing regular treatment would be necessary, and the race was on to develop an easy and cheap method to synthesize cortisone and related drugs.
In Mexico City, Carl Djerassi was using the plant steroid diosgenin from the Mexican yam as the starting point. In 2 years’ time, Syntex achieved the partial synthesis of cortisone, reported in 1951.
14 The Syntex method never reached commercialization, however, because a more efficient process was developed by the Upjohn Company. Djerassi’s productivity at Syntex, 60 publications, attracted a job offer from Wayne State University.
15 Wanting all along to be in the academic world, Djerassi moved to Detroit in January 1951. Five years later, he took a leave of absence to return to Syntex, now Americanowned and a public company. Syntex’s topical corticoid anti-inflammatory products, Synalar and Neosynalar, came from Djerassi’s laboratory. Djerassi maintained his laboratory at Wayne State, and in 1959, when W.S. Johnson at Wisconsin moved to head the chemistry department at Stanford University, Djerassi joined him—a professorial position he held for the next 25 years.
The Upjohn Company and G.D. Searle & Company joined the competition to synthesize cortisone, with Upjohn, the bigger company, devoting over 150 scientists and technicians to the task. Upjohn leadership assigned a symbol to represent the project, a blow torch, making it clear that this was a heated race they wished to win.
16 G.D. Searle was a smaller company, but its participation in this race would cement a long-term relationship with Gregory Pincus.
G.D. Searle was founded in 1888 by Gideon Daniel Searle, a pharmacist in Indiana, to provide elixirs, syrups, and drugs directly to clinicians. Searle’s son, Claude, graduated from Rush Medical College in 1898 and developed a large, successful practice in Sabula, Iowa. In 1909, when his father suffered a stroke, the son returned to Chicago to manage the company, setting up a research department that developed new products. His son, Jack Searle, graduated from the University of Michigan with a degree in pharmacy, and succeeded his father as president of the company in 1936. He recruited Albert L. Raymond from the Rockefeller Institute to serve as director of research, working in new laboratories in Skokie, Illinois. Dramamine, to prevent motion sickness, and Banthine, to treat peptic ulcers, came from these laboratories.
By 1949, Raymond and the G.D. Searle company were supporting steroid research at the Worcester Foundation for Experimental Biology in Massachusetts, and Gregory Pincus, the cofounder of the Worcester Foundation, was a Searle consultant.
17 Pincus and Oscar Hechter had developed a perfusion method, pumping blood, serum, or a serum-like solution through fresh endocrine glands (adrenal glands, testicles, or ovaries) held in a glass apparatus and collecting the perfused fluid. Using the enzymes in the glands, precursors in the perfusing fluid were converted to the final products, hydrocortisone or the sex steroids. This was a method that could be used to produce commercial amounts of cortisone products.
The round-faced, balding, acerbic Oscar Hechter came to the Worcester Foundation in 1944 on a fellowship funded by G.D. Searle. Pincus assigned him the task of perfusing adrenal glands, with the aim of identifying the products of adrenal secretion and the hope of creating a system for commercial production. Five years later, Hechter presented the first positive results at a conference in Detroit in 1949.
18 At that same meeting, Hechter saw Hench’s movie and listened to his results. Hechter returned to the Foundation and urged that his project be given top priority. Pincus’s enduring relationship with Searle that yielded research support and new steroid compounds for almost never-ending testing began in earnest with the race for cortisone and his development of the perfusion system to use animal glands for the synthesis of steroid drugs. The perfusion system was complicated. It required the development of methods to maintain the animal organs, a web of glassware to infuse and collect appropriate perfusing solutions, and the separation and identification of the steroid products. At the moment of its coveted value in 1946, Pincus chose to sell his rights to Searle for only 1 dollar, allowing Searle to patent the process.
19 In return, Pincus obtained and tested steroids that could yield products for clinical use.
Responding to Pincus and Hechter’s success, the Searle company constructed rows of perfusion systems in their Skokie plant. Each contained a periodically replaced fresh beef adrenal gland, producing every few hours a large volume of perfused solution. The long-term plan was to engineer a more economical and profitable system. But in the meantime, Searle was able to provide substantial amounts of cortisone to clinical researchers throughout the United States.
At the same time, Merck ramped up Sarett’s 36-step synthesizing process from bile acids, and by the end of 1950, they were selling cortisone acetate to clinicians for a price that had been reduced from $200 per gram to $35. In Kalamazoo, Michigan, Upjohn chemists were pursuing a method based on the process used to make penicillin, conversion of precursors by microbes to the desired product. The work was headed by Durey H. Peterson, the son of Swedish immigrants. Peterson supported his education by playing semiprofessional baseball.
16 Early in his career, he developed nylon surgical suturing material as well as “Toni,” a product for home permanents to create curly hair. Peterson joined Upjohn in 1946 to work on antibiotics, but he almost immediately became part of the race to synthesize cortisone. Peterson
believed that lower microorganisms might possess the same enzymes used by adrenal glands to make cortisone, especially the difficult step of introducing an oxygen molecule to the structure. When told this could not be done, Peterson said, “The microorganisms do not know this.”
16Using paper chromatography methods developed by Alejandro Zaffaroni, Peterson and H.C. Murray attacked the problem, beginning in 1949. First they needed a microorganism. This they acquired, a fungus of the
Rhizopus species, by leaving an agar plate on the window sill of the “oldest and dirtiest laboratory at the Upjohn Company.”
16 In 1 year’s time, the two chemists proved the value of microorganisms in chemical synthesis. Their method used
Rhizopus nigricans to covert progesterone to 11-hydroxyprogesterone, that could in turn be processed into hydrocortisone, also called cortisol, the major corticosteroid secreted by the adrenal cortex.
By 1955, Upjohn had become the market leader, and Searle shut down its perfusion cells and quit the race. Upjohn’s commercialization of the methods developed by Peterson and Murray led to popular and successful products. But the Searle people had gained valuable experience that would eventually pay off with other synthesized hormones and products.
The Upjohn method used progesterone as the starting point, available in the early 1950s only from Syntex. George Rosenkranz’s laboratory at Syntex was also pursuing the industrial synthesis of cortisone, and in July 1951, Syntex was about to sign a contract with a large chemical firm to begin production. This never happened because of a phone call. Rosenkranz told the story: “I received a phone call from Upjohn asking me whether we would be able to accept an order for 10 tons of progesterone at 48 cents a gram.”
10 The quantity was unheard of, and Upjohn’s order remained a puzzle until the microfermentation method was published. Rosenkranz accepted the order, and Syntex found itself as the key supplier of progesterone to other companies.
The Synthetic Progestational Drugs, Norethindrone and Norethynodrel
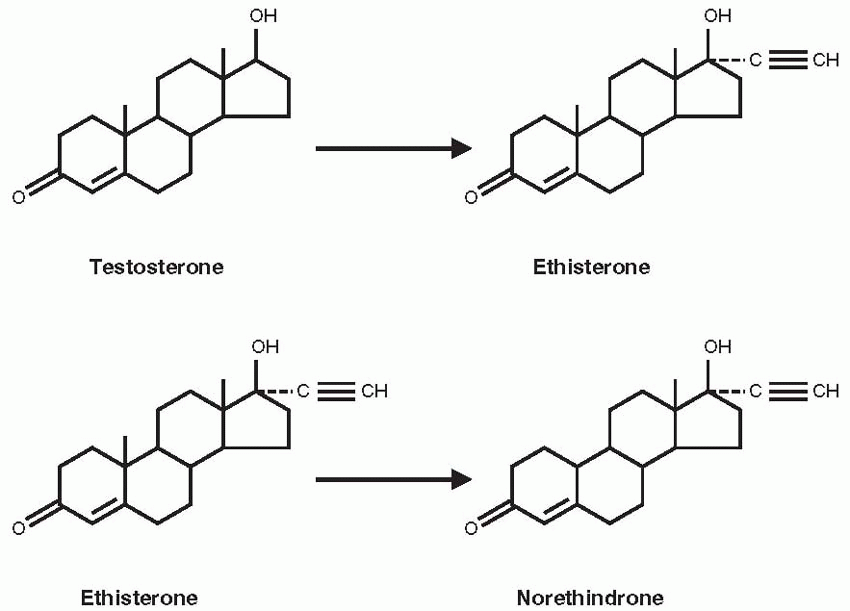
Djerassi and other Syntex chemists turned their attention to the sex steroids. They discovered that the removal of the 19-carbon from yam-derived progesterone increased the progestational activity of the molecule. The clue for this work came from Maximilian Ehrenstein at the University of Pennsylvania, who reported in 1944 that a potent progestational compound he had produced appeared to be progesterone without its carbon at the 19 position; henceforth, the 19-nor family of compounds indicated steroid chemical structures without the carbon atom at the 19 position.
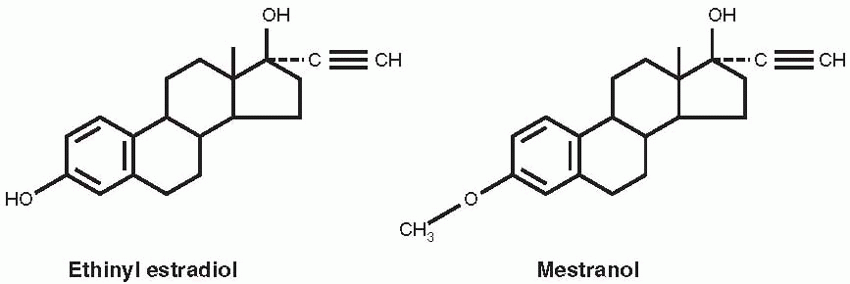
20 Chemists at Schering A.G. in Berlin had produced orally active versions of estradiol and testosterone in 1938, by substituting an acetylene group in the 17-position of the parent compounds. The resulting ethinyl estradiol later became the estrogen component in oral contraceptives. The ethinyl testosterone product was known as ethisterone, marketed in 1941, and the Syntex chemists reasoned that removal of the 19-carbon would increase the progestational potency of this orally active compound.
On October 15, 1951, norethindrone was synthesized at Syntex; the final steps were performed by Luis Miramontes, working on his undergraduate thesis in chemistry under Djerassi’s supervision.
12 The patent application was filed 6 weeks later on November 22, 1951, and the work was presented in April 1952 at the annual meeting of the American Chemical Society and published in 1954.
21 The greater potency of norethindrone, achieved by removing the 19-carbon of ethinyl testosterone, compared with progesterone was demonstrated in monkeys and then four women at the National Institutes of Health, reported in 1953, 1956, and 1957.
22,
23,
24 Syntex supplied norethindrone to many investigators, including Gregory Pincus. Edward T. Tyler first reported its clinical use in 1955 for the treatment of menstrual disorders.
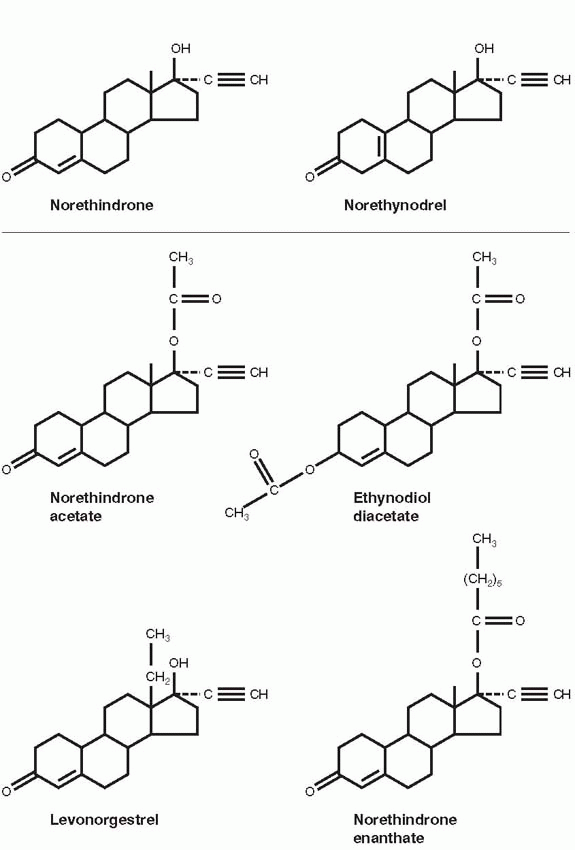
25Frank Colton, a chemist at G.D. Searle & Company, filed a patent for norethynodrel, a compound closely related to norethindrone, differing only in the position of the double bond, on August 31, 1953. The Polish-born Colton received his Ph.D. in chemistry from the University of Chicago. From 1949 to 1951, he was a research fellow working with Edward Kendall at the Mayo Foundation on the synthesis of cortisone. Colton joined Searle in 1951, along with Byron Riegel, to develop steroid drugs, succeeding with Nilevar, the first commercial anabolic agent marketed in 1956 and Aldactone, the antialdosterone antihypertensive agent introduced in 1959.
Norethynodrel was the result of a deliberate and planned program to create orally active agents with progestational activity. Later, Colton pointed out that although the Syntex and Searle chemists followed a similar path, they were independently pursuing the trail blazed by previous scientists.
17 Along the way, hundreds of compounds were sent to Pincus at the Worcester Foundation to test for ovulation inhibition in rabbits. Their best drug, norethynodrel, assigned the number SC-4642, was synthesized at Searle in a process that was considered to be significantly different from the Syntex method.
17Djerassi urged legal proceedings for patent infringement, claiming that norethynodrel was converted to Syntex’s compound, norethindrone, by gastric acid, but Parke-Davis, the American company licensing norethindrone, did not want to make waves presumably because Parke-Davis was supplying the antihistamine component of Searle’s best-selling product for motion sickness, Dramamine.
12 Pincus would ultimately choose the Searle compound, norethynodrel for clinical testing as an oral contraceptive, and Syntex, not having marketing capability, licensed norethindrone to other pharmaceutical companies. Norethindrone was tested as a contraceptive by Edward Tyler in Los Angeles and Joseph Goldzieher in San Antonio, Texas, but Parke-Davis chose not to pursue government approval, probably fearing religious reactions. Subsequently, Syntex turned to the Ortho division of Johnson & Johnson. By 1964, Ortho, Parke-Davis, and Syntex (now in California) were marketing oral contraceptives containing norethindrone or its acetate.
The creation of norethindrone and norethynodrel by the chemists was essential in the development of oral contraception because the natural hormone progesterone is relatively impotent given orally, requiring very large doses that even then do not achieve a uniform response. The synthetic
progestational agents are very active when administered orally, producing reliable effects with small doses.
A Wall Street entrepreneur, Charles Allen, acquired Syntex in 1956 for $2 million cash and a loan of $2 million to be paid from future profits.
9 Rosenkranz became president and CEO, Alejandro Zaffaroni, an Italian who emigrated from Montevideo, Uruguay, executive vice president. Zaffaroni obtained his Ph.D. in 1949 in biochemistry from the University of Rochester, developing a paper chromatography system that soon became a principal method of studying steroid hormones.
26 Rosenkranz met Zaffaroni at the Laurentian Hormone Conference in 1951. Their aim was to develop a pharmaceutical company on a foundation of research. Carl Djerassi, who had left for an academic position at Wayne State University, was recruited back to the company. Rosenkranz said, “We were the brilliant amateurs with a ‘can do anything’ attitude. We were like stem cells (though then none of us really knew the concept). We could differentiate into anything we desired. Production, finance, sales, marketing—all held no fear for us.”
9In 1961, the company moved to Palo Alto, California, influenced by Djerassi who was teaching at Stanford University. The growth of the company was meteoric, with blockbuster hits like Synalar, a topical corticoidsteroid for the treatment of psoriasis, and Naproxen, a nonsteroid, anti-inflammatory drug. Much of this success was to an innovative philosophy in the pharmaceutical business, “patent and publish.”
9 The Syntex scientists were encouraged to promptly publish their results, gaining the peer recognition that is such a motivating force for basic scientists. In 1994, Roche Holdings acquired Syntex for $5.3 billion.
Djerassi eventually left Syntex to become a full-time professor at Stanford University, and is now a playwright and novelist living in San Francisco. Zaffaroni started his own company in 1968, ALZA (after his own name), dedicated to new methods of drug delivery, such as a skin patch. ALZA was acquired by Johnson & Johnson in 2000.
Gregory Pincus
Gregory Goodwin (Goody) Pincus was born in 1903 in New Jersey, the son of Russian Jewish immigrants who lived on a farm colony founded by a Jewish philanthropic organization.
19 Pincus was the oldest of six children and grew up in a home of intellectual curiosity and energy, but even his family regarded him as a genius.
Pincus graduated from Cornell and went to Harvard to study genetics, joining Hudson Hoagland and B.F. Skinner as graduate students of W.J. Crozier in physiology, receiving degrees in 1927. Crozier’s hero was Jacques Loeb who discovered artificial parthenogenesis working with sea urchin eggs. Most importantly, Loeb was a strong believer in applying science to improve human life. Thus, Crozier, influenced by Loeb, taught Pincus, Hoagland, and Skinner (in reproductive biology, neurophysiology, and psychology, respectively) to apply science to human problems. This was to be the cornerstone of Pincus’s own philosophy.
Hoagland, after a short stay at Harvard, spent a year in Cambridge, England, and then moved to Clark University in Worcester, Massachusetts, to be the chair of biology at the age of 31. Pincus went to England and Germany, and returned to Harvard as an assistant professor of physiology.
Pincus performed pioneering studies of meiotic maturation in mammalian oocytes, in both rabbit and human oocytes. In 1934, Pincus reported the achievement of in vitro fertilization of rabbit eggs, earning him a headline in the New York Times that alluded to Haldane and Huxley. An article in Colliers depicted him as an evil scientist. By 1936, Harvard had cited Pincus’s work as one of the university’s outstanding scientific achievements of all time, but Harvard denied him reappointment in 1937.
At Clark University, Hudson Hoagland was in constant conflict with the president of the university, Wallace W. Atwood, the senior author of a widely used textbook on geography. In 1931, the Department of Biology consisted of one faculty member and his graduate student, and their chair, Hudson Hoagland. Hoagland, upset and angry over Harvard’s refusal to grant reappointment to his friend (suspecting that this was because of anti-Semitism), invited Pincus to join him. Hoagland secured funds for Pincus from philanthropists in New York City, enough for a laboratory and an assistant. This success impressed the two men, especially Hoagland, planting the idea that it would be possible to support research with private money.
Min-Chueh Chang was born in Tai Yuan, China, on October 10, 1908. In 1933, he earned a bachelor’s degree in animal psychology from the Tsing Hua University in Peking and stayed at the university as a teacher. Chang won a national competition in 1938 that funded study abroad. He chose to study agricultural science at Edinburgh University. After 1 year, he was pleased to receive an invitation from Arthur Walton to study the physiology of sheep sperm at The University of Cambridge, and he promptly accepted.
Chang received his Ph.D. in animal breeding under the direction of Walton and Sir John Hammond at the University of Cambridge in 1941. It was virtually impossible to leave England during the early years of World War II, and Chang continued to work at the University. In 1944, Chang planned to return to China, but first he wanted to spend a year in the United States. He wrote three letters to American scientists, and only Pincus answered, offering a fellowship at Clark University. Chang mistakenly assumed that a fellowship in the United States was the same as at the University of Cambridge where a Fellow was assured of a lifetime income. The successful recruitment of Chang by Pincus was to pay great dividends. Years later, Chang would direct the testing of new progestins to effectively inhibit ovulation in animals.
Soon Hoagland had put together a group of outstanding scientists, but because of his ongoing antagonism with President Atwood, the group was denied faculty status. Working in a converted barn, they were totally supported by private funds. By 1943, 12 of Clark’s 60 faculty were in the Department of Biology.
Frustrated by the politics of academia, Hoagland and Pincus (who both enjoyed stepping outside of convention) had a vision of a private research center devoted to their philosophy of applied science. Indeed, the establishment of the Worcester Foundation for Experimental Biology, in 1944, can be attributed directly to Hoagland and Pincus, their friendship for each other, and their confidence, enthusiasm, ambition, and drive. It was their spirit that turned many members of Worcester society into financial supporters of biologic science. Hoagland and Pincus accomplished what they set out to do. They created and sustained a vibrant, productive scientific institution in which it was a pleasure to work.
Although named the Worcester Foundation for Experimental Biology, the Foundation was located in the summer of 1945 across Lake Quinsigamond in a house on an estate in Shrewsbury. From 1945 to the death of Pincus in 1967, the staff grew from 12 to 350 (scientists and support people), 36 of whom were independently funded and 45 were postdoctoral fellows. The annual budget grew from $100,000 to $4.5 million. One hundred acres of adjoining land were acquired, and the campus grew to 11 buildings. In its first 25 years, approximately 3,000 scientific papers were published.
But in those early years, Pincus was the animal keeper, Mrs. Hoagland the bookkeeper, Chang was the night watchman, and Hoagland mowed the lawn. During the years of World War II, Pincus and Hoagland combined their interests in hormones and neurophysiology to focus on stress and fatigue in industry and the military.
Katharine Dexter McCormick (1875-1967) was a trained biologist, an early suffragist, and rich, inheriting millions from her mother and a McCormick fortune from her husband. She was the second woman to graduate from the Massachusetts Institute of Technology, socially conscious, and a generous contributor to family planning efforts. Her intervention with money, energy, incisive thinking, and persistent dedication was instrumental in the development of oral contraception. In 1904, she married Stanley McCormick, the son of Cyrus McCormick, the founder of International Harvester. Katharine’s husband suffered from schizophrenia, and she established the Neuroendocrine Research Foundation at Harvard to study schizophrenia. This brought her together with Hoagland, who told her of the work being done by Chang and Pincus who were seeking orally active progestins to inhibit ovulation.
Pincus attributed his interest in contraception to his growing appreciation for the world’s population problem, and to a 1951 visit in New York with Margaret Sanger, at that time president of the Planned Parenthood Federation of America. Sanger promised a small amount of money and expressed hope that a method of contraception could be derived from the laboratory work being done by Pincus and Chang. During this meeting, Pincus formulated his thoughts derived from his mammalian research. He envisioned a progestational agent in pill form as a contraceptive, acting like progesterone in pregnancy.
Margaret Sanger brought Pincus and Katharine McCormick together. On June 7, 1953, when 78-year-old Katharine met with 50-old Pincus at the Worcester Foundation and wrote him a check for $20,000; she promised him
another $20,000. A week later, Pincus and Hoagland met with Katharine and her lawyer. They signed a contract outlining the goals, the decision-making process, and the timetable. Pincus received a second check for $20,000, and Katharine agreed to fund laboratory improvements, which ended up as the completion of a new building in 1955.
Katharine’s contract with the Worcester Foundation stipulated that Pincus would provide written reports every 2 weeks. In addition, Pincus and John Rock, the Boston gynecologist performing the initial oral contraceptive studies in his patients, made many visits to Katharine’s home office on Beacon Street across the street from the Harvard Club. Katharine had Sara De Laney, her secretary, take careful notes in shorthand, and at the next visit De Laney read the transcribed notes to her boss so that she would be prepared. Periodically the principals met at the Worcester Foundation. Katharine peppered Pincus, Chang, and Rock with questions and urged them to stop wasting time. She found Pincus “imaginative and inspirational; Rock was informative and very realistic about medical work.” By now everyone was familiar with Katharine’s methods. She had earned their respect, and detailed reports on laboratory results, clinical planning, and budgets were immediately forthcoming. Time and time again, Katharine proved that she handled delays poorly, but she approached each meeting with an eagerness that slowly but surely was rewarded with success after 7 years and an expenditure of about $2 million of Katharine’s money.
In her last years, Katharine continued to support the work of Pincus and Chang. When testing the hundreds of compounds that yielded the progestational agents in birth control pills, Chang observed that some of them prevented implantation of fertilized eggs in rabbits. From 1962 to 1966, Chang and Pincus were pursuing a drug that could prevent pregnancy with one administration, a day or two after sexual intercourse. With Pincus’s death, this project was abandoned. It is not certain whether Chang and Pincus coined the phrase the “morning after” pill, but it is accurate to state that the concept came from Chang.
When Pincus and Chang began their studies, the focus was on inhibition of ovulation, first by progesterone, and then by synthetic progestins. Chang’s contribution was easy to overlook. Chang worked away in his laboratory, and it was Pincus who was highly visible, raising the money and providing direction. Chang started by repeating the experiments reported by Makepeace in 1927, documenting that progesterone could inhibit ovulation.
60 The first experiment was on April 25, 1951, and Chang quickly moved to testing the newly synthesized progestins from Searle and Syntex.
By December 1953, three synthetic progestins were selected as the most potent and effective in inhibiting ovulation: norethindrone from Syntex, and Searle’s norethynodrel and norethandrolone. The animal and human results were published in
Science in 1956.
27,
28 In 1957, these three compounds were approved for the treatment of menstrual disorders with the trade names of Norlutin, Enovid, and Nilevar, respectively.
It was Pincus who made the decision to involve a physician because he knew human experiments would be necessary. John Rock, chief of gynecology and obstetrics at Harvard, met Pincus at a scientific conference
and discovered their mutual interest in reproductive physiology. Rock and his colleagues pursued Pincus’s work. Using oocytes from oophorectomies, they reported in vitro fertilization in 1944, the first demonstration of fertilization of human oocytes in vitro. Rock was interested in the work with progestational agents, not for contraception, however, but because he hoped the female sex steroids could be used to overcome infertility.
In their first collaborative study, Pincus and Rock administered oral progesterone, 300 mg/d. Pincus suggested a 20-day regimen beginning on day 5 of the menstrual cycle.
29 He had two reasons for choosing this regimen: (1) it covered the time period during which nearly all, if not all, ovulations occurred, and (2) the withdrawal menstrual bleed at the conclusion of the treatment period would mimic the timing of a normal menstrual cycle and reassure the women that they were not pregnant. The first study involved 33 volunteers who ovulated regularly but had been infertile for 2 years. The women were treated for one to three cycles after a baseline control month. About 85% of the treated women did not ovulate during the treatment cycles. Not one became pregnant during treatment, pleasing Pincus who all along was aiming for contraception, and four became pregnant after treatment, pleasing Rock who initially was motivated by his pursuit of the “rebound” phenomenon for the treatment of infertility.
Sanger and McCormick needed some convincing that Rock’s Catholicism would not be a handicap, but they were eventually won over because of his stature. Rock was a physician who literally transformed his personal values in response to his recognition of the problems secondary to uncontrolled reproduction. With the help of Luigi Mastroianni, the first administration of synthetic progestins to women was to Rock’s patients in 1954. Of the first 50 patients to receive 10 to 40 mg of synthetic progestin (a dose extrapolated from the animal data) for 20 days each month, all failed to ovulate during treatment (causing Pincus to begin referring to the medication as “the pill”), and 7 of the 50 became pregnant after discontinuing the medication, again pleasing Rock, who all along was motivated to treat his infertile patients.
In 1956, with Celso-Ramon Garcia and Edris Rice-Wray, working in Puerto Rico, the first human trial was performed. The initial progestin products were contaminated with about 1% mestranol. In the amounts being used, this added up to 50 to 500 µg of mestranol, a sufficient amount of estrogen to inhibit ovulation by itself. When efforts to provide a more pure progestin lowered the estrogen content and yielded breakthrough bleeding, it was decided to retain the estrogen for cycle control, thus establishing the principle of the combined estrogen-progestin oral contraceptive. Early clinical trials were also conducted by J.W. Goldzieher in San Antonio and E.T. Tyler in Los Angeles.
Pincus, a longtime consultant to Searle, picked the Searle compound for extended use, and with great effort, convinced Searle that the commercial potential of an oral contraceptive warranted the risk of possible negative public reaction. Pincus also convinced Rock, and together they pushed the U.S. Food and Drug Administration (FDA) for acceptance of oral contraception. In 1957, Enovid was approved for the treatment of miscarriages and menstrual
disorders, and on June 23, 1960, for contraception. Neither Pincus nor the Worcester Foundation got rich on the pill; alas, there was no royalty agreement.
The pill did bring Pincus fame and travel. There is no doubt that he was very much aware of the accomplishment and its implications. As he traveled and lectured in 1957, he said: “How a few precious facts obscurely come to in the laboratory may resonate into the lives of men everywhere, bring order to disorder, hope to the hopeless, life to the dying. That this is the magic and mystery of our time is sometimes grasped and often missed, but to expound it is inevitable.”
30Pincus was the perfect person to bring oral contraception into the public world, at a time when contraception was a private, suppressed subject. Difficult projects require people like Pincus. A scientific entrepreneur, he could plow through distractions. He could be hard and aggressive with his staff. He could remain focused. He hated to lose, even in meaningless games with his children. Yet he combined a gracious, warm, charming manner with his competitive hardness. He was filled with the kind of self-confidence that permits an individual to forge ahead, to translate vision into reality. Pincus died in 1967 (as did Katharine McCormick at the age of 92) of myeloid metaplasia. Rock died in 1984, at the age of 94. Chang died in 1991 at the age of 82, and was buried in Shrewsbury, near his laboratory and close to the grave of Pincus.
Pincus wrote his book,
The Control of Fertility, in 1964-1965, because “a break came in the apparent dam to publication on reproductive physiology and particularly its subdivisions concerned with reproductive behavior, conception, and contraception.”
30
“We have conferred and lectured in many countries of the world, seen at first hand the research needs and possibilities in almost every European, Asiatic, Central, and South American country. We have faced the hard fact of overpopulation in country after country, learned of the bleak demographic future, assessed the prospects for the practice of efficient fertility control. This has been a saddening and a heartening experience; saddening because of the sight of continuing poverty and misery, heartening because of the dedicated colleagues and workers seeking to overcome the handicap of excess fertility and to promote healthy reproductive function. Among these we have made many friends, found devoted students.”
30
Syntex, a wholesale drug supplier, was without marketing experience or organization. By the time Syntex had secured arrangements with Ortho for a sales outlet, Searle marketed Enovid in 1960 (150 µg mestranol and 9.85 mg norethynodrel). Ortho-Novum, using norethindrone from Syntex, appeared in 1962. Wyeth Laboratories introduced norgestrel in 1968, the same year in which the first reliable prospective studies were initiated. It was not until the late 1970s that a dose-response relationship between problems and the amount of steroids in the pill was appreciated. Health care providers and patients, over the years, have been confronted by a bewildering array of different products and formulations. The solution to this clinical dilemma is relatively straightforward, the theme of this chapter: use the lowest doses that provide effective contraception.