Background
Fetal-neonatal alloimmune thrombocytopenia affects approximately 1 of 1000 live births, most of which are not severely thrombocytopenic. Despite effective treatment with intravenous gammaglobulin and/or prednisone, antenatal management of a subsequent affected pregnancy is complicated by the risks associated with fetal blood sampling. Furthermore, there are no biomarker(s) of high risk other than the occurrence of intracranial hemorrhage in a previous sibling. Management of these high-risk pregnancies requires intensive treatment initiated at 12 weeks of gestation.
Objective
The objective of the study was to evaluate whether empiric escalation of therapy at 32 weeks allows the omission of fetal blood sampling in all fetal-neonatal alloimmune thrombocytopenia–affected patients. Specifically, we sought to determine whether intensive intravenous gammaglobulin–based regimens for the treatment of a subsequent fetal-neonatal alloimmune thrombocytopenia–affected pregnancy followed by empirically escalated intravenous gammaglobulin and prednisone treatment would increase the fetal platelet count and thus safely allow omission of fetal blood sampling in the antepartum management of these patients.
Study Design
In this prospective, multicenter, randomized controlled study, 99 women with fetal-neonatal alloimmune thrombocytopenia whose prior affected child did not have an intracranial hemorrhage were randomized to receive an intensive intravenous gammaglobulin–based regimen: 2 g/kg per week or intravenous gammaglobulin 1 g/kg per week plus prednisone 0.5 mg/kg per day, starting at 20–30 weeks of gestation. Escalated therapy (intravenous gammaglobulin 2 g/kg per week plus prednisone 0.5 mg/kg per day) was recommended and usually initiated at 32 weeks when fetal counts were <50,000/mL 3 or when fetal blood sampling was not performed. The preliminary report of this study from 2007 demonstrated the efficacy of both intravenous gammaglobulin–based regimens in most patients. Most patients who underwent fetal sampling had adequate fetal counts and therefore did not have their treatment escalated. This post hoc analysis describes the 29 fetuses who had their treatment escalated either because they had low counts at 32 weeks or when sampling was not performed. This study explored whether the empiric escalation of treatment at 32 weeks was sufficiently effective in increasing fetal platelet counts in these patients.
Results
Mean fetal and birth counts of fetuses randomized to each of the 2 initial treatment groups were all >100,000/mL 3 . Three neonates had an intracranial hemorrhage; all 3 were grade 1 and all had birth platelet counts >130,000/mL 3 . In a post hoc analysis, 19 fetuses undergoing fetal blood sampling at 32 weeks had fetal platelet counts <50,000/mL 3 despite their initial treatment. Of these 19, birth platelet counts were >50,000/mL 3 in 11 of 13 fetuses who received escalated treatment compared with only 1 of 6 of those who did not ( P = .01); only 3 fetuses that received initial therapy followed by escalated treatment had birth platelet counts <50,000/mL 3 and none had an intracranial hemorrhage. The platelet counts of 14 of 15 fetuses that received empirically escalated treatment without sampling were >50,000/mL 3 at birth. In addition, none of these had an intracranial hemorrhage.
Conclusion
The 2 recommended protocols of intensive initial treatment followed by empiric escalation of therapy at 32 weeks of gestation are reasonably safe, effective in increasing fetal platelet counts, and allow omission of fetal blood sampling by increasing the fetal platelet count in almost all cases.
Fetal and neonatal alloimmune thrombocytopenia results from parental human platelet antigen incompatibility, usually of human platelet antigen-1a. If maternal sensitization occurs, mothers produce anti–human platelet antigen-1a antibodies directed at fetal platelets. Fetal-neonatal alloimmune thrombocytopenia affects approximately 1 in 1000 live births; most are not severely affected. Without universal screening, diagnosis usually occurs after the birth of a markedly thrombocytopenic neonate; as many as 11–21% experience intracranial hemorrhage, most commonly in utero.
When managing the mother’s subsequent fetal-neonatal alloimmune thrombocytopenia–affected pregnancy, sequential studies over 30 years have developed intravenous immunoglobulin–based protocols that increase the fetal platelet count and largely avoid intracranial hemorrhage. Intravenous gammaglobulin 1 g/kg per week, commonly used to treat the next fetal-neonatal alloimmune thrombocytopenia–affected pregnancy, is insufficient to increase the platelet count in the most severely thrombocytopenic fetuses that are most at risk of hemorrhage. Current recommendations therefore advocate more intensive initial therapy as in this study.
In early studies, knowledge of the fetal platelet count before and 4–6 weeks after initiating treatment was critical to assessing response and determining whether additional therapy was required. However, complications associated with fetal blood sampling such as fetal demise, hemorrhage, bradycardia, need for cesarean delivery, were frequent and concerning. Therefore, it seemed appropriate to empirically initiate an aggressive treatment early in gestation without determining the fetal platelet count. With this approach, fetal blood sampling was to be performed at 32 weeks because if complications necessitated immediate delivery, the neonatal outcome would likely be good, given the relatively advanced gestational age.
A noninvasive approach to antenatal management of fetal-neonatal alloimmune thrombocytopenia using intravenous gammaglobulin alone at a dose of 1 g/kg per week has been reported from The Netherlands ; however, 13% of their treated subjects had birth platelet counts <50,000/mL 3 . Intravenous gammaglobulin 1 g/kg per week was inadequate in 11 of 13 cases in which the initial fetal platelet count was <20,000/mL 3 . In addition, cases of intracranial hemorrhage have occurred in patients receiving this treatment.
This study used initial intensive intravenous gammaglobulin–based therapy for fetal-neonatal alloimmune thrombocytopenia–affected mothers with a prior affected child who had not experienced an intracranial hemorrhage. They were randomized between 2 initial regimens, each of which was more intensive than 1 g/kg per week of intravenous gammaglobulin: (1) intravenous gammaglobulin infusion 1 g/kg twice per week, and (2) intravenous gammaglobulin infusion 1 g/kg once per week with prednisone 0.5 mg/kg per day. Therapy was escalated if the fetal platelet count was low.
Data from a preliminary report of the 67 delivered patients revealed the following: (1) the regimens were similar in efficacy, and (2) despite intensive initial treatment, 20% of the patients in each arm had unacceptably low fetal platelet counts at 32 weeks. Ten patients who underwent fetal blood sampling had complications, necessitating emergency caesarean delivery (although there was no mortality and no intracranial hemorrhage).
The objective of this report is to evaluate whether intensive initial therapy followed by empiric escalation at 32 weeks allows the omission of fetal sampling in all patients.
Materials and Methods
From May 2001 through July 2013, 102 mothers with documented fetal-neonatal alloimmune thrombocytopenia and no prior children who had had an intracranial hemorrhage participated in this randomized controlled study ( ClinicalTrials.gov , number NCT00194987 , under biologic-based investigational new drug number 3446). Three randomized women, all from arm B, chose to discontinue the study. Five pregnancies of twin births were included in the study; an analysis of outcomes with inclusion of only the first twin or both twins did not change the significance of the findings of any analyses.
Patients were treated in 35 American and 1 Canadian center. The institutional review boards of each participating center approved the protocol, and local informed consent was obtained from each participant.
The inclusion criteria required parental human platelet antigen incompatibility and a maternal antibody directed against the incompatible human platelet antigen or a mother with 2 successive thrombocytopenic neonates whose thrombocytopenia eventually resolved. Paternal heterozygosity or unknown paternal type mandated amniocentesis to determine the fetal human platelet antigen genotype.
Randomization was computer generated into 2 treatment arms. Group A received intravenous gammaglobulin 2 g/kg per week divided into 2 infusions per week, and group B received intravenous gammaglobulin 1 g/kg per week with prednisone 0.5 mg/kg per day. Therapy without prior fetal blood sampling was initiated at 20, but not later than 30, weeks of gestation. Mothers initially underwent fetal blood sampling at approximately 32 weeks of gestation. Betamethasone was administered prior to the procedure.
Response to the initial therapy was originally defined as a fetal platelet count of ≥30,000/mL 3 , but this was increased to ≥50,000/mL 3 when a fetus in group A with a fetal platelet count of 48,000/mL 3 at 32 weeks that did not receive escalated therapy was found to have a platelet count of 14,000/mL 3 at birth.
The protocol called for all nonresponders discovered at fetal blood sampling to be managed by escalating therapy to a total of 2 g/kg per week of intravenous gammaglobulin and 0.5 mg/kg per day of prednisone. In addition, some mothers refused to undergo fetal blood sampling or were unable to undergo the procedure for technical reasons, and all of these women were empirically escalated to the same regimen given to the documented nonresponders. Increasingly after 2005, mothers declined fetal blood sampling because of concern for the complications associated with the procedure ( Supplemental Table 1 ).
The primary endpoint of the randomized study was the number of patients who had a birth platelet count ≥50,000/mL 3 , a number considered adequate to prevent intracranial hemorrhage and to allow vaginal delivery. If an in utero platelet transfusion was administered within 1 week prior to delivery for fetal thrombocytopenia, the pretransfusion platelet count was considered the birth count. Screening for fetal intracranial hemorrhage was performed with serial ultrasound examinations in utero and on all neonates after birth, regardless of their platelet count. Secondary outcome variables included fetal platelet counts determined by fetal blood sampling, adverse events occurring within 2 weeks of the fetal blood sampling, and adverse events associated with maternally administered intravenous gammaglobulin infusions ( Supplemental Table 2 ).
For the post hoc analysis of this study, fetuses and newborns were divided into 3 nonrandomized groups: those that underwent fetal blood sampling and had platelet counts ≥50,000/mL 3 (these patients were not considered in the analysis), those that underwent fetal blood sampling and had platelet counts <50,000/mL 3 , and those that did not undergo fetal blood sampling. The analyses compared the outcomes of those who did and did not receive escalated therapy among 19 fetuses with a fetal platelet count of <50,000/mL 3 as well as describing those who empirically received escalated therapy without sampling.
Statistical analyses included a Student t test, Fisher exact test, and Wilcoxon rank sum test. In view of the multiple analyses, a 2-tailed value of P ≤ .01 was considered significant, whereas values of P > .01 and P ≤ .10 were a trend. A 2007 analysis compared the 2 groups, focusing on efficacy. The study was closed at 102 pregnancies when it became clear that it was futile to continue the study to demonstrate a difference between the 2 arms.
Results
Overall results
Ninety-nine women completed the study ( Figure , A and B). Group A contained 51 women (55 fetuses including 4 sets of twins, Figure , A) and group B contained 48 women (49 fetuses including 1 set of twins, Figure , B). Ninety-three cases were a result of human platelet antigen-1a incompatibility (47 in arm A and 46 in arm B), 3 of which were also human platelet antigen-5a incompatible, 7 were human platelet antigen-5a alone, 1 was human platelet antigen-11b incompatibility, and 1 was unknown.
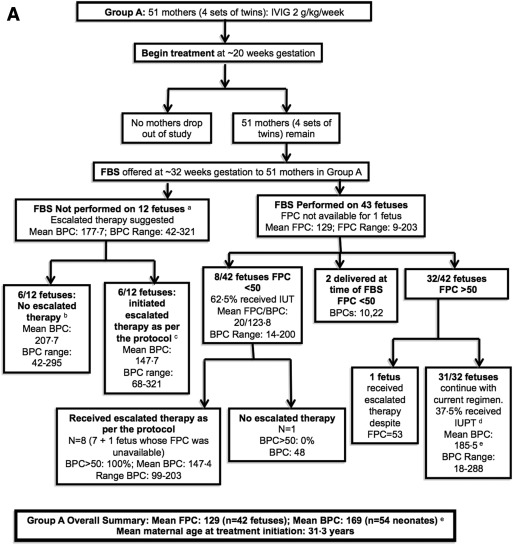
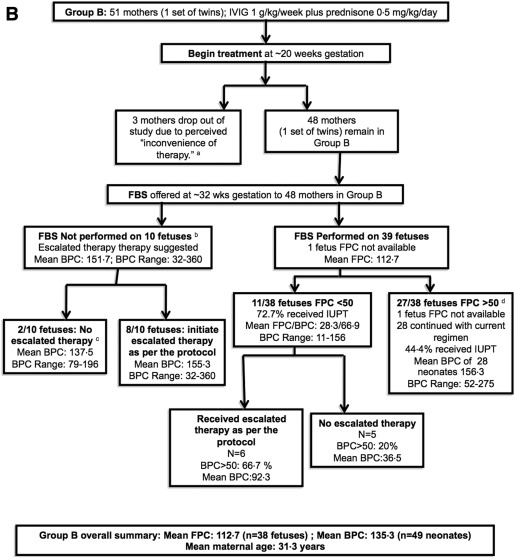
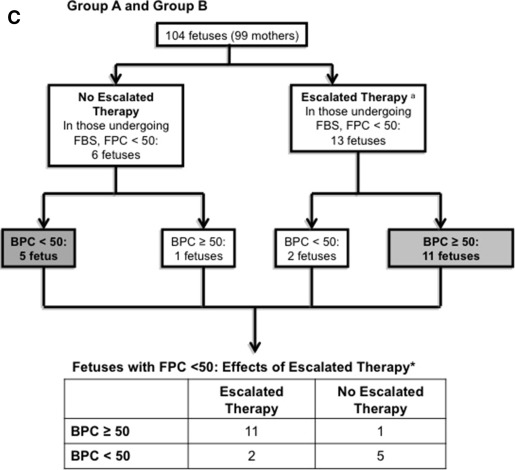
Both regimens were equally safe and effective
The average gestational age at delivery, number of deliveries by cesarean delivery, and average birthweights were similar between the groups ( Table ). The birth platelet counts of the treated neonates were substantially higher than those of the prior affected but untreated siblings: group A, 169,000/mL 3 vs 28,000/mL 3 ( P < .001), and group B, 135,000/mL 3 vs 39,000/mL 3 ( P < .001).
| Group A (IVIG 2 g/kg per wk) (n = 55) a | Group B (IVIG 1 g/kg per wk plus prednisone 0.5 mg/kg per d) (n = 49) | |
|---|---|---|
| FBS | ||
| Average GA, wks, at FBS (range) | 31.7 (n = 42) | 32.1 (n = 38) |
| Average FPC at FBS (range) | 129 (9–303) | 112.7 (11–257) |
| Birth | ||
| Average GA, wks, at delivery (range) | 36.1 (29.4–40) (n = 55) | 36.1 (32–39) (n = 49) |
| Number of deliveries via cesarean delivery | 43 | 32 |
| Average birthweight, kg (range) | 2.90 (0.96–4.31) (n = 54) | 2.89 (1.81–4.05) (n = 45) |
| BPC <50 × 10 9 /L, % b | 6 (11.1) | 7 (14.3) |
| Mean BPC c | 169 | 135.3 |
| Sibling | ||
| Mean/median sibling BPC, n | 27.6/17 (47) | 39.4/20.5 (44) |
a BPC unavailable for 1 neonate in arm A
b If an in utero platelet transfusion was administered within 1 week prior to delivery, the pretransfusion platelet count was considered the birth platelet count
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


