Mechanisms of Normal Hemostasis
A major concept in understanding the pathophysiology and management of obstetrical hemorrhage is the mechanism by which hemostasis is achieved after normal delivery. Recall that near term an incredible amount of blood—at least 600 mL/min—flows through the intervillous space (Pates, 2010). As described in Chapter 5 (p. 96), this prodigious flow circulates through the spiral arteries, which average 120 in number. Also recall that these vessels have no muscular layer because of their endotrophoblastic remodeling, which creates a low-pressure system. With placental separation, these vessels at the implantation site are avulsed, and hemostasis is achieved first by myometrial contraction, which compresses this formidable number of relatively large vessels (Chap. 2, p. 26). Contractions are followed by clotting and obliteration of vessel lumens.
If after delivery, the myometrium within and adjacent to the denuded implantation site contracts vigorously, fatal hemorrhage from the placental implantation site is unlikely. Importantly, an intact coagulation system is not necessary for postpartum hemostasis unless there are lacerations in the uterus, birth canal, or perineum. At the same time, however, fatal postpartum hemorrhage can result from uterine atony despite normal coagulation. Adhered placental pieces or large blood clots that prevent effective myometrial contraction will serve to impair hemostasis at the implantation site.
 Definition and Incidence
Definition and Incidence
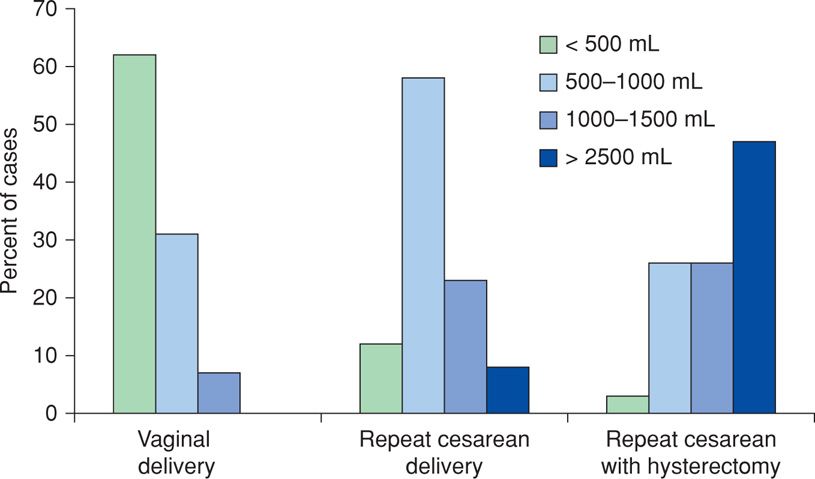
Traditionally, postpartum hemorrhage has been defined as the loss of 500 mL of blood or more after completion of the third stage of labor. This is problematic because almost half of all women delivered vaginally shed that amount of blood or more when losses are measured quantitatively (Pritchard, 1962). These results are depicted in Figure 41-1 and show further that approximately 5 percent of women delivering vaginally lose more than 1000 mL of blood. These studies also showed that estimated blood loss is commonly only approximately half the actual loss. Because of this, estimated blood loss in excess of “average” or 500 mL should alert the obstetrician to possible excessive bleeding. Calibrated delivery-drape markings improve estimation accuracy, but even this technique underestimates blood loss compared with more precise methods (Sosa, 2009; Toledo, 2007).
FIGURE 41-1 Blood loss associated with vaginal delivery, repeat cesarean delivery, and repeat cesarean delivery plus hysterectomy. (Data from Pritchard, 1962.)
The blood volume of a pregnant woman with normal pregnancy-induced hypervolemia usually increases by half, but increases range from 30 to 60 percent—1500 to 2000 mL for an average-sized woman (Pritchard, 1965). The equation to calculate blood volume is shown in Table 41-1. It is axiomatic that a normal pregnant woman tolerates, without any decrease in postpartum hematocrit, blood loss at delivery that approaches the volume of blood that she added during pregnancy. Thus, if blood loss is less than the pregnancy-added volume, the hematocrit remains the same acutely and during the first several days. It then increases as nonpregnant plasma volume normalizes during the next week or so. Whenever the postpartum hematocrit is lower than one obtained on admission for delivery, blood loss can be estimated as the sum of the calculated pregnancy-added volume plus 500 mL for each 3 volume percent decrease of the hematocrit. Blood loss in these women can also be calculated using the formula of Hernandez and associates (2012). Briefly, these investigators used admission-to-discharge red cell volumes to compute total blood loss.
TABLE 41-1. Calculation of Maternal Total Blood Volume
Nonpregnant blood volumea:
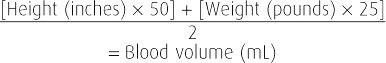
Pregnancy blood volume:
Average increase is 30 to 60 percent of calculated nonpregnant volume
Increases across gestational age and plateaus at approximately 34 weeks
Usually larger with low normal-range hematocrit (–30) and smaller with high normal-range hematocrit (–40)
Average increase is 40 to 80 percent with multifetal gestation
Average increase is less with preeclampsia—volumes vary inversely with severity
Postpartum blood volume with serious hemorrhage:
Assume acute return to nonpregnant total volume after fluid resuscitation
Pregnancy hypervolemia cannot be restored postpartum
Excessive blood loss has been estimated by several methods that include measuring loss directly, using a predetermined decline in hematocrit or hemoglobin concentration, or counting the number of women transfused. Of these, direct measurement was performed in a two-center investigation done in Argentina and Uruguay (Sosa, 2009). Specially constructed drapes were used to collect blood at vaginal delivery. These investigators reported that 10.8 percent of women had hemorrhage in excess of 500 mL, whereas 1.9 percent lost > 1000 mL. These estimates likely are too low.
Tita and colleagues (2012) used a 6- volume percent decrease in the postpartum hematocrit to define clinically significant blood loss with vaginal delivery. This decrease easily signifies greater than 1000-mL blood loss in the averaged-sized women. They documented this amount in a fourth of women, which agrees with the findings in Figure 41-1.
A final marker used to estimate the hemorrhage incidence is the transfusion rate. Because of prevailing conservative attitudes toward blood replacement, rates cited more recently are lower than transfusion rates described in older studies. In the study by Tita and colleagues (2012) cited above, more than 6 percent of women delivered vaginally underwent interventional treatment and blood transfusions. In a study of more than 66,000 women delivered at Parkland Hospital from 2002 to 2006, Hernandez and associates (2012) reported that 2.3 percent overall were given blood transfusions for hypovolemia. Half of these women had undergone cesarean delivery. Importantly, for those transfused, these investigators calculated blood loss to average approximately 3500 mL! In a recent study from Australia, 1.6 percent of women delivered in 2010 were transfused (Patterson, 2014). Last, from a registry-based Norwegian study, Skjeldestad and Øian (2012) reported that blood loss exceeded 1250 mL in 2.7 percent of women undergoing cesarean delivery.
Thus, it is apparent that significant blood loss accompanies about a fourth of vaginal deliveries. The amounts and proportions for cesarean delivery are much greater.
When audits of discharge statistics are evaluated, it is apparent that hemorrhage is also underreported. In one example, Bateman and associates (2010) queried the Nationwide Inpatient Sample for 2004 and found a 2.9-percent reported rate of postpartum hemorrhage. Similarly, using a sample of nearly 185,000 delivery outcomes from the National Hospital Discharge Summary database that represented more than 40 million deliveries, Berg and coworkers (2009) reported postpartum hemorrhage incidence of 2.0 and 2.6 percent, for two epochs. In a population-based Canadian study, the incidence of postpartum hemorrhage from atony increased from 3.6 to 4.8 percent from 2001 to 2009 (Mehrabadi, 2013). In contrast, a study encompassing 1999 to 2008 by Kramer and associates (2013) cited a rate of severe postpartum hemorrhage that increased from only 1.9 to 4.2 per 1000 births. At the same time, reported transfusion rates between 1991 and 2003 increased from only 3 to 5 per 1000 deliveries—all rather meager compared with rates in previously cited prospective studies.
Predisposing Conditions
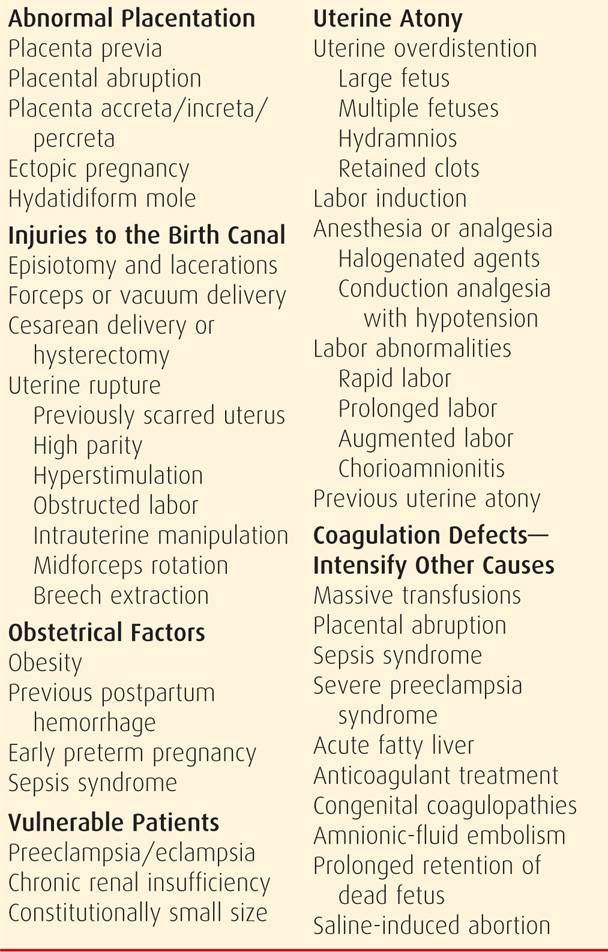
There are myriad clinical circumstances in which risks for obstetrical hemorrhage and its consequences are appreciably increased. The imposing list shown in Table 41-2 illustrates that hemorrhage can manifest at any time throughout pregnancy, delivery, and the puerperium. Thus, any description of obstetrical hemorrhage should include gestational age. Use of terms such as third-trimester bleeding is not recommended because of imprecision.
TABLE 41-2. Obstetrical Hemorrhage: Causes, Predisposing Factors, and Vulnerable Patients
Importantly, inadequate obstetrical and anesthetic services in some areas contribute to maternal death from hemorrhage. This undoubtedly includes facilities in the United States. It was quantified in the United Kingdom by the 2002 Confidential Enquiry into Maternal and Child Health, which concluded that most maternal deaths from hemorrhage were associated with substandard care (Weindling, 2003). Similar experiences were reported from Japan and from the Netherlands (Nagaya, 2000; Zwart, 2008).
 Timing of Hemorrhage
Timing of Hemorrhage
It is has been traditional to classify obstetrical hemorrhage as antepartum—such as with placenta previa or placental abruption, or as postpartum—commonly caused by uterine atony or genital tract lacerations. In individual women, however, these terms are nonspecific, and it is reasonable to specify the cause and gestational age as descriptors.
Antepartum Hemorrhage
Bleeding during various times in gestation may give a clue as to its cause. Many aspects of bleeding during the first half of pregnancy from abortion or ectopic pregnancy are discussed in detail in Chapters 18 and 19, respectively. Discussions that follow concern pregnancies with a viable mature living fetus. In these cases, rapid assessment should always consider the deleterious fetal effects of maternal hemorrhage.
Slight vaginal bleeding is common during active labor. This “bloody show” is the consequence of effacement and dilatation of the cervix, with tearing of small vessels. Uterine bleeding, however, coming from above the cervix, is concerning. It may follow some separation of a placenta previa implanted in the immediate vicinity of the cervical canal, or it may be from a placental abruption or uterine tear. Rarely, there may be velamentous insertion of the umbilical cord, and the involved placental vessels may overlie the cervix—vasa previa. Fetal hemorrhage follows laceration of these vessels at the time of membrane rupture. In many women near term, the source of uterine bleeding is not identified, symptomless bleeding ceases, and no apparent anatomical cause is found at delivery. In most of these cases, bleeding likely resulted from a slight marginal placental separation. Despite this, any pregnancy with antepartum bleeding remains at increased risk for an adverse outcome even though bleeding has stopped and placenta previa has been excluded sonographically.
Several reports address mid- to early third-trimester bleeding. Lipitz and colleagues (1991) reported that a fourth of 65 consecutive women with uterine bleeding between 14 and 26 weeks had placental abruption or previa, and a third of all 65 fetuses ultimately were lost. Similar outcomes were described subsequently (Ajayi, 1992; Leung, 2001). The Canadian Perinatal Network described 806 women with hemorrhage between 22 and 28 weeks’ gestation (Sabourin, 2012). Placental abruption (32 percent), previa (21 percent), and cervical bleeding (6.6 percent) were the most common causes identified, and in a third, no cause was found. Of all women, 44 percent were delivered ≤ 29 weeks. Clearly, second- and third-trimester bleeding are associated with a poor pregnancy prognosis.
Postpartum Hemorrhage
In most cases, the cause of postpartum hemorrhage can and should be determined. Frequent causes are uterine atony with bleeding from the placental implantation site, genital tract trauma, or both (see Table 41-2). Postpartum hemorrhage is usually obvious. Important exceptions are unrecognized intrauterine and intravaginal blood accumulation and uterine rupture with intraperitoneal bleeding. Initial assessment should attempt to differentiate uterine atony from genital tract lacerations. Primary considerations are the predisposing risk factors shown in Table 41-2, lower genital tract examination, and uterine tone assessment. Atony is identified by a boggy, soft uterus during bimanual examination and by expression of clots and hemorrhage during uterine massage.
Persistent bleeding despite a firm, well-contracted uterus suggests that hemorrhage most likely is from lacerations. Bright red blood further suggests arterial bleeding. To confirm that lacerations are a source of bleeding, careful inspection of the vagina, cervix, and uterus is essential. Sometimes bleeding may be caused by both atony and trauma, especially after forceps or vacuum-assisted vaginal delivery. Importantly, if significant bleeding follows these deliveries, then the cervix and vagina are carefully examined to identify lacerations. This is easier if conduction analgesia was given. If there are no lower genital tract lacerations and the uterus is contracted, yet supracervical bleeding persists, then manual exploration of the uterus is done to exclude a uterine tear. This also is completed routinely after internal podalic version and breech extraction.
 Blood Loss Estimation
Blood Loss Estimation
Estimated blood loss—like beauty—is in the eye of the beholder.
Estimation is notoriously inaccurate, especially with excessive bleeding (p. 781). Instead of sudden massive hemorrhage, postpartum bleeding is frequently steady. If atony persists, bleeding may appear to be only moderate at any given instant but may continue until serious hypovolemia develops. Bleeding from an episiotomy or a vaginal laceration can also appear to be only minimal to moderate. However, constant seepage can lead to enormous blood loss relatively quickly. In some cases, after placental separation, blood may not escape vaginally but instead may collect within the uterine cavity, which can become distended by 1000 mL or more of blood. In others, postpartum uterine massage is applied to a roll of abdominal fat mistaken for the uterus. Thus, monitoring of the uterus immediately postpartum must not be left to an inexperienced person (Chap. 27, p. 547).
All of these factors can lead to an underappreciation of the magnitude of hemorrhage over time. The effects of hemorrhage depend to a considerable degree on the maternal nonpregnant blood volume and the corresponding degree of pregnancy-induced hypervolemia as previously discussed. For this and other reasons, hypovolemia may not be recognized until very late. A treacherous feature of postpartum hemorrhage is the failure of the pulse and blood pressure to undergo more than moderate alterations until large amounts of blood have been lost. The normotensive woman initially may actually become somewhat hypertensive from catecholamine release in response to hemorrhage. Moreover, women with preeclampsia may become “normotensive” despite remarkable hypovolemia.
Late Postpartum Hemorrhage
Bleeding after the first 24 hours is designated late postpartum hemorrhage. Such bleeding is found in up to 1 percent of women and may be serious (American College of Obstetricians and Gynecologists, 2013b). It is discussed in Chapter 36 (p. 670).
Special Considerations
In certain conditions, a pregnant woman may be particularly susceptible to hemorrhage because her blood volume expansion is less than expected. This situation is most commonly encountered in small women—even those with normal pregnancy-induced hypervolemia. Women with severe preeclampsia or eclampsia are also more vulnerable to hemorrhage because they frequently do not have a normally expanded blood volume. Specifically, Zeeman and associates (2009) documented a mean increase above nonpregnant volume of only 10 percent in eclamptic women (Chap. 40, p. 737). A third example is the moderate to severely curtailed pregnancy-induced volume expansion in women with chronic renal insufficiency (Chap. 53, p. 1061). When excessive hemorrhage is suspected in these high-risk women, crystalloid and blood are promptly administered for suspected hypovolemia.
CAUSES OF OBSTETRICAL HEMORRHAGE
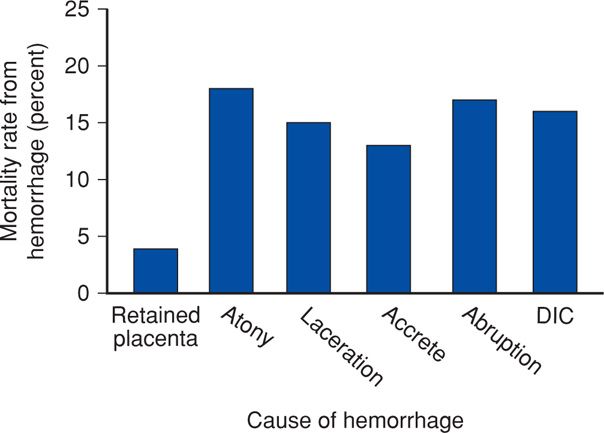
The most important causes—because of their incidence or consequence—of severe obstetrical hemorrhage and their contribution to maternal mortality rates are shown in Figure 41-2. Fatal hemorrhage is most likely in circumstances in which blood or components are not immediately available. As discussed on page 815, the ability to provide prompt resuscitation of hypovolemia and blood administration is an absolute requirement for acceptable obstetrical care.
FIGURE 41-2 Contributions to maternal death from various causes of obstetrical hemorrhage. Percentages are approximations because of different classification schemata used. DIC = disseminated intravascular coagulation. (Data from Al-Zirqi, 2008; Berg, 2010; Chichakli, 1999; Zwart, 2008.)
 Uterine Atony
Uterine Atony
The most frequent cause of obstetrical hemorrhage is failure of the uterus to contract sufficiently after delivery and to arrest bleeding from vessels at the placental implantation site (p. 780). That said, some bleeding is inevitable during third-stage labor as the placenta begins to separate. Blood from the implantation site may escape into the vagina immediately—the Duncan mechanism of placental separation, or it remains concealed behind the placenta and membranes until the placenta is delivered—the Schultze mechanism.
With bleeding during the third stage, the uterus should be massaged if it is not contracted firmly. After the signs of separation, placental descent is indicated by the cord becoming slack. Placental expression should be attempted by manual fundal pressure (Chap. 27, p. 546). If bleeding continues, then manual removal of the placenta may be necessary. Separation and delivery of the placenta by cord traction, especially when the uterus is atonic, may cause uterine inversion, which is discussed on page 787.
Third-Stage Bleeding
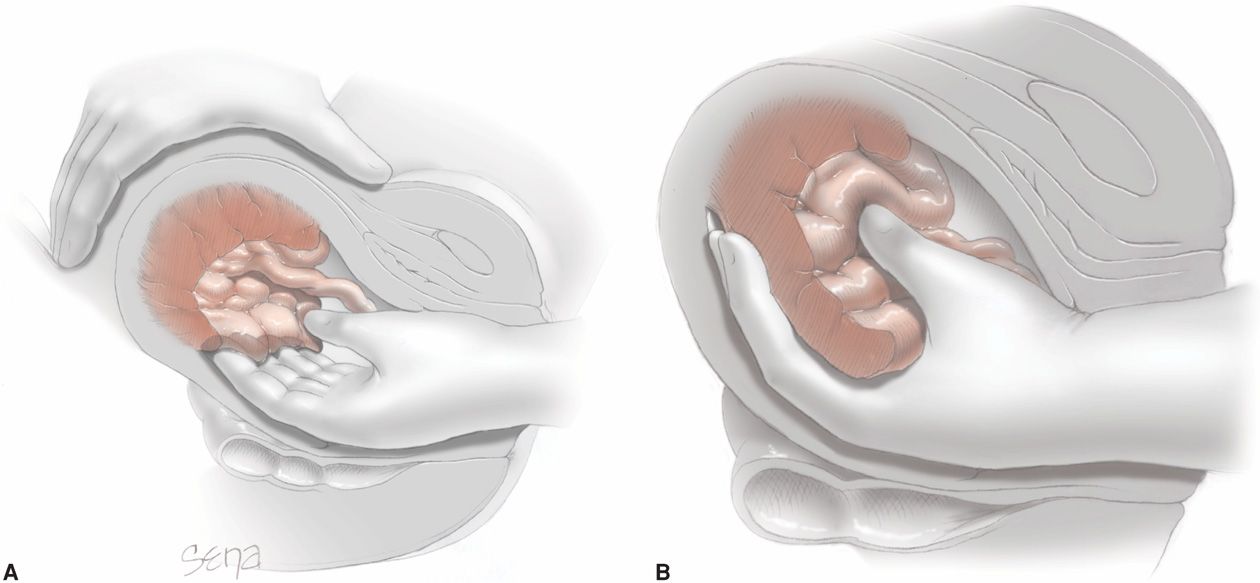
If significant bleeding persists after delivery of the infant and while the placenta remains partially or totally attached, then manual placental removal is indicated (Chap. 27, p. 546). For this, adequate analgesia is mandatory, and aseptic surgical technique should be used. As illustrated in Figure 41-3, the placenta is peeled off its uterine attachment by a motion similar to that used in separating the pages of a book. After its removal, trailing membranes are removed by carefully teasing them from the decidua and using ring forceps for this as necessary. Another method is to wipe out the uterine cavity with a gauze-wrapped hand.
FIGURE 41-3 Manual removal of placenta. A. One hand grasps the fundus. The other hand is inserted into the uterine cavity, and the fingers are swept from side to side as they are advanced. B. When the placenta has become detached, it is grasped and removed.
Uterine Atony after Placental Delivery
The fundus should always be palpated following placental delivery to confirm that the uterus is well contracted. If it is not firm, then vigorous fundal massage usually prevents postpartum hemorrhage from atony (Hofmeyr, 2008). Simultaneously, 20 units of oxytocin in 1000 mL of crystalloid solution will often be effective given intravenously at 10 mL/min for a dose of 200 mU/min. Higher concentrations are minimally more effective (Tita, 2012). Oxytocin should never be given as an undiluted bolus dose because serious hypotension or cardiac arrhythmias may develop.
Risk Factors
In many women, uterine atony can at least be anticipated well in advance of delivery (see Table 41-2). In one study, however, up to half of women who had atony after cesarean delivery were found to have no risk factors (Rouse, 2006). Thus, the ability to identify which individual woman will experience atony is limited. Risk factors for retained placenta are similar (Endler, 2012).
The magnitude of risk imposed by each of these factors shown in Table 41-2 varies considerably between reports. Primiparity has been cited as a risk factor (Driessen, 2011). High parity is a long-known risk factor for uterine atony. The incidence of postpartum hemorrhage was reported to increase from 0.3 percent in women of low parity to 1.9 percent with parity of 4 or greater. It was 2.7 percent with parity of 7 or greater (Babinszki, 1999). The overdistended uterus is prone to hypotonia after delivery, and thus women with a large fetus, multiple fetuses, or hydramnios are at greater risk. Labor abnormalities predispose to atony and include hyper- or hypotonic labor. Similarly, labor induction or augmentation with either prostaglandins or oxytocin is more likely to be followed by atony (Driessen, 2011). Finally, the woman who has had a prior postpartum hemorrhage is at risk for recurrence with a subsequent delivery.
Evaluation and Management
With immediate postpartum hemorrhage, careful inspection is done to exclude birth canal laceration. Because bleeding can be caused by retained placental fragments, inspection of the placenta after delivery should be routine. If a defect is seen, the uterus should be manually explored, and the fragment removed. Occasionally, retention of a succenturiate lobe may cause postpartum hemorrhage (Chap. 6, p. 117). During examination for lacerations and causes of atony, the uterus is massaged and uterotonic agents are administered.
 Uterotonic Agents
Uterotonic Agents
There are several compounds that prompt the postpartum uterus to contract (Chap. 27, p. 547). One of these is routinely selected and given to prevent postpartum bleeding by ensuring uterine contractions. Most of these same agents are also used to treat uterine atony with bleeding. Moreover, because many trials combine results from atony prophylaxis and treatment, evaluation of these studies is problematic. For example, oxytocin has been used for more than 50 years, and in most cases, it is infused intravenously or given intramuscularly after placental delivery. Neither route has been shown to be superior (Oladapo, 2012). This or other uterotonins given prophylactically will prevent most cases of uterine atony.
When atony persists despite preventive measures, ergot derivatives have been used for second-line treatment. These include methylergonovine—Methergine—and ergonovine. Only methylergonovine is currently manufactured in the United States. Given parenterally, these drugs rapidly stimulate tetanic uterine contractions and act for approximately 45 minutes (Schimmer, 2011). A common regimen is 0.2 mg of either drug given intramuscularly. A caveat is that ergot agents, especially given intravenously, may cause dangerous hypertension, especially in women with preeclampsia. Severe hypertension is also seen with concomitant use of protease inhibitors given for human immunodeficiency viral (HIV) infection (Chap. 65, p. 1279). These adverse effects notwithstanding, it is speculative whether there are superior therapeutic effects of ergot derivatives compared with oxytocin.
In more recent years, second-line agents for atony have included the E- and F-series prostaglandins. Carboprost tromethamine—Hemabate—is the 15-methyl derivative of prostaglandin F2α. It was approved more than 25 years ago for uterine atony treatment in a dose of 250 μg (0.25 mg) given intramuscularly. This dose can be repeated if necessary at 15- to 90-minute intervals up to a maximum of eight doses. Oleen and Mariano (1990) reported its use for postpartum hemorrhage from 12 obstetrical units. Bleeding was controlled in 88 percent of 237 women treated. Another 7 percent required other uterotonics to control hemorrhage, and the remaining 5 percent required surgical intervention. Carboprost causes side effects in approximately 20 percent of women. These include, in descending order of frequency, diarrhea, hypertension, vomiting, fever, flushing, and tachycardia (Oleen, 1990). Another pharmacological effect is pulmonary airway and vascular constriction. Thus, carboprost should not be used for asthmatics and those with suspected amnionic-fluid embolism (p. 812). We have occasionally encountered severe hypertension with carboprost given to women with preeclampsia, and it has also been reported to cause arterial oxygen desaturation that averaged 10 percent (Hankins, 1988).
E-series prostaglandins have been used to prevent or treat atony. Dinoprostone—prostaglandin E2—is given as a 20-mg suppository per rectum or per vaginam every 2 hours. It typically causes diarrhea, which is problematic for the rectal route, whereas vigorous vaginal bleeding may preclude its use per vaginam. Intravenous prostaglandin E2—sulprostone—is used in Europe, but it is not available in this country (Schmitz, 2011).
Misoprostol—Cytotec—is a synthetic prostaglandin E1 analogue that has also been evaluated for both prevention and treatment of atony and postpartum hemorrhage (Abdel-Aleem, 2001; O’Brien, 1998). Most studies have addressed prevention and have conflicting conclusions. In a Cochrane review, Mousa and Alfirevic (2007) reported no added benefits to misoprostol use compared with oxytocin or ergonovine. Derman and coworkers (2006) compared a 600-μg oral dose given at delivery against placebo. They found that the drug decreased hemorrhage incidence from 12 to 6 percent and that of severe hemorrhage from 1.2 to 0.2 percent. In another study, however, Gerstenfeld and Wing (2001) concluded that 400-μg misoprostol administered rectally was not more effective than intravenous oxytocin in preventing postpartum hemorrhage. From a systematic review, Villar (2002) found that oxytocin and ergot preparations administered after delivery were more effective than misoprostol for prevention of postpartum hemorrhage (Chap. 27, p. 547).
Bleeding Unresponsive to Uterotonic Agents
Hemorrhage that persists despite uterine massage and continued uterotonin administration may be from a yet unrecognized genital tract laceration, for example, uterine rupture. Thus, if bleeding persists after initial measures for atony have been implemented, then the following management steps are performed immediately and simultaneously:
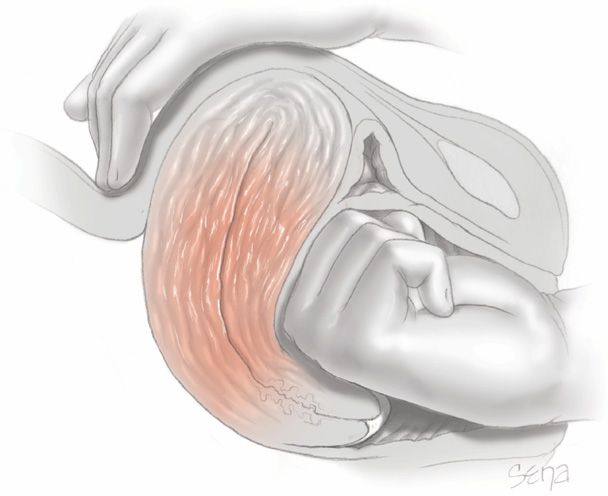
1. Begin bimanual uterine compression, which is easily done and controls most cases of continuing hemorrhage (Fig. 41-4). This technique is not simply fundal massage. The posterior uterine wall is massaged by one hand on the abdomen, while the other hand is made into a fist and placed into the vagina. This fist kneads the anterior uterine wall through the anterior vaginal wall. Concurrently, the uterus is also compressed between the two hands.
2. Immediately mobilize the emergent-care obstetrical team to the delivery room and call for whole blood or packed red cells.
3. Request urgent help from the anesthesia team.
4. Secure at least two large-bore intravenous catheters so that crystalloid with oxytocin is continued simultaneously with blood products. Insert an indwelling Foley catheter for continuous urine output monitoring.
5. Begin volume resuscitation with rapid intravenous infusion of crystalloid (p. 815).
6. With sedation, analgesia, or anesthesia established and now with optimal exposure, once again manually explore the uterine cavity for retained placental fragments and for uterine abnormalities, including lacerations or rupture.
7. Thoroughly inspect the cervix and vagina again for lacerations that may have escaped attention.
8. If the woman is still unstable or if there is persistent hemorrhage, then blood transfusions are given (p. 815).
FIGURE 41-4 Bimanual compression for uterine atony. The uterus is positioned with the fist of one hand in the anterior fornix pushing against the anterior wall, which is held in place by the other hand on the abdomen. The abdominal hand is also used for uterine massage.
At this juncture, after causes other than atony have been excluded and after hypovolemia is reversed, several other measures are considered if bleeding continues. Their use depends on several factors such as parity, desire for sterilization, and experience with each method.
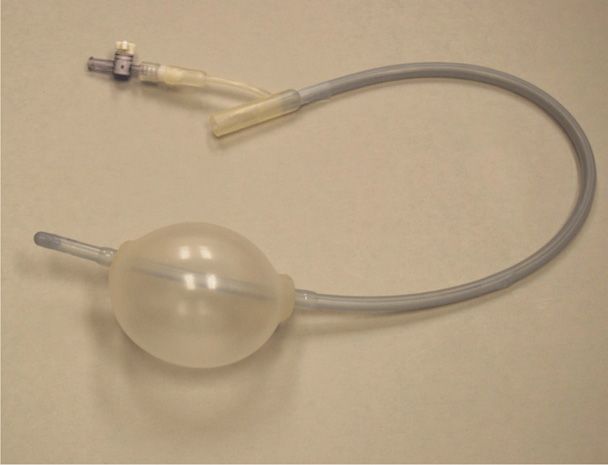
Uterine Packing or Balloon Tamponade. Uterine packing was popular to treat refractory uterine atony during the first half of the 20th century. It subsequently fell from favor because of concerns regarding concealed bleeding and infection (Hsu, 2003). Newer techniques have been described that alleviate some of these concerns (Zelop, 2011). In one technique, the tip of a 24F Foley catheter with a 30-mL balloon is guided into the uterine cavity and filled with 60 to 80 mL of saline. The open tip permits continuous drainage of blood from the uterus. If bleeding subsides, the catheter is typically removed after 12 to 24 hours. Similar devices that have been used for tamponade include Segstaken-Blakemore and Rusch balloons and condom catheters (Georgiou, 2009; Lo, 2013a,b). Alternatively, the uterus or pelvis may be packed directly with gauze (Gilstrap, 2002).
Enthusiasm has developed for specially constructed intrauterine balloons to treat hemorrhage from uterine atony and other causes (Barbieri, 2011). A Bakri Postpartum Balloon (Cook Medical) or BT-Cath (Utah Medical Products) may be inserted and inflated to tamponade the endometrial cavity and stop bleeding (Fig. 41-5). Insertion requires two or three team members. The first performs abdominal sonography during the procedure. The second places the deflated balloon into the uterus and stabilizes it. The third member instills fluid to inflate the balloon, rapidly infusing at least 150 mL followed by further instillation over a few minutes for a total of 300 to 500 mL to arrest hemorrhage.
There have been few prospective studies with these uterine balloons, and thus data are observational (Aibar, 2013; Grönvall, 2013; Laas, 2012). In all, more than 150 women were managed for postpartum hemorrhage. Perhaps a fourth were caused by uterine atony. For all causes, the success rate was noted to be approximately 85 percent. Combinations of balloon tamponade and uterine compression sutures have also been described (Diemert, 2012; Yoong, 2012). Failures for all of these require various surgical methods including hysterectomy. Well-designed studies are needed before instituting intrauterine balloon tamponade as a second-line treatment for atony.
Surgical Procedures. Several invasive procedures can be used to control hemorrhage from atony. These include uterine compression sutures, pelvic vessel ligation, angiographic embolization, and hysterectomy. These are discussed on page 818.
 Uterine Inversion
Uterine Inversion
Puerperal inversion of the uterus is considered to be one of the classic hemorrhagic disasters encountered in obstetrics. Unless promptly recognized and managed appropriately, associated bleeding often is massive. Risk factors include alone or in combination:
1. Fundal placental implantation,
2. Delayed-onset or inadequate uterine contractility after delivery of the fetus, that is, uterine atony,
3. Cord traction applied before placental separation, and
4. Abnormally adhered placentation such as with the accrete syndromes (p. 804).
Depending on which of these factors is contributory, the incidence and severity of uterine inversion varies, with the worst scenario being complete inversion with the uterus protruding from the birth canal as shown in Figure 41-6. There is progressive severity of inversion as shown in Figure 41-7.
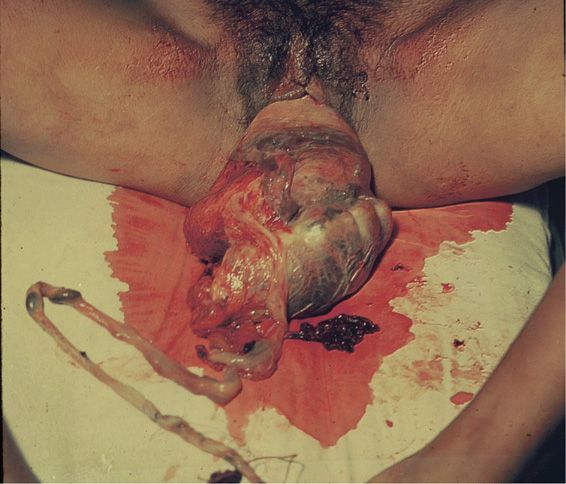
FIGURE 41-6 Maternal death from exsanguination caused by uterine inversion associated with a fundal placenta accreta during a home delivery.
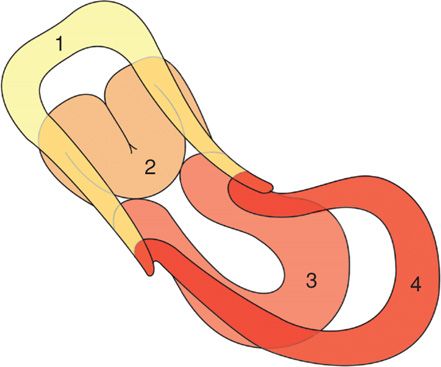
FIGURE 41-7 Progressive degrees of uterine inversion depicted. After the fundus begins and continues to invert (Nos. 1 and 2), it would not be visible externally until it was at the level of the introitus (No. 3) or completely inverted (No. 4).
The incidence of uterine inversion is variable and is usually different for vaginal and for cesarean delivery. Incidences range from approximately 1 in 2000 to 1 in 20,000 deliveries (Baskett, 2002; Ogah, 2011; Rana, 2009; Witteveen, 2013). Our experiences at Parkland Hospital comport with the higher 1:2000 incidence. This is despite our policy to discourage placental delivery by cord traction alone and before certainty of its separation. It is unknown if active management of third-stage labor with cord traction applied ostensibly after signs of placental separation increases the likelihood of uterine inversion (Deneux-Tharaux, 2013; Gülmezoglu, 2012; Peña-Marti, 2007; Prick, 2013).
Recognition and Management
Immediate recognition of uterine inversion improves the chances of a quick resolution and good outcome. That said, inexperienced attendants may have a delayed appreciation for an inverting uterus, especially if it is only partial and thus not protruding through the introitus. In these cases, continued hemorrhage likely will prompt closer examination of the birth canal. Even so, the partially inverted uterus can be mistaken for a uterine myoma, and this can be resolved by sonography (Pauleta, 2010; Rana, 2009). Many cases are associated with immediate life-threatening hemorrhage, and at least half require blood replacement (Baskett, 2002). It was previously taught that associated shock was disproportionate to blood loss because of parasympathetic stimulation from stretching pelvic tissues. This has been refuted (Watson, 1980).
Once any degree of uterine inversion is recognized, several steps must be implemented urgently and simultaneously:
1. Immediate assistance is summoned, including obstetrical and anesthesia personnel.
2. Blood is brought to the delivery suite in case it may be needed.
3. The woman is evaluated for emergency general anesthesia. Large-bore intravenous infusion systems are secured to begin rapid crystalloid infusion to treat hypovolemia while awaiting arrival of blood for transfusion.
4. If the recently inverted uterus has not contracted and retracted completely and if the placenta has already separated, then the uterus may often be replaced simply by pushing up on the inverted fundus with the palm of the hand and fingers in the direction of the long axis of the vagina (Fig. 41-8). Some use two fingers rigidly extended to push the center of the fundus upward. Care is taken not to apply so much pressure as to perforate the uterus with the fingertips.
5. If the placenta is still attached, it is not removed until infusion systems are operational and a uterine relaxant drug administered. Many recommend a trial of an intravenously administered tocolytic drug such as terbutaline, magnesium sulfate, or nitroglycerin for uterine relaxation and repositioning (Hong, 2006; You, 2006). If these fail to provide sufficient relaxation, then a rapidly acting halogenated inhalational agent is administered.
6. After removing the placenta, steady pressure with the fist, palm, or fingers is applied to the inverted fundus in an attempt to push it up into and through the dilated cervix as described in Step 4.
7. Once the uterus is restored to its normal configuration, tocolysis is stopped. Oxytocin is then infused, and other uterotonics may be given as described for atony (p. 785). Meanwhile, the operator maintains the fundus in its normal anatomical position while applying bimanual compression to control further hemorrhage until the uterus is well contracted (see Fig. 41-4). The operator continues to monitor the uterus transvaginally for evidence of subsequent inversion.
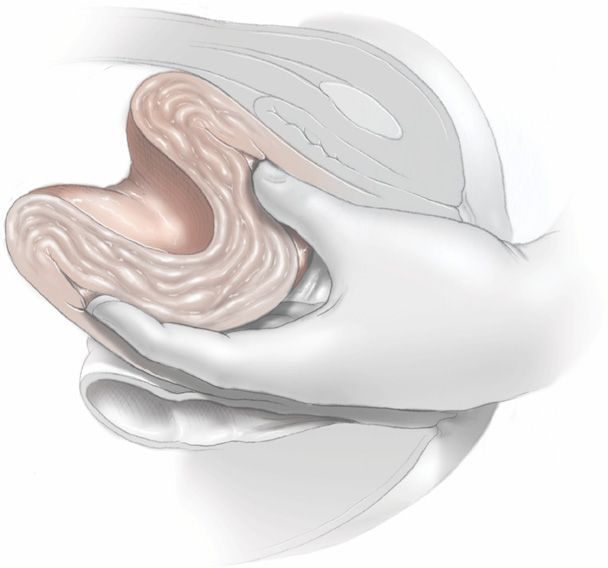
FIGURE 41-8 The incompletely inverted uterus is repositioned by using the abdominal hand for palpation of the crater-like depression while gently pushing the inverted fundus up out of the lower segment and to its normal anatomical position.
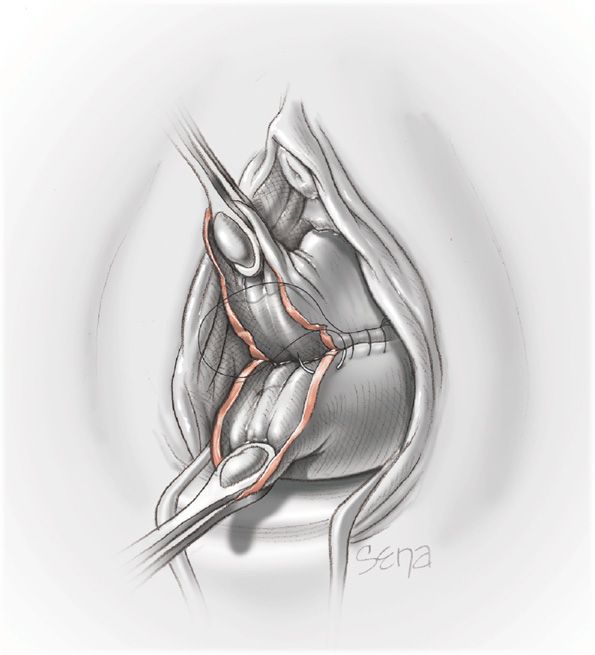
Surgical Intervention
In most cases the inverted uterus can be restored to its normal position by the techniques described above. Occasionally, manual replacement fails. One cause is a dense myometrial constriction ring (Kochenour, 2002). At this point, laparotomy is imperative. The anatomical configuration found at surgery can be confusing as shown in Figure 41-9. With agents given for tocolysis, a combined effort is made to reposition the uterus by simultaneously pushing upward from below and pulling upward from above. Application of atraumatic clamps to each round ligament and upward traction may be helpful—the Huntington procedure. In some cases, placing a deep traction suture in the inverted fundus or grasping it with tissue forceps may be of aid. However, these may be technically difficult (Robson, 2005). If the constriction ring still prohibits repositioning, a longitudinal surgical cut—Haultain incision—is made posteriorly through the ring to expose the fundus and permit reinversion (Sangwan, 2009). After uterine replacement, tocolytics are stopped, oxytocin and other uterotonics are given, and the uterine incision is repaired. Risks of separation of this posterior hysterotomy incision during subsequent pregnancy, labor, and delivery are unknown.
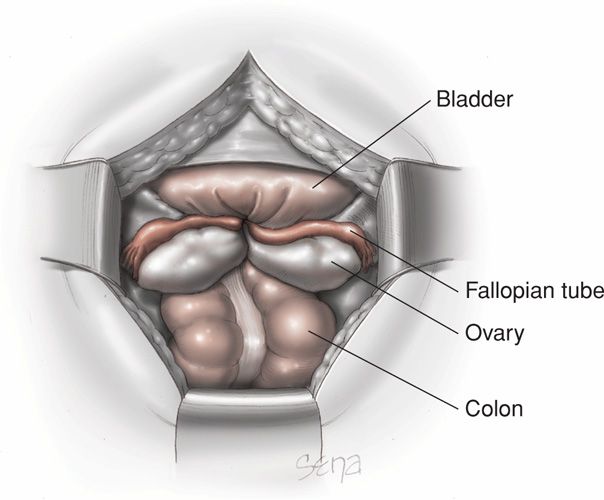
FIGURE 41-9 Surgical anatomy of a completely inverted uterus viewed from above at laparotomy. The uterine fundus is not visible and the tubes and ovaries have been drawn into the birth canal.
In some cases, the uterus will again invert almost immediately after repositioning. If this is a problem, then compression sutures shown on page 819 can be used to prevent another inversion (Matsubara, 2009; Mondal, 2012). Occasionally, chronic puerperal uterine inversion may become apparent weeks after delivery.
 Injuries to the Birth Canal
Injuries to the Birth Canal
Childbirth is invariably associated with trauma to the birth canal, which includes the uterus and cervix, vagina, and perineum. Injuries sustained during labor and delivery range from minor mucosal tears to lacerations that create life-threatening hemorrhage or hematomas.
Vulvovaginal Lacerations
Small tears of the anterior vaginal wall near the urethra are relatively common. They are often superficial with little to no bleeding, and their repair is usually not indicated. Minor superficial perineal and vaginal lacerations occasionally require sutures for hemostasis. Those large enough to require extensive repair are typically associated with voiding difficulty, and an indwelling bladder catheter will obviate this.
Deeper perineal lacerations are usually accompanied by varying degrees of injury to the outer third of the vaginal vault. Some extend to involve the anal sphincter or varying depths of the vaginal walls. In more than 87,000 deliveries in the Consortium on Safe Labor database, the frequency of third- or fourth-degree perineal lacerations was 5.7 percent in nulliparas and 0.6 percent in multiparas (Landy, 2011). Bilateral vaginal lacerations are usually unequal in length, and they are separated by a tongue of vaginal tissue. Lacerations involving the middle or upper third of the vaginal vault usually are associated with injuries of the perineum or cervix. These sometimes are missed unless thorough inspection of the upper vagina and cervix is performed. Bleeding despite a firmly contracted uterus is strong evidence of genital tract laceration. Those that extend upward usually are longitudinal. They may follow spontaneous delivery but frequently result from injuries sustained during operative vaginal delivery with forceps or vacuum extractor. Most involve deeper underlying tissues and thus usually cause significant hemorrhage. Bleeding is typically controlled by appropriate suture repair.
Extensive vaginal or cervical tears should prompt a careful search for evidence of retroperitoneal hemorrhage or peritoneal perforation or hemorrhage. If this is strongly suspected, then laparotomy is considered (Rafi, 2010). Extensive vulvovaginal lacerations should also prompt intrauterine exploration for possible uterine tears or rupture. For deep vulvovaginal lacerations, suture repair is usually required, and effective analgesia or anesthesia, vigorous blood replacement, and capable assistance are mandatory. In the study reported by Melamed and colleagues (2009), 1.5 percent of women with these lacerations required blood transfusions. Repair of vulvovaginal lacerations is detailed in Chapter 27 (p. 548).
Levator Sling Injuries
The levator ani muscles, described in Chapter 2 (p. 22), are usually involved with deep vaginal vault lacerations. They also sustain stretch injuries that result from overdistention of the birth canal. Muscle fibers are torn and separated, and their diminished tone may interfere with pelvic diaphragm function to cause pelvic relaxation. If the injuries involve the pubococcygeus muscle, urinary incontinence also may result.
Cervical Lacerations
Superficial lacerations of the cervix can be seen on close inspection in more than half of all vaginal deliveries. Most of these are less than 0.5 cm and seldom require repair (Fahmy, 1991). Deeper lacerations are less frequent, but even these may be unnoticed. Due to ascertainment bias, variable incidences are described. For example, in the Consortium database, the incidence of cervical lacerations was 1.1 percent in nulliparas and 0.5 percent in multiparas (Landy, 2011). But the overall incidence reported by Melamed and coworkers (2009), in a study of more than 81,000 Israeli women, was only 0.16 percent. Parikh and associates (2007) reported a 0.2-percent incidence of lacerations that needed repair.
Cervical lacerations are not usually problematic unless they cause hemorrhage or extend to the upper third of the vagina. Rarely, the cervix may be entirely or partially avulsed from the vagina—colporrhexis—in the anterior, posterior, or lateral fornices. These injuries sometimes follow difficult forceps rotations or deliveries performed through an incompletely dilated cervix with the forceps blades applied over the cervix. In some women, cervical tears reach into lower uterine segment and involve the uterine artery and its major branches. They occasionally extend into the peritoneal cavity. The more severe lacerations usually manifest as external hemorrhage or as a hematoma, however, they may occasionally be unsuspected. In the large Israeli study reported by Melamed and colleagues (2009), almost 11 percent of women with a cervical laceration required blood transfusions.
Other serious cervical injuries fortunately are uncommon. In one, the edematous anterior cervical lip is caught during labor and compressed between the fetal head and maternal symphysis pubis. If this causes severe ischemia, the anterior lip may undergo necrosis and separate from the rest of the cervix. Another rare injury is when the entire vaginal portion of the cervix is avulsed. Such annular or circular detachment of the cervix is seen with difficult deliveries, especially forceps deliveries.
Diagnosis. A deep cervical tear should always be suspected in women with profuse hemorrhage during and after third-stage labor, particularly if the uterus is firmly contracted. It is reasonable to inspect the cervix routinely following major operative vaginal deliveries even if there is no third-stage bleeding. Thorough evaluation is necessary, and often the flabby cervix interferes with digital examination. The extent of the injury can be more fully appreciated with adequate exposure and visual inspection. This is best accomplished when an assistant applies firm downward pressure on the uterus while the operator exerts traction on the lips of the cervix with ring forceps. A second assistant can provide even better exposure with right-angle vaginal wall retractors.
Management. In general, cervical lacerations of 1 and even 2 cm are not repaired unless they are bleeding. Such tears heal rapidly and are thought to be of no significance. When healed they result in the irregular, sometime stellate appearance of the external cervical os that indicates previous delivery of a viable-size fetus.
Deep cervical tears usually require surgical repair. When the laceration is limited to the cervix or even when it extends somewhat into the vaginal fornix, satisfactory results are obtained by suturing the cervix after bringing it into view at the vulva as depicted in Figure 41-10. While cervical lacerations are repaired, associated vaginal lacerations may be tamponaded with gauze packs to arrest their bleeding. Because hemorrhage usually comes from the upper angle of the wound, the first suture using absorbable material is placed in tissue above the angle. Subsequently, either interrupted or continuous locking sutures are placed outward toward the operator. If lacerations extend to involve the lateral vaginal sulcus, then attempts to restore the normal cervical appearance may lead to subsequent stenosis.
FIGURE 41-10 Repair of cervical laceration with appropriate surgical exposure. Continuous absorbable sutures are placed beginning at the upper angle of the laceration.
Most cervical lacerations are successfully repaired using sutures. However, if there is uterine involvement and continued hemorrhage, some of the methods described on page 818 may be necessary to obtain hemostasis. For example, Lichtenberg (2003) described successful angiographic embolization for a high cervical tear after failed surgical repair.
 Puerperal Hematomas
Puerperal Hematomas
Pelvic hematomas can have several anatomical manifestations following childbirth. They are most often associated with a laceration, episiotomy, or an operative delivery. However, they may develop following rupture of a blood vessel without associated lacerations (Nelson, 2012; Propst, 1998; Ridgway, 1995). In some cases, they are quickly apparent, but in others, hemorrhage may be delayed. Occasionally, they are associated with an underlying coagulopathy. Faulty clotting may be acquired, such as with consumptive coagulopathy from placental abruption or fatty liver failure, or may stem from a congenital bleeding disorder such as von Willebrand disease.
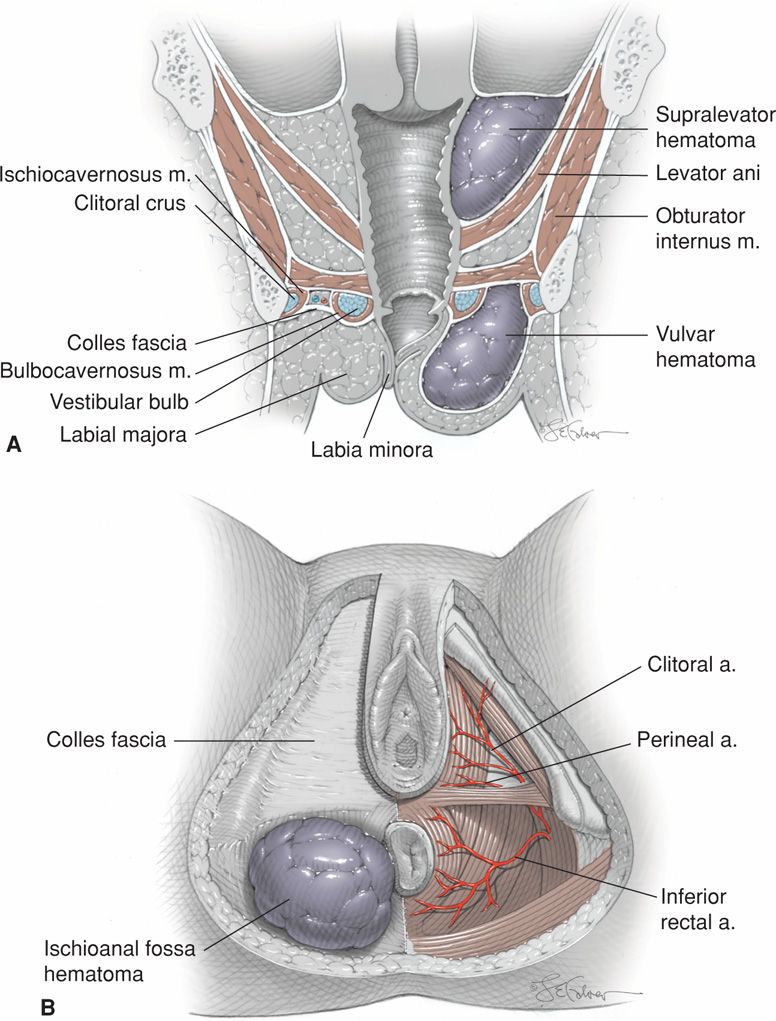
One classification of puerperal hematomas includes vulvar, vulvovaginal, paravaginal, and retroperitoneal hematomas. Vulvar hematomas may involve the vestibular bulb or branches of the pudendal artery, which are the inferior rectal, perineal, and clitoral arteries (Fig. 41-11). Paravaginal hematomas may involve the descending branch of the uterine artery (Zahn, 1990). In some cases, a torn vessel lies above the pelvic fascia, and a supralevator hematoma develops. These can extend into the upper portion of the vaginal canal and may almost occlude its lumen. Continued bleeding may dissect retroperitoneally to form a mass palpable above the inguinal ligament. Finally, it may even dissect up behind the ascending colon to the hepatic flexure at the lower margin of the diaphragm (Rafi, 2010).
FIGURE 41-11 Schematic showing types of puerperal hematoma. A. Coronal view showing supralevator and anterior perineal triangle hematomas on the right. B. Perineal view shows anterior perineal triangle anatomy and an ischioanal fossa hematoma on the left.
Vulvovaginal Hematomas
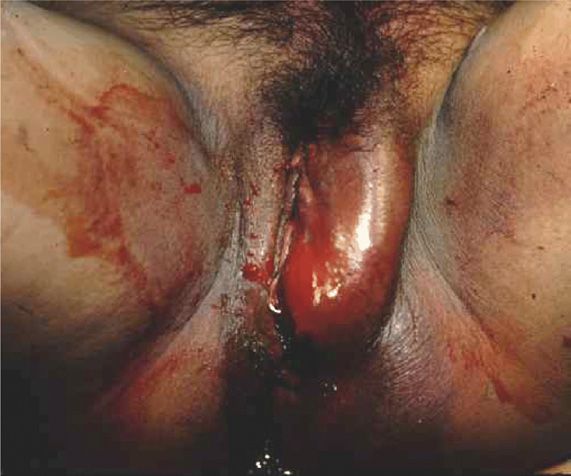
These may develop rapidly and frequently cause excruciating pain, as did the one shown in Figure 41-12. If bleeding ceases, then small- to moderate-sized hematomas may be absorbed. However, we have encountered a few that rebled up to 2 weeks postpartum. In others, the tissues overlying the hematoma may rupture from pressure necrosis. At this time, profuse hemorrhage may follow, but in other cases, the hematoma drains in the form of large clots and old blood. In those that involve the paravaginal space and extend above the levator sling, retroperitoneal bleeding may be massive and occasionally fatal.
FIGURE 41-12 Left-sided anterior perineal triangle hematoma associated with a vaginal laceration following spontaneous delivery in a woman with consumptive coagulopathy from acute fatty liver of pregnancy.
Diagnosis. A vulvar hematoma is readily diagnosed by severe perineal pain. A tense, fluctuant, and tender swelling of varying size rapidly develops and is eventually covered by discolored skin. A paravaginal hematoma may escape detection temporarily. However, symptoms of pelvic pressure, pain, or inability to void should prompt evaluation with discovery of a round, fluctuant mass encroaching on the vaginal lumen. When there is a supralevator extension, the hematoma extends upward in the paravaginal space and between the leaves of the broad ligament. The hematoma may escape detection until it can be felt on abdominal palpation or until hypovolemia develops. Imaging with sonography or computed tomographic (CT) scanning can be useful to assess hematoma location and extent. As discussed, supralevator hematomas are particularly worrisome because they can lead to hypovolemic shock and death.
Management. Vulvovaginal hematomas are managed according to their size, duration since delivery, and expansion. In general, smaller vulvar hematomas identified after the woman leaves the delivery room may be treated expectantly (Propst, 1998). But, if pain is severe or the hematoma continues to enlarge, then surgical exploration is preferable. An incision is made at the point of maximal distention, blood and clots are evacuated, and bleeding points ligated. The cavity may then be obliterated with absorbable mattress sutures. Often, no sites of bleeding are identified. Nonetheless, the evacuated hematoma cavity is surgically closed, and the vagina is packed for 12 to 24 hours. Supralevator hematomas are more difficult to treat. Although some can be evacuated by vulvar or vaginal incisions, laparotomy is advisable if there is continued hemorrhage.
Blood loss with large puerperal hematomas is nearly always considerably more than the clinical estimate. Hypovolemia is common, and transfusions are frequently required when surgical repair is necessary.
Angiographic embolization as described on page 820 has become popular for management of some puerperal hematomas. Embolization can be used primarily, or more likely secondarily, if surgical attempts at hemostasis have failed or if the hematoma is difficult to access surgically (Distefano, 2013; Ojala, 2005). The use of a Bakri balloon for a paracervical hematoma has also been described (Grönvall, 2013).
 Rupture of the Uterus
Rupture of the Uterus
Uterine rupture may be primary, defined as occurring in a previously intact or unscarred uterus, or may be secondary and associated with a preexisting myometrial incision, injury, or anomaly. Some of the etiologies associated with uterine rupture are presented in Table 41-3. Importantly, the contribution of each of these underlying causes has changed remarkably during the past 50 years. Specifically, before 1960, when the cesarean delivery rate was much lower than it is currently and when women of great parity were numerous, primary uterine rupture predominated. As the incidence of cesarean delivery increased and especially as a subsequent trial of labor in these women became prevalent through the 1990s, uterine rupture through the cesarean hysterotomy scar became preeminent. As discussed in detail in Chapter 31 (p. 617), along with diminished enthusiasm for trial of labor in women with prior cesarean delivery, the two types of rupture likely now have equivalent incidences. Indeed, in a 2006 study of 41 cases of uterine rupture from the Hospital Corporation of America, half were in women with a prior cesarean delivery (Porreco, 2009).
TABLE 41-3. Some Causes of Uterine Rupture
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree