Obstetric Analgesia and Anesthesia
Joy L. Hawkins
The purpose of this chapter is to acquaint the obstetrician with the various techniques of obstetric analgesia (pain relief) and anesthesia (for surgical procedures) and to describe their indications, advantages, disadvantages, and complications. The technical aspects, including the methods of administration, will not be described in detail. Readers seeking specific information on how to perform the various obstetric anesthetic techniques are referred to one of the basic obstetric anesthesia textbooks.
Obstetric analgesia or anesthesia refers to the multiple techniques useful for the alleviation of pain associated with labor, delivery, or surgery. The choice of an appropriate analgesic technique must be made by the patient, the obstetrician, and the anesthesiologist and should take into consideration the patient’s anatomy and physiology, the status of her fetus, the obstetric plan for delivery, and the pharmacology of the drugs to be employed. Both the American College of Obstetricians and Gynecologists (ACOG) and the American Society of Anesthesiologists (ASA) have guidelines for the practice of obstetric anesthesia.
Pain of Parturition
Increasing dilation of the cervix; contraction and distention of the uterus; and distention or tearing of the vagina, vulva, and perineum causes the pain that occurs during labor and delivery. Pain may also be generated through stretching or application of pressure to adjacent pelvic organs.
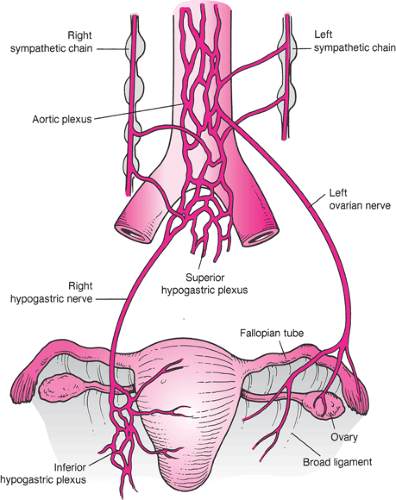
The pain that occurs in the first stage of labor increases in severity as the cervix becomes more dilated. The onset of pain lags approximately 15 to 30 seconds behind the onset of the uterine contraction and is first perceived when the intra-amniotic pressure reaches 15 mm Hg above that of resting tonus. The pain of uterine contractions is conducted through small sensory nerve fibers of the paracervical and inferior hypogastric plexuses to join the sympathetic nerve chain at L2–3. The ascending fibers enter the spinal cord through the nerve roots of T-10 to T-12, with a variable contribution from L-1 (Fig. 3.1). Because the cutaneous branches of the lower thoracic and upper lumbar nerves migrate caudally for a considerable distance before they innervate the skin, the pain of uterine contractions is often referred to the area over the upper sacrum and the lower lumbar spine.
Pain from the uterus and cervix is transmitted through the small-diameter myelinated A-delta fibers and unmyelinated C fibers. Because there are relatively fewer nociceptive afferent nerves from visceral structures than from somatic structures, visceral pain is perceived as being diffuse and difficult to localize. These visceral afferents also synapse on and excite the same dorsal horn neurons as afferents from somatic structures. This arrangement is responsible for the phenomenon of referred pain.
In the second stage of labor, sharp pain occurs as the tissues of the vagina and perineum are stretched. Stretching stimulates the second, third, and fourth sacral nerve roots, which carry nociceptive information to the spinal cord through the sensory fibers of the pudendal nerve. Adnexal pressure and traction on the bladder, urethra, rectum, and peritoneum also contribute to the pain of parturition. Compression of the lumbosacral plexus by the fetal head, particularly in the occiput posterior position, may cause pain even before the onset of labor.
The gate theory of Melzack and Wall holds that stimulation of the large cutaneous A-beta nerve fibers closes a “gate” in the substantia gelatinosa of the spinal cord, preventing pain impulses from being carried rostrally by
the A-delta and C nerve fibers. This theory forms the basis for the use of acupuncture, transcutaneous electrical nerve stimulation (TENS), and intracutaneous nerve stimulation with sterile water injections for the relief of pain associated with parturition.
the A-delta and C nerve fibers. This theory forms the basis for the use of acupuncture, transcutaneous electrical nerve stimulation (TENS), and intracutaneous nerve stimulation with sterile water injections for the relief of pain associated with parturition.
Pregnancy appears to reduce anesthetic requirements. It has been postulated that high progesterone levels lead to increased quantities of endogenous endorphins, which may increase the maternal threshold to pain. One study correlated pain intensity during labor and plasma levels of β-endorphin. The lowest endorphin levels were found after abolition of labor pain by epidural analgesia. The highest concentrations were observed in the first few minutes after delivery, immediately after cessation of the severe pain of expulsive labor.
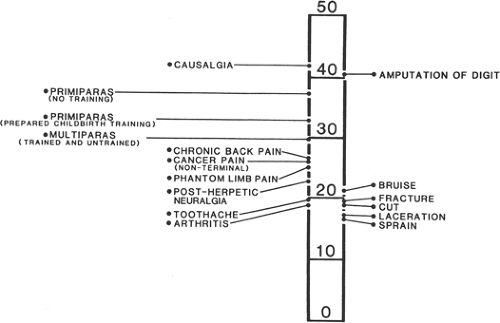
The nature of the pain of labor varies in intensity with the stages of labor. The intensity of pain is related to physical factors such as the strength and duration of uterine contractions; the rapidity of cervical dilation; the degree of distention of the vaginal and perineal tissues; the requirement for operative delivery; and the size, presentation, and position of the infant. Augmentation of labor with oxytocin increases the strength and pain of uterine contractions. The primiparous woman may perceive greater pain than the multipara who enters labor with more advanced cervical dilation and who may also be more psychologically prepared (Fig. 3.2). Exhaustion, lack of social supports, other psychologic factors, and protracted nausea and vomiting may also increase the parturient’s perception of labor pain.
Pain management is an important part of modern obstetric care. A joint statement by the ACOG and the ASA notes that “Labor results in severe pain for many women. There is no other circumstance in which it is considered acceptable for a person to experience untreated severe pain, amenable to safe intervention, while under a physician’s care. In the absence of a medical contraindication, maternal request is a sufficient medical indication for pain relief during labor.” Most women now request some form of analgesia during childbirth (Table 3.1). The obstetrician should appreciate the importance of providing pain relief during labor through the use of nonpharmacologic techniques, systemic analgesics, or regional block analgesia. During the second stage of labor, additional analgesia
may also be needed through perineal extension of a segmental epidural, spinal analgesia, or through the use of pudendal or local infiltration.
may also be needed through perineal extension of a segmental epidural, spinal analgesia, or through the use of pudendal or local infiltration.
Systemic Analgesia and Sedation
In the management of labor pain, systemic narcotics are usually considered to be the first step beyond the less invasive or “natural” methods such as massage, water baths, and birth attendants (doulas). They may also be necessary for patients who are not candidates for regional analgesia. Research indicates that the analgesic effects of parenteral agents used in labor is limited, and the primary mechanism of action is heavy sedation. Although narcotics may be effective for some patients in relieving the pain of labor, their side effects prohibit the use of large doses. The physician must balance maternal sedation, nausea, and respiratory depression and neonatal respiratory depression with effective relief of pain. The pain of labor occurs intermittently with contractions. Maternal hyperventilation during a contraction lowers pCO2 and leads to hypoventilation for 2 to 3 minutes between contractions, especially when narcotics have shifted the carbon dioxide response curve. There are advantages and disadvantages of all available narcotics, and a drug should be chosen with knowledge of its side effects and pharmacokinetics (Table 3.2).
TABLE 3.1 Types of Labor Analgesia Provided by Size of Hospital in Three Time Periods | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Use of Systemic Medications
The use of systemic narcotics and tranquilizers does not require special training or the availability of anesthesia personnel, and they are used extensively in the United States to provide labor analgesia, either alone or before provision of neuraxial analgesia (Table 3.1). These compounds do not induce fetal heart rate (FHR) abnormalities other
than reductions in variability and rarely sinusoidal heart rate patterns, nor do they cause fetal acidosis. The drug-related adverse maternal effects of narcotics can include decreased gastric motility, nausea, vomiting, pruritus, sedation, loss of protective airway reflexes, and hypoxia due to respiratory depression. The adverse neonatal effects of these agents include central nervous system (CNS) depression, respiratory depression, impaired early breast-feeding, altered neuroadaptive behavior, and decreased ability to regulate body temperature. To minimize these side effects, the lowest effective dosage should be employed, and the timing with respect to delivery must be carefully considered. Resuscitation equipment should be kept at hand, and naloxone (Narcan), used to antagonize opioids, should be readily available. Benzodiazepine effects may be reversed with flumazenil (Romazicon).
than reductions in variability and rarely sinusoidal heart rate patterns, nor do they cause fetal acidosis. The drug-related adverse maternal effects of narcotics can include decreased gastric motility, nausea, vomiting, pruritus, sedation, loss of protective airway reflexes, and hypoxia due to respiratory depression. The adverse neonatal effects of these agents include central nervous system (CNS) depression, respiratory depression, impaired early breast-feeding, altered neuroadaptive behavior, and decreased ability to regulate body temperature. To minimize these side effects, the lowest effective dosage should be employed, and the timing with respect to delivery must be carefully considered. Resuscitation equipment should be kept at hand, and naloxone (Narcan), used to antagonize opioids, should be readily available. Benzodiazepine effects may be reversed with flumazenil (Romazicon).
TABLE 3.2 Parenteral Medications for Labor Analgesia | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
Systemic Narcotics
Meperidine
Meperidine (Demerol) has achieved wide popularity for systemic analgesia during labor. It is preferred over morphine because it produces less emesis and may not depress newborn carbon dioxide response curves as much as morphine. It can be administered intravenously or intramuscularly during labor. Current usage consists of small, incremental intravenous doses of 25 to 50 mg. Small doses (12.5 mg) can also be used to treat shivering. Placental transfer of meperidine occurs rapidly. Maximal depression of the infant occurs when delivery takes place 2 to 4 hours after maternal intravenous or intramuscular administration. Delivery of the infant within 1 hour of administration produces little evidence of newborn depression. A study using meperidine for labor analgesia by a patient-controlled infusion device found 5% of infants required naloxone at delivery.
Meperidine has as its principal metabolite the compound normeperidine, which is equipotent with meperidine in its ability to produce respiratory depression and can also cause seizures. Repeated intravenous administration of small doses of meperidine leads to increasing maternal and fetal levels of normeperidine. Meperidine has an elimination half-life in neonatal blood of 22.7 hours, and that of normeperidine is measured in days, reflected in abnormal neurobehavioral scores for up to 3 days.
Morphine
Morphine is pharmacologically more potent than meperidine by a factor of approximately 10. Morphine may depress the newborn carbon dioxide response curve more
than meperidine, perhaps due to greater permeability of the infant brain to morphine. Because of this reputation, morphine is rarely used by the obstetrician for the management of pain during labor.
than meperidine, perhaps due to greater permeability of the infant brain to morphine. Because of this reputation, morphine is rarely used by the obstetrician for the management of pain during labor.
Fentanyl
Fentanyl (Sublimaze) is a potent synthetic narcotic with analgesic activity approximately 100 times that of morphine. Its onset of action is rapid, and its duration of activity is short (i.e., 20 to 30 minutes) because of its rapid distribution from plasma. The terminal drug elimination half-life after a single small dose is 1 to 2 hours. Fentanyl is highly bound to protein, which may limit its placental transfer. It has no active metabolites. Fetal–maternal blood concentration ratios average 0.31 over the first 10 minutes after intravenous administration. Fentanyl produces moderate analgesia and mild sedation. There may be a brief period of decreased FHR variability, but no other disturbing FHR patterns have been reported. Comparative studies with meperidine indicate that the need for newborn naloxone administration is less after use of fentanyl.
Nalbuphine
Nalbuphine (Nubain) is a potent narcotic agonist–antagonist agent which, at equianalgesic doses, produces respiratory depression equivalent to that of morphine. The advantage and disadvantage of nalbuphine is that as the dosage is increased, a ceiling effect is seen for respiratory depression and unfortunately also for analgesia. Maximal respiratory depression occurs with a dose of 30 mg in a 70-kg adult. Sedation and dysphoric reactions may also occur. Reversal of other opioid effects may precipitate withdrawal in opioid-tolerant patients.
Butorphanol
Butorphanol (Stadol) is another synthetic narcotic with agonist–antagonist properties. It is five times more potent than morphine. It has achieved moderate popularity in the United States in the management of the pain of the first stage of labor. It is usually administered intravenously in doses of 1 to 2 mg. Butorphanol exhibits the same ceiling effect for analgesia and respiratory depression as nalbuphine. Maternal side effects may include sedation, dysphoric reactions, and reversal of other opioid effects.
Patient-controlled Intravenous Analgesia
Intravenous patient-controlled analgesia (PCA) is widely available and provides pain relief through self-administration of small doses of intravenous opioids. Fentanyl, remifentanil, and meperidine are the analgesics most commonly employed with this technique. The infusion pump is programmed so that the patient receives an incremental dose when she pushes a button, followed by a lockout interval when additional requests by the patient will not be administered. An hourly maximum may also be programmed. A basal infusion is rarely used in labor because of the risk of respiratory depression between contractions. The actual settings are dictated by the pharmacokinetics of the narcotic chosen. The main advantage of PCA is improved patient satisfaction due to a feeling of control and not having to wait for a nurse to bring pain medication. Use of PCA may also decrease nursing staffing requirements.
In a study by Rosenblatt and colleagues, metoclopramide (Reglan) was used as an antiemetic and analgesic adjunct to PCA for patients undergoing prostaglandin induction of labor for second-trimester termination of pregnancy. Patients were given intravenous metoclopramide, 10 mg, or saline placebo followed by PCA-administered morphine. Those receiving metoclopramide used 54% less morphine and had lower pain scores.
Narcotic Antagonists
Naloxone
Because all narcotics cross the placenta and can produce respiratory depression in the neonate, availability of an effective antagonist is essential. Naloxone also reverses analgesia, thus its prophylactic use is not advised. Naloxone may be administered to the parturient as an intravenous bolus of 0.1 to 0.4 mg to treat severe maternal respiratory depression, using the lowest possible dose. Care must be taken to titrate naloxone to the desired effect, since large doses have been implicated in the causation of myocardial infarction, pulmonary edema, and severe hypertension. Naloxone, 0.01 mg per kg, may also be administered intravenously, intramuscularly, or through the endotracheal tube to the newborn to reverse the respiratory depressant effects of placentally transferred narcotics. The effect is usually apparent within a few minutes and persists for as long as 2 hours. The neonate must be carefully observed for evidence of renarcotization, because the half-life of naloxone is less than that of most narcotics.
Sedative Drugs
Benzodiazepines
The principal benzodiazepine drugs are diazepam (Valium) and midazolam (Versed). Diazepam has been used extensively in other parts of the world for seizure prophylaxis in patients with severe preeclampsia. However, because of its side effects on the newborn, it has found little favor in the United States. Newborns exposed to diazepam characteristically exhibit hypotonicity, hypoactivity, and impaired temperature regulation and metabolic response to cold stress.
Midazolam is a newer benzodiazepine anxiolytic, a sedative drug with significant amnestic properties. It is five times more potent than diazepam and is soluble in water, a property that reduces pain associated with intravenous administration. Midazolam crosses the sheep placenta,
achieving a fetal–maternal concentration ratio of 0.15. Its metabolites are inactive, and the drug is excreted more rapidly than diazepam. Midazolam has been used in large doses as an induction agent for cesarean delivery, but because of its ability to cross the placenta, it has produced neonatal respiratory depression and decreased body tone and temperature. Midazolam has not been recommended for use as a tranquilizer-sedative in labor, because its amnestic properties are unacceptable to most parturients.
achieving a fetal–maternal concentration ratio of 0.15. Its metabolites are inactive, and the drug is excreted more rapidly than diazepam. Midazolam has been used in large doses as an induction agent for cesarean delivery, but because of its ability to cross the placenta, it has produced neonatal respiratory depression and decreased body tone and temperature. Midazolam has not been recommended for use as a tranquilizer-sedative in labor, because its amnestic properties are unacceptable to most parturients.
Barbiturates
Barbiturates may be used in the latent phase of labor. Although they cause maternal sedation and decreased anxiety, barbiturates lack analgesic properties and may increase the perception of pain when given without concomitant administration of a narcotic. Most barbiturates have long elimination half-lives and readily cross the placenta. Prolonged neonatal effects have led to the virtual elimination of these drugs from use during labor.
Other Sedatives
Phenothiazine derivatives, such as promethazine (Phenergan), have been used in obstetrics to provide sedation and decrease nausea. Maternal sedation is achieved without significant maternal or newborn side effects. It is important to remember that none of these drugs provides analgesia, and some parturients may object to the heavy sedation they cause. In addition, promethazine is a very painful intramuscular injection. It should have little place in the management of labor pain.
Ketamine (Ketalar), when administered intermittently at low doses (10 to 20 mg), can produce analgesia in parturients without causing maternal loss of consciousness or neonatal respiratory depression. For patients who are opioid tolerant, or for whom parenteral narcotics have proven inadequate for pain control, 20 mg ketamine intravenously followed by a low-dose ketamine infusion of 20 mg per hour may be helpful. However, the profound amnesia and potential for dysphoria or other psychomimetic effects when using ketamine limit its general use for labor analgesia. It is most often used for short painful procedures such as urgent forceps delivery or manual removal of the placenta.
Inhalational Agents
Nitrous oxide can be inhaled periodically during contractions in a 50% mixture with oxygen. During a painful contraction, the mother breathes from a mask connected to the regulator valve of a breathing circuit. A scavenging system is required by the Occupational Safety and Health Administration (OSHA) to eliminate exhaled waste anesthetic gases. When nitrous oxide is used in conjunction with narcotics, maternal oxygen saturation may decrease. Use of a pulse oximeter to ensure adequate maternal oxygenation is recommended. In practical terms, since almost all deliveries in the United States now take place outside the operating room, an anesthesia machine will probably not be available to safely administer inhalational agents.
Regional Analgesia
Local Anesthetic Agents
Most local anesthetic agents share a common structure consisting of a hydrophilic amino group connected by an intermediate chain to a lipophilic aromatic residue. Their mechanism of action is to block exchange of sodium and potassium ions across the cell membrane, probably through mechanical interruption of ion flow through cell wall channels. Local anesthetic drugs are manufactured as chloride salts. The nonionized base is able to diffuse across tissues, while the ionized form is actually the active component. The amounts of nonionized (mobile) and ionized (active) drug depend on the pKa of the local anesthetic and tissue pH. After injection of lidocaine (Xylocaine), the sensory nerve action potential decreases more sharply in pregnant women than in nonpregnant women. This implies that pregnant women have an increased susceptibility to the effects of local anesthetic agents.
Local anesthetics belong principally to two groups, those of ester and amide configurations. Ester drugs are generally characterized by their rapid onset of action, short duration, and low toxicity. Chloroprocaine (Nesacaine) is a representative of this group. It is rapidly metabolized by serum pseudocholinesterase, forming para-aminobenzoic acid. Lidocaine, bupivacaine, and ropivacaine are representatives of the amide group. These drugs are more highly bound to protein and have a slower onset and a longer duration of action. They are metabolized in the liver. Toxicity is usually greater for amides than for drugs of the ester group (Table 3.3).
Adverse Effects of Local Anesthetic Drugs
Systemic Toxicity
Systemic complications of local anesthetics include toxic blood levels of the drug and allergic reactions as well as reactions due to epinephrine that is often added to local anesthetic solutions to retard systemic absorption and prolong duration of action.
Maximal safe doses for healthy young adults are approximately 7 mg/kg (500 mg) of lidocaine with epinephrine, 2 to 3 mg/kg (200 mg) of bupivacaine, and 20 mg/kg (1500 mg) of chloroprocaine (Table 3.3).
The most common reason for high blood levels of local anesthetic drugs is accidental intravascular injection. This usually occurs during a pudendal block or when an epidural catheter has been placed or migrated into a vein. To minimize accidental intravenous injection, gentle aspiration
should be undertaken before each injection. Injection should be done slowly and incrementally with no more than 5 mL of local anesthetic drug to reduce the chance of a sudden increase in plasma levels. A marker such as epinephrine may be added to the local anesthetic solution so that intravascular injection will manifest as tachycardia.
should be undertaken before each injection. Injection should be done slowly and incrementally with no more than 5 mL of local anesthetic drug to reduce the chance of a sudden increase in plasma levels. A marker such as epinephrine may be added to the local anesthetic solution so that intravascular injection will manifest as tachycardia.
TABLE 3.3 Characteristics of Local Anesthetics Commonly Used in Obstetric Anesthesia | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||