Fig. 6.1
Stage 4 vaginal vault prolapse
Some type of preoperative evaluation for stress urinary incontinence (SUI), even in patients that report continence, is recommended due to the high rate of occult SUI in women with POP. The rate of occult stress urinary incontinence in the setting of Stage 2 prolapse or greater ranges from 33.5 to 67.9 % [18, 29–32]. A simple cough stress test with a full bladder and the prolapse reduced is often sufficient in patients with uncomplicated, demonstrable SUI. Patients with voiding dysfunction, mixed incontinence, incomplete bladder emptying, or prior urologic surgery should undergo a more thorough investigation with urodynamics.
The data is varied and the true predictive value of preoperative urodynamics remains unclear. Reena et al. studied women both before and after they underwent prolapse repairs without anti-incontinence procedures and found that 64.2 % of patients with documented occult SUI also demonstrated SUI postop [30]. In a small series of patients, Chaikin et al. reported that no patients with negative preoperative testing developed postoperative SUI [29]. Similarly, Hafidh et al. found a very low rate of postoperative SUI (4 %) in patients with no SUI demonstrated on preoperative urodynamics [33]. In contrast, studies by Wei and Al-Mandeel found a high incidence of postoperative SUI, 38 % and 42 % respectively, in patients with preoperative testing that was negative for SUI [32, 34]. What is clear, however, is that it is reasonable to place a midurethral sling at the time of prolapse repair in women with clinical SUI or documented occult SUI. In a 100 women with occult SUI who underwent TVT, Croutz et al. report a 83 % success rate for absence of postoperative SUI and only 2 % of patients with persistent SUI were symptomatic [35]. Meschia et al. also reported high rates of postoperative continence (objective 92 %, subjective 96 %) in patients who underwent TVT placement for occult SUI [36].
It remains controversial whether to place a sling in patients without clinical SUI or documented occult stress incontinence. The large, randomized CARE trial looked at women who were stress continent preoperatively and found decreased rates of postoperative SUI in women who underwent prophylactic Burch procedure at the time of sacrocolpopexy versus those who did not (32 % versus 45.2 %) [37]. However, midurethral slings are the most common anti-incontinence procedures performed, and it is unclear if this data can be extrapolated to colpocleisis and midurethral slings. In another large, randomized trial Wei et al. also looked at stress continent women undergoing prolapse repair and randomized patients to sling versus no sling. They also found a significantly lower rate of urinary incontinence in the sling patients (27.3 % versus 43 %), but at the expense of increased adverse advents including bladder perforation, urinary tract infection, major bleeding complications, and incomplete bladder emptying [32]. Another argument for prophylactic sling placement at the time of colpocleisis is the issue of access to the suburethral area. Successful colpocleisis is dependent on aggressive closure of the genital hiatus with levator placation [43]. Depending on the degree of closure, this can make it very challenging to access the midurethra for future placement of a sling.
Management of the Uterus
In women with a uterus it is prudent to confirm that there is no cervical or endometrial pathology which would be a contraindication to leaving the uterus in situ. Closure of the vagina will severely limit the ability to perform future surveillance via the traditional routes (pap smear, endometrial biopsy). A complete history should be taken regarding any history of abnormal pap smears as well as any episodes of postmenopausal bleeding. Benign cervical cytology should be documented in a patient with a history of any abnormal pap smears or a previous treatment for cervical intraepithelial neoplasia (CIN). The most recent guidelines from the American College of Obstetricians and Gynecologists (ACOG) recommend that women with a history of CIN2, CIN3, or adenocarcinoma in situ should have 20 years of negative screening following treatment prior to discontinuation of cervical cancer screening [38]. Therefore, it is recommended that any woman who would need continued surveillance based on her history should have a hysterectomy at the time of colpocleisis.
Women with a history of endometrial hyperplasia or any episodes of postmenopausal bleeding should have a preoperative assessment of the endometrium. This can be accomplished with endometrial sampling via endometrial biopsy or dilation and curettage of the uterus. Alternatively, the least invasive approach is to evaluate the endometrial thickness via transvaginal ultrasound. In women with postmenopausal bleeding, endometrial sampling is not required if an endometrial thickness of less than or equal to 4 mm is found on transvaginal ultrasound [39]. The decision to screen asymptomatic women with transvaginal ultrasound for assessment of the endometrial thickness may be left to the discretion of the surgeon. As reported by ACOG, the significance of an endometrial thickness greater than 4 mm in a postmenopausal woman without bleeding has not been established and does not routinely need evaluation in the absence of risk factors [39]. Concurrent hysterectomy is recommended for women with the finding of endometrial hyperplasia. Patients with the diagnosis of atypical endometrial hyperplasia should be referred to a gynecologic oncologist for surgical management due to the high rate (42.6 % [40]) of concurrent carcinoma.
It is important to note that hysterectomy with concurrent colpocleisis does not improve success rates over colpocleisis alone [14, 41], and the combination of procedures may significantly increased blood loss and patient transfusion requirements [14]. Due to this increased morbidity, exceptions to the above recommendations may be reasonable in patients who are of advanced age or debilitated and should be a joint decision between the patient and the surgeon.
Because colpocleisis eliminates the possibility of future vaginal intercourse, preoperative counseling is extremely important and patient selection is key. There is no identified minimum age requirement for consideration of the procedure. With colpocleisis, as in all cases of prolapse repair and reconstruction, the treatment plan must be individualized for each patient. Preoperative counseling should be specific and thorough including information on potential pessary management, alternative options for repair, possibility of postoperative urinary incontinence, and recurrence risk.
The option for concurrent midurethral sling placement should also be discussed with patients. Specifically, in the situation of demonstrated SUI in the setting of incomplete bladder emptying as well as patients with no preoperative urinary incontinence. The addition of a midurethral sling does not appear to cause a high risk of urinary retention and preoperative incomplete bladder emptying seems to resolve in most patients [31, 42]. In a series of 38 women who underwent colpocleisis and midurethral sling placement, Abbasy et al. reported a 2 % rate of elevated PVR postoperatively. Additionally, they saw a 90 % postoperative resolution of preoperative incomplete bladder emptying (defined as PVR greater than 100 ml) [42]. In a much larger series of 210 women, Smith et al. found a de novo voiding dysfunction rate of 1.9 % in women who underwent colpocleisis and midurethral sling. Similarly, they found a 91 % resolution of preoperative incomplete emptying [31]. An alternative, nonpermanent approach is to offer periurethral bulking injections to patients for whom the risk of retention is thought to be particularly high.
The decision whether to offer a midurethral sling to continent patients at the time of colpocleisis remains controversial. As detailed previously, the risk for de novo SUI may be quite significant; however, midurethral slings are not without complications or sequela. A large randomized controlled trial by Wei et al. specifically addressed this question by randomizing women without SUI who were undergoing vaginal prolapse repair to either have a midurethral sling or sham sling incisions. The sling group had significantly decreased rates of urinary incontinence at both 3 [23.6 % versus 49.4 % (p < 0.001)] and 12 months [27.3 % versus 43.0 % (p = 0.002)] [32]. However, the sling group did have significantly higher rates of complications including: bladder perforation, urinary tract infection, major bleeding complications, and incomplete bladder emptying for up to 6 weeks following surgery. Also of note, 5 % of patients in the sham group had a sling placement within the first year after surgery, but only 2.4 % of patients in the sling group required sling revision for voiding dysfunction. A detailed discussion of all the possible risks and benefits should be carried out with patients when making the determination of whether to place a sling in this population.
Surgical Procedures
All patients receive a preoperative prophylactic broad-spectrum antibiotic. Additionally, all patients have DVT prophylaxis; our standard is to use compression stockings and sequential compression devices on the lower extremities. Table 6.1
Table 6.1
Risk classification for venous thromboembolism
Level of risk | Definition | Prevention strategies |
|---|---|---|
Low | Surgery less than 30 min in patients younger than 40 years with no additional risk factors | No specific prophylaxis, early mobilization |
Moderate | Surgery lasting less than 30 min in patients with additional risk factors | Low-dose unfractionated heparin: (5,000 units every 12 h) OR Low molecular weight heparin: (2,500 units dalteparin or 40 mg enoxaparin daily) OR Graduated compression stockings OR Intermittent pneumatic compression device |
Surgery lasting less than 30 min in patients aged 40–60 years with no additional risk factors | ||
Major surgery in patients younger than 40 years with no additional risk factors | ||
High | Surgery lasting less than 30 min in patients older than 60 years or with additional risk factors | Low-dose unfractionated heparin: (5,000 units every 8 h) OR Low molecular weight heparin: (5,000 units dalteparin or 40 mg enoxaparin daily) OR Intermittent pneumatic compression device |
Major surgery in patients older than 40 years or with additional risk factors | ||
Highest | Major surgery in patients older than 60 years plus prior venous thromboembolism, cancer, or molecular hypercoagulable state | Low-dose unfractionated heparin: (5,000 units every 8 h) OR Low molecular weight heparin: (5,000 units dalteparin or 40 mg enoxaparin daily) OR Intermittent pneumatic compression device/graduated compression stockings + low-dose unfractionated heparin or low molecular weight heparin Consider continuing prophylaxis for 2–4 weeks postop |
For a patient in whom the uterus is to remain in situ, a LeFort colpocleisis is performed (Fig. 6.2a–e). To begin, outward traction is placed on the cervix using a tenaculum or Allis clamp. Two rectangles (anterior and posterior) are outlined with a surgical marker starting approximately 2 cm distal to the cervix and extending to the bladder neck anteriorally and mirroring this posteriorly. This will aid in maintaining orientation during removal of the vaginal epithelium. Laterally, there should be at least 2 cm of epithelium separating anterior from posterior rectangles in order to allow adequate tissue for creation of the drainage channels. Starting with the posterior wall 1 % lidocaine with a 1:200,000 dilution of epinephrine is infiltrated in to the subepithelial space to aid in hemostasis and hydrodissection. The demarcated areas are circumscribed with knife and sharp dissection is performed to start the removal of the vaginal epithelium from the underlying fibromuscularis layer. We use a number 10 blade to make the initial incisions. Dissection is initiated with tenotomy scissors for precision in finding the appropriate plane and then is completed with curved mayo scissors which are safer for combined blunt and sharp dissection. It can be helpful to refrain from making all incisions initially but rather to proceed in a systematic fashion (posterior to anterior) in order to decrease blood loss and improve visualization during dissection. Typically, a combination of sharp and blunt finger dissection with a sponge can be employed to facilitate removal of the epithelium once the appropriate plane is achieved. Hemostasis is maintained with meticulous use of monopolar cautery throughout the dissection. With the LeFort procedure only the areas of anterior and posterior rectangles are denuded.
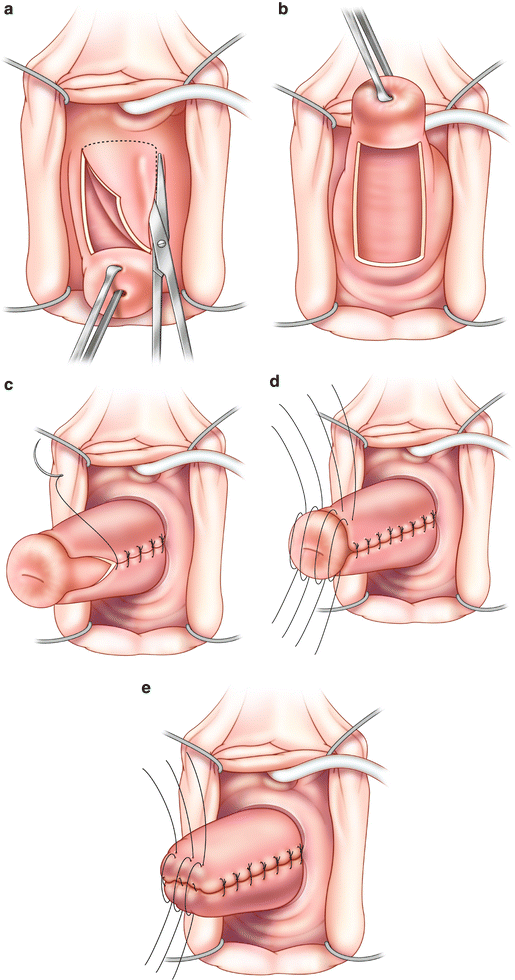

Fig. 6.2
LeFort colpocleisis. (a) Removal of the anterior rectangle of vaginal epithelium. (b) After removal of the posterior rectangle at least 2 cm of epithelium should remain laterally separating the denuded anterior and posterior rectangles. (c) Creation of the drainage channels: an interrupted or running stitch is used to tubularize the remaining, lateral strips of epithelium. (d) Anterior to posterior imbricating sutures to reduce the epithelialized cervix. (e) Further reduction of the uterus with continued anterior to posterior imbrications
To continue the LeFort procedure, channels are created after the removal of the epithelium and prior to starting closure of the vagina. Absorbable suture is used to tubularize the lateral strips of epithelium by suturing the epithelial edges together, superior to inferior. This may be done with an interrupted or running stitch. Our preference is to use 2-0 polyglycolic acid suture on a CT2 needle and run this closure towards the cervix, thus allowing the surgeon to sew towards him/herself. These channels will allow the drainage of cervical and uterine secretions. Care should be taken to continue to identify the location of the channels throughout the rest of the procedure in order to avoid inadvertently suturing them closed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


