Novel Pharmacological Approaches for Treatment of Obstructive Sleep Apnea
Introduction
Adenotonsillar hypertrophy has been identified as the major pathophysiological factor in the causation of upper airway obstruction in pediatric obstructive sleep apnea (OSA). Over the last few decades, novel immunological techniques have enabled identification of some of the specific tonsillar cells underlying inflammatory immune responses in specific contextual settings; however, the exact mechanisms leading to the adenotonsillar cell proliferation are still not fully understood.1 It is apparent that a combination of structural and neuromuscular abnormalities contributes to the occurrence of OSA in children.2 Tonsils and adenoids continue to grow from birth to 12 years of age, with the greatest increase in size during the ages of 2 and 8, a process that is paralleled by the surrounding structures that constitute the upper airway. During this period of time, the gradual growth in the size of the skeletal boundaries of the upper airway and the occurrence of disproportionate growth of adenoids and tonsils relative to the other upper airway structures will result in a relatively narrower upper airway, even if, as mentioned, the growth of all structures during this period is parallel to each other.3 The relative physiological narrowing of the upper airway space in children is, however, compensated by the activation of the upper airway muscles and through increased central ventilatory drive, preventing the excessive upper airway collapsibility when compared to adults.4 As such, OSA only occurs when the interaction between the anatomical restriction and the neuromuscular reflexes fails to preserve airway patency, a state-dependent process, since it does not occur during wakefulness.
Nevertheless, the size of the adenoids and tonsils plays a significant role in the severity of OSA.5,6 This excessive tissue enlargement can be induced by proliferation of specific cellular components and is due to various isolated or recurrent bacterial or viral infections as well as exposure to environmental irritants such as allergens, cigarette smoke and air pollution.7–11 The location of the adenoids and tonsils at the entrance of the respiratory and alimentary tracts positions them as the first site of contact with a variety of microorganisms and antigenic substances that are present in food and inhaled air, resulting in proliferation and growth in these organs.
Currently, surgical extirpation of these tissues is the first line of treatment for either recurrent infection or pediatric sleep apnea;12 however, the efficacy of adenotonsillectomy has recently been challenged, with informal assessments of striking reductions in estimated success rates to less than 30% of all cases,13,14 further intensifying the need for developing non-surgical therapeutic options more than ever.
Inflammation in Pediatric OSA
Since adenotonsillar hypertrophy and hyperplasia are the primary causes of OSA in children, the mechanisms leading to the enlargement of these complex lymphoid structures has been a main focus of investigation among researchers in the field. The earlier studies were primarily focused on assessment of bacterial infections as the underlying cause of recurrent tonsillitis and as contributing factors to recurrent chronic otitis media and their epidemiological links with adenotonsillar hypertrophy.15–19 Since then, different theories have evolved, particularly in relation to the development of adenotonsillar hypertrophy that underlies OSA in children. Indeed, current opinion surmises that a low-grade systemic inflammation is present in addition to local upper inflammation in pediatric OSA. Multiple studies have investigated the cause and effect of mechanical vibration due to intermittent collapse and occlusion of the upper airway manifested by snoring as a potential primary source of inflammation in children with OSA. In this context, localized inflammation of the upper airway tissue would occur as a result of continuous and periodic mechanical insult due to tissue vibration and intra-luminal pharyngeal pressure swings from the repeated upper airway obstructive events.20,21 Thus, the initiation of mild snoring would promote inflammation that would progressively aggravate the snoring, leading to a vicious cycle of disease progression. Although this theory is definitely attractive, there is no available epidemiological evidence supporting such a temporal trajectory of progressive worsening of OSA in children. Indeed, the time course of disease initiation and progression has yet to be investigated. Some investigators argue that snoring-related mechanical trauma to the soft palate and uvula may not be the sole factor underlying upper airway inflammation considering the fact that both nasal and oropharyngeal mucosal inflammation are also present in patients with OSA, and that the nasal mucosa is not subjected to repeated snoring-induced injury.22–24 Conversely, the alternations between hypoxia and re-oxygenation could produce excessive free radicals mediated through several intracellular pathways that potentially will lead to both local and systemic inflammation.25 Another possible mechanism that has been advanced links early life infections with respiratory viruses as eliciting enduring immune cell-mediated amplificatory memory responses that will be triggered upon exposure to inhaled stimuli such as environmental pollution or recurrent viral infections.26
Evidence of Local Upper Airway and Systemic Inflammation in Pediatric OSA
In adults with OSA, increased concentrations of pro-inflammatory markers such as interleukin-6 and 8-isopentane, and oxidative stress markers have been reported in the exhaled upper airway condensate.27–29 Higher levels of nuclear factor kappaB-dependent genes such as tumor necrosis factor-alpha (TNF-α) and interleukin 6 (IL-6) are also present,30 and treatment of OSA with continuous positive airway pressure was associated with reductions in serum levels of high-sensitivity C-reactive protein (hsCRP) and IL-6 concentrations,31 while local airway inflammation was improved, as evidenced by reduced numbers of neutrophils.32 Thus, adults with OSA present evidence of both local (i.e., upper airway) and systemic low-grade inflammatory changes.
What about children? Since the initial study by Tauman and colleagues, who reported increased plasma hsCRP levels in children with OSA compared to controls, multiple studies have corroborated such findings.33 Interestingly, improved hsCRP levels and cardiovascular markers after effective treatment of OSA were also demonstrated.33–36 Recent studies further suggest that variances in the hsCRP levels or in polymorphisms within the NADPH oxidase gene or its functional subunits such as p22phox, both of which reflect systemic inflammatory responses, appear to account for important components of the differences in cognitive function deficits associated with OSA in children.36–38 Thus, the degree of systemic inflammation and oxidative stress may play a major contributor to the presence of morbidity in children with OSA and, as a result, targeting of such pathways may provide a viable therapeutic strategy towards prevention of end-organ morbidity.37,38
Another significant contributor to the pathophysiology of pediatric OSA and the treatment outcomes is obesity, which should be viewed as yet another systemic inflammatory condition. The rather accelerated increase over the last two decades in the prevalence of pediatric obesity has led to substantial changes in the cross-sectional demographic and anthropometric characteristics of the children who are referred for evaluation of suspected OSA.39,40 Obesity is a proven and definitive risk factor that operates in a synergistic fashion among children at risk for OSA, and such interactions could, in fact, reflect augmented activation of inflammatory pathways.41–43 Thus, considering the deleterious consequences of OSA in children if left untreated, the use of therapeutic agents towards reduction in inflammation or specific oxidative stress may yield to reverse or palliated morbidities.
Leukotriene and Leukotriene Receptors
Cysteinyl leukotrienes are major mediators of inflammation in both humans and animals, and serve as potent neutrophil chemoattractants and activators.44 The cysteinyl leukotriene receptors 1 and 2 are expressed on several tissues including the nasal mucosa and the lungs.45,46 The leukotrienes B4 and the Cys LTs, C4, D4, and E4 can mediate inflammation in both the upper and lower airways by binding to Cys Lt receptors, although LTB4 has 2 cognate receptors that exhibit much higher affinity to this leukotriene. A considerable overlap and interdependency between asthma and OSA47–49 as well as compelling evidence of the favorable effect of CYS LT1-R antagonists in reducing inflammation in children with inflammatory conditions such as asthma and allergic rhinitis,50,51 suggested a potential role for leukotriene modifiers in the management of pediatric OSA.
Assessment of the LT1-R and LT2-R expression in tonsils and adenoids of children with OSA compared to children with recurrent infectious tonsillitis without OSA revealed higher protein expression levels of LT1-R and LT2-R, along with a distinct topographical pattern of distribution, suggesting that different mechanisms promoting inflammation are operational in OSA versus infection-induced tonsillitis.52 In this context, it would appear that leukotrienes may contribute to the higher proliferative pattern of upper airway lymphoid tissues than is present in the generation of adenotonsillar hypertrophy that characterizes the majority of children with OSA. Indeed, immunohistochemical characterization of germinal centers in tonsils of children with OSA showed a peripheral location of LT1 receptor (Figure 36-1), which might be due to either its occurrence during late stages of maturation of lymphoid tissues or, as proposed by others, LT1-R-positive cells might have migrated from the vasculature to occupy sites within the tonsils, where their activation may be of functional importance.53 The initial characterization of heightened expression of leukotrienes and their receptors in the adenoids and tonsils of OSA patients was subsequently confirmed by Kaditis and colleagues who showed that tonsils of children with OSA display an enhanced expression of cysteinyl leukotriene receptors in T lymphocytes without an associated increase in serum hsCRP concentrations.54 Of note, an increased preponderance of CD8-positive lymphocytes is present in tonsils of children with OSA (see Figure 37-1). Increased concentrations of either LTB4 or LTC4/D4/E4 in the adenotonsillar tissues of children with OSA could promote upper airway lymphoid hypertrophy/hyperplasia when compared with children with recurrent infectious tonsillitis.55 Up-regulation of LT1-R and LT2-R expression could potentially promote tonsillar enlargement in children with OSA by promoting the proliferation or the pro-inflammatory activity of T-cell lymphocytes within the tonsillar and adenoidal tissues.56 Of note, increases in leukotriene concentrations LTB4 and LTC4/LTD4/LTE4 are readily identified in the exhaled breath condensates of children with OSA.57 Interestingly, and similar to children, LTB4 concentrations were also found to be elevated in the exhaled condensates of adults with OSA and appear to be correlated with the severity of OSA.58
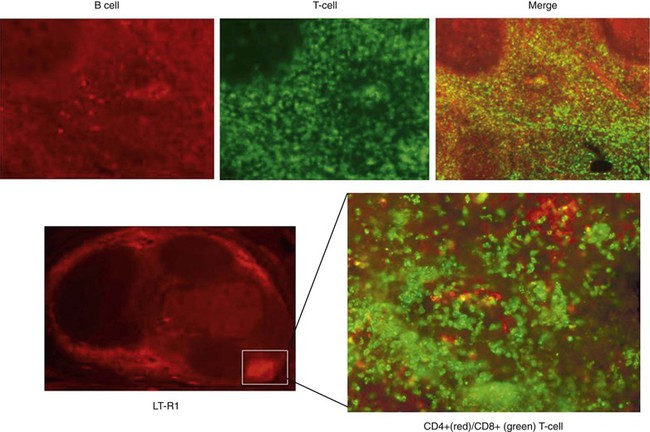
Figure 36-1 Immunihistochemical stains of a tonsil from a child with OSA showing the disproportionate abundance of T-cell lymphocytes, particularly CD8-positive lymphocytes, and the high level of expression of leukotriene receptors in the peripheral regions surrounding the germinal centers.
To further assess the effects of LTD4 and several LT receptor antagonists on lymphoid tissue proliferation, Gozal and colleagues developed a mixed-cell culture model using freshly dissociated tonsils or adenoids harvested during adenotonsillectomy from children with polysomnographically diagnosed OSA or recurrent tonsillitis. Cellular proliferation and release of inflammatory cytokines were assessed in cell culture supernatants using standard enzyme-linked immunosorbent assays.59,60 In this ex vivo model, LTD4 elicited dose-dependent increases in adenotonsillar cell proliferation that were markedly enhanced in children with OSA.
On the other hand, LT antagonists exhibited dose-dependent reductions in adenotonsillar cellular proliferation rates, with montelukast showing superior potency compared to the other antagonists tested, suggesting that LT-dependent pathways underlie components of the intrinsic proliferative and inflammatory signaling pathways and play a significant role in adenotonsillar hypertrophy in children.61
Interestingly, the cell substrates mediating the hyperplastic responses of these tissues demonstrate a T-cell preponderance of proliferation of CD3-, CD4-, and CD8-positive lymphocytes in OSA, while B-cell lymphocytes were more likely to be proliferative in RI.54,60 Circulating levels of LTB4 and Cys LT were also found to be elevated in children with mild and moderate to severe OSA compared to controls, and decreased after treatment.62
Therefore, based on the aforementioned studies, it is plausible to conclude that lipoxygenase-dependent pathways are involved in the pathophysiology of OSA in children, and may serve as targets for treatment of this condition, particularly in selected subgroups of children. Improving the severity of residual OSA after adenotonsillectomy with leukotriene antagonists was shown as an effective approach, justifying the use of leukotriene receptor antagonists as potential adjuncts to pre- and to post-T&A in pediatric OSA, in an effort to improve the outcomes associated with this surgery.63 Leukotriene modifiers could also be considered as a direct therapeutic option in mild OSA as an alternative to T&A.10 A recent randomized double-blind, placebo-controlled trial with oral montelukast in children with mild OSA showed significant improvements in apnea index and in adenoid size.64



