Restless Legs Syndrome, Periodic Leg Movements and Periodic Limb Movement Disorder
Introduction
A feeling of restlessness can describe both a physical and a psychological sensation that prompts movement or the urge to move. This ubiquitous human experience is at once recognizable as normal yet, in genetically susceptible individuals, an exaggeration of these (sometimes painful) sensations results in intrusive compensatory movements and the degradation of rest, sleep, performance and health. It is in these instances – when feelings of internal restlessness and the urge to move interfere with routine activities – that we use the term restless legs syndrome (RLS) or Willis–Ekbom disease. The characteristic symptoms of RLS have been known for hundreds of years and were first reported in medicine in the 1600s. The Swedish neurologist Karl Ekbom formally described the clinical, epidemiologic and pathophysiologic correlates of the condition in 1945.1
The syndrome has four well-known clinical criteria: (1) an uncomfortable sensation or unexplainable urge to move the legs or other affected body part; (2) increasing symptoms with rest or inactivity; (3) a reduction of symptoms with movement; and (4) a circadian enhancement of symptoms in the evening or night. Ekbom reported all aspects of RLS occurring in children, but it was not until the mid 1990s that the first case reports of children with RLS were published and research began to focus on the potential genetic causes for this familial disorder.2,3
Periodic limb movement disorder (PLMD), which is clinically defined as a disorder distinct from RLS, is noted in children as well as adults. The diagnostic criteria for PLMD include increased PLMS for age (>5 per hour) and a clinical sleep disturbance that is not accounted for by another sleep disorder, including RLS. Clinical case studies suggest that children may manifest PLMD before developing RLS later in childhood.4 Observations such as these help our understanding of the biological relationships between the sensory and motor components of these seemingly distinct but related disorders. Due to the limited available pediatric research, this chapter provides consensus opinion-based – as well as current evidence-based – information on the subject of childhood RLS and PLMD. Prevalence, pathophysiology, diagnosis, treatment and clinical associations of these conditions are also discussed.
Symptoms and Prevalence of RLS and PLMD in Children
Restless legs syndrome and PLMD are very common in northern European populations and are believed to be among the most common inherited conditions known. Surveys show that between 4% and 15% of adults in the US and Western Europe have symptoms consistent with RLS.5–7 In addition, there is evidence that up to 40% of adult RLS sufferers may have had the onset of symptoms in childhood or adolescence.8,9 Prevalence rates vary from population to population due to genetic heterogeneity as well as to differences in survey tools used.
Validated RLS inventories, such as the International Restless Legs Syndrome Study Group Rating Scale (IRLS),10 have allowed investigators to utilize common tools in adult studies. The occurrence of RLS is increased in women compared to men (3 : 2 female : male). In younger populations the female : male ratio is closer to 2 : 1. Studies of adults over the age of 65 years show RLS prevalence increasing up to 10–20%.11
Approximately 8% to 20% of adults fulfill standard RLS criteria,12,13 but only 2.7–3.9% of adults meet criteria for moderate to severe RLS (episodes twice or more per week with moderate to severe distress).12,14 Not all people with RLS symptoms require medical attention.
Picchietti and colleagues – using the NIH consensus criteria for the diagnosis of definite RLS in children – performed the most comprehensive prevalence survey to date of RLS in children from the US and UK.15 They demonstrated that 1.9% of 8–11-year-olds and 2% of 12–17-year-olds fulfilled these criteria. The prevalence of moderately severe RLS was 0.5% and 1% in 8–11-year-olds and 12–17-year-olds, respectively. There was no gender preference noted, unlike in adult RLS. In addition, the data showed a potentially strong genetic predisposition for RLS with 71–80% of children having at least one affected parent.15 In 2010, an RLS symptom severity scale for children and adolescents was developed,16 although large-scale validation studies have yet to be performed.
Periodic Limb Movement Disorder and Periodic Leg Movements
PLMD is delineated as a separate sleep-related movement disorder,17 although many experts in the field consider PLMD to exist on a continuum with RLS. Both disorders are associated with low ferritin levels, respond to dopaminergic medications, share similar genetics, and are more common in Caucasian children than in other racial groups (OR = 9.5).18 The supposition of a continuum from PLMD to RLS is further bolstered by clinical evidence that some children manifest PLMD or PLMS years before the symptoms of RLS develop.4 In a retrospective longitudinal study, Picchietti and Stevens identified PLMD or probable/possible RLS in 18 children (mean age = 10.3 years) and subsequently diagnosed these children with definite RLS an average of 11.6 years later. In addition, these children had many of the comorbidities commonly associated with RLS such as ADHD, parasomnias, and a low serum ferritin level.4 Thus, despite the differentiation of RLS from PLMD on clinical grounds, this evidence illustrates their potentially common pathophysiology and treatment.
With the recent discovery of a dose-dependent association between the BTBD9 gene (on chromosome 6p) and the findings in RLS of both PLMS and low serum ferritin, there remains no doubt that the motor and sensory features of RLS are related.19 In addition, given that the vast majority of RLS sufferers have PLMS on polysomnography (PSG) testing (reports vary from 80% to 100%), the presence of a common neural mechanism for both sensory and motor symptoms is suggested. In contrast, PLMS are noted in a number of other disorders (such as Parkinson’s disease and Tourette’s syndrome), in association with certain medical conditions (such as pregnancy), in association with other sleep disorders (such as narcolepsy, sleep deprivation, and obstructive sleep apnea (OSA)), as a result of OSA treatment with continuous positive airway pressure),20 and as a result of certain medications (such as selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs)).21–27
Since PLMS are not disease-specific, some authorities question whether or not PLMS (and thus PLMD) should be considered abnormal. They also increase with age and occur in up to 30% of adults over the age of 50 years,8 suggesting neurological deterioration that is part of normal aging.
Counter to the argument – that PLMS represent normal motor activity – are data that demonstrate the impact of PLMS and RLS on both health and psychological well-being. Sympathetic over-activation may explain the association between PLMS and chronic cardiovascular conditions in adults;28 and, in RLS sufferers, this relationship is thought to elevate the risk for stroke and even the risk for insulin resistance and type II diabetes.29 The natural state of sympathetic hyperactivity associated with youth may actually place children and younger adults at an even higher risk.30 A number of adult and pediatric studies demonstrate a strong relationship with ADHD, behavioral disorders, and cognitive deficits as well as with depression and anxiety in RLS populations.31–34 Adults with RLS carry a 4–5-fold increased risk for depression and a 13-fold increased risk for panic disorder.35–38 Patient-reported outcome measures demonstrate a significant impact of RLS in adults.12,39–42 Studies demonstrating that RLS causes clinical morbidity in pediatric populations, however, are lacking (Figure 43-1).
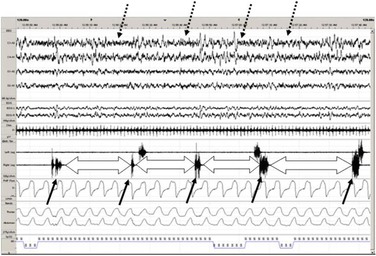
Figure 43-1 Periodic leg movements during sleep (PLMS) depicted on 2-minute PSG recording page. Each solid arrow demonstrates an individual PLM in a sequence of PLMS recorded with right and left anterior tibialis EMG. Block arrows denote the inter-movement interval, which is very consistent as noted in RLS. Also, note cortical arousals (dashed arrows) associated with PLMS that are thought to confer excessive autonomic activity and sleep fragmentation. From: Durmer JS and Quraishi GH. Restless legs syndrome, periodic leg movements and periodic leg movement disorder in children. Pediatric Clinics of North America 2011;58:591–620.
Measuring Periodic Leg Movements in Sleep
PLMS in children are not uncommon, especially in children with RLS; and, studies suggest that between 8.4% and 11.9% of children may have PLMD.43 Normative pediatric data from studies that record PLMS via PSG and/or accelerometry (also referred to as actigraphy and actometry) demonstrate that most children and adolescents exhibit a periodic leg movement index (PLMi) no greater than 5/hour.43–47 By contrast, up to 74% of children with definite RLS have a PLMi in excess of 5/hour.48 When a PLMi are noted in children, clinicians should consider further investigation into the possibility of RLS.
Standardized criteria commonly utilized to score PLMS recorded via bilateral anterior tibialis electromyography (EMG)49 have been modified in the past 5 years. Specifically, four movements must be scored in a row to qualify as periodic leg movements, and each of these movements must have an EMG amplitude greater than 8 microvolts, the movements must be separated by between 5 and 90 seconds, and individual movements must last between 0.5 seconds and 10 seconds.50 These criteria allow for significant variability in the expression and periodicity of PLMS. Investigation based on rhythmic firing characteristics of neural systems provides a less variable measure of PLMS and provides a more scientific assessment for the potential generation of PLMS. Using the technique of a Markov-based stochastic mathematical process to measure inter-movement intervals and characterize the periodicity of PLMS,51 researchers have demonstrated that subjects with RLS-related PLMS (and likely PLMD) have less inter-movement interval variability (with intervals clustering between 24 and 28 seconds) than is seen in PLMS due to other conditions (such as narcolepsy and ADHD).52–54 There has been speculation that this particular frequency range is caused by neural pattern generators in the spinal cord and/or diencephalon.
PLMS demonstrate marked night-to-night variability, in children as well as in adults, and multiple nights of testing may be required to accurately quantify and diagnose PLMS.55 Ambulatory or home-based PLMS measurements made using accelerometry in adults correlate with PLMS measured by PSG, and the same technique may be useful in the clinical evaluation of a child.56,57
Pathophysiology of RLS/PLMD
The neurobiological mechanisms leading to the motor and sensory symptoms of RLS/PLMD remain the topic of scientific inquiry. Clinical observations demonstrate that most primary cases of RLS respond to dopaminergic treatments such as levodopa/carbidopa, ropinirole and pramipexole. These observations suggest that monoaminergic neurotransmitter systems within the central nervous system play a pivotal role in the expression of RLS/PLMD symptoms. Neuroanatomic and physiologic models of diencephalic and spinal cord dopaminergic systems support an intriguing hypothesis related to the sole source of dopamine innervation in the spinal cord, namely the bilateral A-11 hypothalamic cell groups. These cells project to all levels of the spinal cord and provide dorsal (sensory), ventral (motor) and mediolateral (sympathetic) dopaminergic activity. This important neuroanatomic property suggests that the A-11 dopaminergic cell groups may be major contributors to the development of RLS.58 Animal models using dopamine receptor (D2-like) knock-out mice suggest that the loss of spinal cord gating via D2-like receptors may precipitate the sensory and motor symptoms of RLS/PLMD (Figure 43-2).59
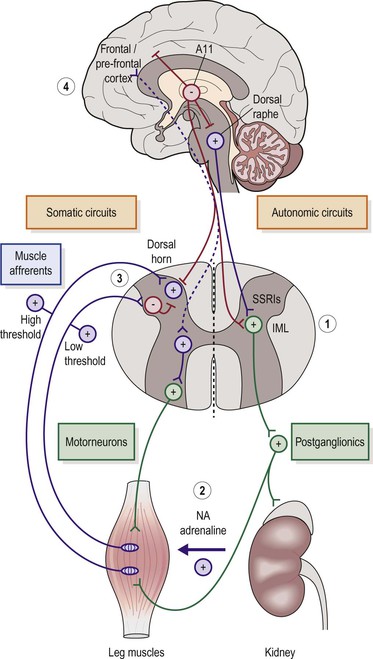
Figure 43-2 The proposed role of the diencephalic dopaminergic cell group A-11 in the pathophysiology of RLS. A11 neurons project caudally to inhibit the dorsal raphae nucleus. This results in less sympathetic excitation via the spinal cord intermediolateral cell column (IML). A-11 neurons also project to all levels of the spinal cord and inhibit dorsal horn sensory transmission as well as the afferent projections of the IML (#1). As proposed in RLS, a loss of A-11 dopaminergic inhibition results in increased sensory input to cortex (uncomfortable sensations or urge to move), increased sensory activation of the spinal cord reflex arc (#3) (causing PLMS), and increased sympathetic activity (#2) (accentuating PLMS and associated medical conditions such as hypertension and pro-inflammatory states). The loss of rostral A11 projections would also enhance cortically mediated sensory discomfort associated with RLS (#4). From: Clemens S, Rye D, Hochman S. RLS revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67:125-130.
An association between iron deficiency and RLS was first noted by Nordlander in 1954.60 Impairment of brain iron availability is hypothesized to play a role in the pathogenesis of RLS and PLMD, based on several studies in animals and humans. Serum iron indices, such as total iron, hemoglobin levels, and hematocrit, are usually within the normal ranges in RLS patients. Nevertheless, brain iron deficiency has been implicated in humans from investigations using cerebrospinal fluid analysis of iron and ferritin,61,62 magnetic resonance imaging and ultrasound of the substantia nigra,63–65 and autopsy examination of brain tissue from RLS subjects.66,67 The link between iron deficiency and dysfunction of central dopaminergic systems is based on evidence that iron is a cofactor for the rate-limiting enzyme in dopamine synthesis, tyrosine hydroxylase, and is required for postsynaptic D2 receptor function. Iron deficiency results in down-regulation of striatum and nucleus accumbens dopamine receptors as well as dysregulation of dopamine vesicular release.68–70 Correlations between peripheral serum ferritin levels and cerebrospinal ferritin levels in RLS patients demonstrate that serum ferritin levels below 50 ng/mL correlate with relative body iron storage deficiency.61,62
Recent evidence suggests that lower ferritin status in RLS may not only correlate with alterations in dopamine metabolism and neural transmission but may also be associated with an inability to retain intracellular ferritin.71 In a study comparing 24 women with early-onset RLS with a control group of 25 women without RLS, Earley et al. demonstrated there were marked differences in proteins associated with iron trafficking into cells (soluble transferrin receptor (TfR) and divalent metal transporter 1 protein (DMT-1)) and out of cells (ferroportin protein). RLS patients had higher TfR and DMT-1 levels consistent with an increased cellular need for iron. Paradoxically, they also had higher ferroportin protein levels, which would normally signify high intracellular iron levels since this protein regulates iron efflux. Thus, patients with RLS seem to have an intracellular need for iron; yet, the proteins responsible for regulating cellular iron content create a situation tantamount to a leaky bucket.
The Genetics of RLS/PLMD
Genetic investigations over the past 12 years using twin concordance, family association, familial linkage, and genomic association methods have created a more complex picture. Twin studies suggest a heritability of approximately 54% for RLS.72 Additional genomic and linkage studies suggest that different sensorimotor phenotypes may be linked to different genetic loci. Ten different genetic loci for RLS have been identified (on multiple chromosomes) using familial linkage analysis, and these findings suggest that RLS/PLMD is a complex genetic trait that interacts with environmental factors.73–86
Initial evidence from a familial segregation analysis in Germany demonstrated that early-onset RLS (patients <30 years old) supported a single major gene model with an autosomal dominant mode of inheritance.87 Late-onset RLS did not demonstrate this effect. On further analysis, two distributions of RLS were identified, based on age of onset (with the dividing point at 26.3 years). These data suggest that RLS is primarily genetic in younger age-of-onset groups but that environmental components influence the expression of RLS and may be more relevant in the later age-of-onset groups.88 Many studies of familial RLS demonstrate genetic anticipation where subsequent generations show a progressively earlier ages of symptom onset.89–92 Thus far, there is no evidence to support a trinucleotide repeat mechanism for RLS (as is the case in certain other disorders that also demonstrate genetic anticipation, such as spinocerebellar ataxia and Huntington’s disease).93,94
To understand if there are more common RLS alleles with particular phenotypes, genomic association studies using single nucleotide polymorphisms (SNPs) have been performed since 2007. An Icelandic investigation of 306 RLS cases and 15,664 controls identified three genes (BTBD9, GLO1, and DNAH8) on chromosome 6p that were associated with RLS patients with PLMS as a major component of their phenotype.19 This study demonstrated dose-dependent associations among the BTBD9 allele, PLMS, and serum ferritin levels. Heterozygous individuals for this allele showed twice the risk for RLS with PLMS, while those homozygous for this variant had four times the risk. Serum ferritin levels were also lower in those with the additional BTBD9 allele. In a second genomic study performed in a German cohort of 401 familial RLS sufferers and 1644 controls, four genes (MEIS1 – ch 2p, BTBD9 – ch 6p, MAP2K5 – ch15q, and LBXCOR1 – ch 15q) were found in association with RLS.95 In both the German and Icelandic investigations, the BTBD9 allele, which is widely distributed in the brain and body, was found to be associated with RLS.
Replication studies of the familial and genomic findings from adult studies have proven inconclusive in children. One study assessed gene variants in 23 children and found an 87% positive family history of RLS and a trend toward association with MEIS1 and MAP2K/LBX-COR1 variants, but no association was found with BTBD9.96 Another study of 386 children with ADHD and RLS did not find a genetic association.97
The Diagnosis of RLS/PLMD in Children
As stated above, the diagnosis of PLMD in children and adolescents formally requires: (1) PLMS documented by polysomnography and exceeding a PLMi of 5 per hour, (2) clinical sleep disturbance, and (3) the absence of another primary sleep disorder or reason for the PLMS (including RLS). In 1995, the International Restless Legs Syndrome Study Group developed standardized criteria for the diagnosis of RLS in adults.98 In addition to the four essential features (noted above), five additional clinical features of RLS were included: (1) sleep disturbance, or daytime results of sleep disturbance, (2) involuntary movements during sleep (PLMS) and during wake (periodic leg movements during wakefulness (PLMW)), (3) neurological examination findings consistent with RLS, (4) typical clinical course and exacerbating factors, and (5) positive family history. Idiopathic or primary RLS was also distinguished from reactive or secondary RLS (which may be caused by conditions such as uremia, neuropathy, medications, and anemia).
In 2003, an NIH workshop produced expert consensus criteria for the diagnosis of RLS in children and special populations.99 Categories of diagnostic certainty for RLS in children aged 2–12 years old were established based on varying levels of clinical evidence (see Box 43-1). The essential four adult criteria were retained for the diagnosis of definite RLS in adolescents (13–18 years old). In addition, categories of possible and probable RLS were established, suggesting that individuals with incomplete RLS should be followed for progression of symptoms.
In 2010, a multi-dimensional, self-administrated, patient-reported outcome questionnaire (the Pediatric Restless Legs Syndrome Severity Scale (P-RLS-SS)) was published to assess pediatric RLS symptom severity and impact.16 In addition to establishing a metric, this study demonstrated that children experience RLS symptoms during both daytime and nighttime. They experience pain in association with RLS and often utilize countermeasures such as rubbing or moving. Many children provided non-verbal descriptions of their symptoms using a visual analog scale and free-hand drawings of their experiences.100 Despite variations of interpretation, such visual approaches may be more appropriate for diagnosing RLS in younger or less fluent children, especially when one is searching for a starting point in the diagnostic process (Figure 43-3).
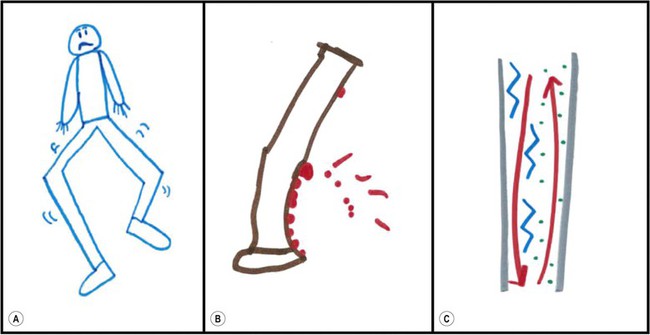
Figure 43-3 Children’s drawings of RLS symptoms: (a) From an 8-year-old boy: ‘It’s like my legs are wiggly;’ (b) From an 11-year-old girl: ‘Well this picture shows like, see like it’s ant bites that’s kind of showing you that it’s really hurting me like in those areas;’ (c) From a 14-year-old girl: ‘I feel stuff going up and down my legs where it just tingles. And that’s more when it starts feeling like a little numb and then these represent my tingles. And then the red would be just when it hurts.’ Adapted from: Picchietti DL, Arbuckle RA, Abetz L, Durmer JS, Ivanchenko A, Owens J, Croenlein J, Allen RP, Walters AS. Pediatric restless legs syndrome: analysis of symptom descriptions and drawings. Journal of Child Neurology 2011;26(11):1365–76.
In the diagnostic interview for RLS it is important to provide a non-leading introduction in order to allow the child to express his or her own experience. Adult family members should be questioned as well since they may have similar symptoms or even have an RLS diagnosis themselves. However, clinicians should direct their inquiry toward the child to help them recreate the last time they experienced something that made it hard ‘to fall asleep’ or ‘to lie still in bed.’ Often, the presenting complaint from the child or parent may not appear to be related to ‘restlessness’ or ‘kicking.’ Common complaints in pediatric RLS are difficulty falling asleep, not wanting to go to sleep, and (occasionally) difficulty remaining asleep. By directing the conversation to a description of the bedtime routine, a young or forgetful child can start by reporting the bedroom surroundings to contextualize the sleep-onset experience. Since this is the most common time for symptoms to emerge, the child may then recall the last time he or she had a problem falling asleep. The clinician can use similar techniques to help the child remember symptoms that occur at other times of the day, such as sitting in class at a desk, attempting to nap, or while doing homework after school. Children provide imaginative descriptions of RLS symptoms – e.g., ‘soda bubbles in my legs,’ ‘ants biting my leg,’ ‘just want to move,’ or ‘got to kick.’4,15,101 It is not common for children to use the term ‘urge to move’ in relationship to their description of RLS symptoms, but it is helpful to allow them to physically demonstrate compensatory maneuvers such as wiggling, rubbing, kicking, hitting and even constantly moving ‘to find the cool spot.’ After exhausting one’s direct inquiry with a child it is sometimes helpful to incorporate the parent into the discussion to remind the child of bedtime rituals and activities that may provide some recall.
Clinicians must be aware of causes of secondary or reactive RLS in children since their treatment may be quite different from that of primary RLS. Age-associated causes of secondary RLS include joint pain and arthritis, Osgood–Schlatter disease, dysasthesias related to peripheral neuropathy or radiculopathy, akathesia related to anti-dopaminergic medications, and cutaneous pain related to dermatitis or rashes (see Box 43-2). Identification of one of these entities or RLS should prompt clinicians to ask for symptoms of the other. Another condition commonly associated with, and often possibly identical to, RLS is that of growing pains. Children diagnosed with growing pains unrelated to any other identifiable disorder commonly demonstrate a strong family history of RLS and, in many cases, fulfill the diagnostic criteria for RLS.102 The prevalence of growing pains in children ages 4–6 years measured with validated instruments is as high as 37%.103 And, the presence of typical growing pains in children with either a family history of growing pains or RLS should prompt clinicians to consider a diagnosis of RLS.
It is helpful to note that, in children, symptoms commonly associated with RLS may also be seen with other sleep or medical/psychiatric disorders. Irritability, depression, anxiety, and hyperactivity may occur secondary to sleep deprivation; they may also be symptoms of comorbid conditions such as panic disorder, generalized anxiety disorder, ADHD, ODD and depression. Parasomnias, sleep-related movement disorders, and the insomnias also may be the presenting symptoms in some cases of childhood RLS.4,101,104 It is important to note mood and behavioral symptoms since these secondary symptoms of RLS may be most prominent.
Although RLS is a clinical diagnosis that does not require testing, it is not possible to determine the presence or effect of PLMS on a child’s sleep without a test. Parental reports and even clinical evaluation by trained sleep clinicians are not adequate predictors of PLMS.105,106 Sleep testing is also required when symptoms suggest other sleep disorders. Traditionally, PSG is employed to detect PLMS; however, accelerometry may also be considered.



