Non-Invasive Positive Airway Pressure Treatment
Introduction
Non-invasive positive pressure ventilation (NIPPV) encompasses a variety of noninvasive techniques of ventilatory therapy delivered through an interface, usually a nasal mask and less frequently through a full face mask or nasal prongs. Treatment of obstructive sleep apnea (OSA) by nasal CPAP (nCPAP) was first reported in 1981 in adults.1 Guilleminault and colleagues reported the first use in children with OSA in 1986.2 In OSA, CPAP prevents upper airway obstruction by acting as a pneumatic splint, thereby maintaining an open airway. Further advances in non-invasive respiratory support both for sleep-disordered breathing (SDB) and for other pulmonary or neurologic diseases have brought about new NIPPV technologies. In this chapter the term NIPPV refers to and includes all of these techniques including CPAP, automatically titrating CPAP devices, BIPAP techniques, adaptive servo-ventilation (ASV), average volume-assured pressure support (AVAPS) and bi-level positive airway pressure with pressure release technology (Bi-Flex). The new techniques have been used mainly in adults, and have led to changes in pressure titration protocols, allowing for machine-driven pressure titration in the home environment.
Clinical Indications in Sleep Medicine
Delineation of the specific indications for NIPPV implementation in children has yet to be formally defined in any consensus statement. A recent paper has further emphasized the need for such consensus since it pointed out for the first time that NIPPV leads to amelioration of cognitive deficits in children with SDB.3 The indications for NIPPV are summarized in Box 35-1.
Use in Obstructive Sleep Apnea Syndrome (OSAS)
Most children with OSAS respond to adenotonsillectomy. A minority who fail surgery and children with other causes of OSAS such as obesity, craniofacial anomalies or hypotonia may require NIPPV.4 This indication is becoming increasingly relevant due to the secular trends in the prevalence of pediatric obesity around the world.
Use in Central and Peripheral Nocturnal Apnea/Hypoventilation
One of the major applications of NIPPV in children is the treatment of central hypoventilation.5–7 However, caution must be used when applying NIPPV to very young infants with severe central hypoventilation since such patients might not synchronize the opening of the upper airways and glottis at inspiration.
NIPPV is also extremely useful in severe and progressive forms of neuromuscular, skeletal, lung and airway diseases. In most of these situations, the disease progresses over time, and commonly, the first indication of ventilatory insufficiency is manifested by nocturnal hypoventilation that usually resolves during wakefulness. As the disease progresses, both nocturnal and daytime hypoventilation emerge. Symptoms may include headaches, decreased performance and cardiovascular complications. Although during the first stage, when hypoventilation occurs only during sleep, clinical symptoms may be mild, daytime sleepiness and fatigue are not infrequent. Assisted bi-level positive airway pressure delivered by nasal mask may be used successfully to treat sleep-disordered breathing in these patients. The added value of IPAP techniques that provide support also during the inspiratory phase operates primarily through reduction in the work of breathing.7 Of note, hypoventilation in these patients may appear first or be more severe during REM sleep. Therefore, the indication to start ventilatory support is based on carbon dioxide and saturation during sleep as observed by polysomnography together with the clinical presentation. The main goal of NIPPV in these situations is to improve arterial blood gases to near normal values without discomfort and in the absence of sleep disruption. It should be noted that specifically in these diseases, isolated low oxygenation during sleep without increased paCO2 might not suggest nocturnal hypoventilation, but rather reflects an underlying pulmonary problem with ventilation/perfusion mismatching that might be adequately treated by supplemental oxygen without NIPPV. Some children may require oxygen supplementation being bled into the ventilator circuit in addition to satisfactory NIPPV due to the concurrent presence of parenchymal lung disease. Since these diseases might progress over time, clinical as well as polysomnographic follow-up should be regularly conducted at a rate that depends on the type of disease, age and progression.
Hardware
Interface
The interface is the device positioned on the patient’s nose and mouth in order to connect the patient to the ventilator. It includes the device itself and straps that secure the interface to the face. Choosing the correct interface is crucial for patient compliance and effectiveness of treatment. The ideal interface should have minimal leaks, be comfortable and easy to wear.7 Types of interfaces include: nasal prongs (usually for smaller children), nasal mask (the most popular for sleep disturbances in adults and older children), nasal pillows, oro-nasal mask, full-face mask and helmet. Factors contributing to the selection of the interface include: patient age, neurological status, and pulmonary or facial abnormalities. For example, a nasal mask may not be suitable for young and uncooperative children due to mouth air-leaks, and the risk of vomiting and aspiration in disabled patients should be considered when using a full-face mask.8 Infants and small children might better be treated with masks that have a small dead space. Mask type and size will also need to be adjusted over time as the child grows.
Other features of the interface are: anti-asphyxia valves that allow external air to enter the mask in case of ventilator failure, and ports to connect lines for oxygen supplementation and for capnography. The combination of mask and circuit must have only one exhalation port for venting (producing intentional, calibrated leak). Unfortunately, there are only a few products specifically designed for young children.8
Ventilator
There are two basic types of ventilator, time-cycled-pressure-limited and volume-controlled.9 The former delivers gas using a preset constant pressure; hence, the volume-delivered, is determined by respiratory mechanics and interface properties. Volume-cycled ventilators provide constant volume and will adjust the driving pressure accordingly until a set-up limit is reached. While the pressure-controlled machines compensate for small to medium air leaks (a pressure drop due to leak will drive the machine to continue delivering gas until the set-up pressure is reached again), the volume-controlled ventilators do not, since the machine cannot differentiate between inhaled gas and lost gas due to leak resulting in reduced tidal volume and minute ventilation. Although studies in adults comparing these methods did not show clear benefit for one method over the other,10,11 the pressure mode is usually preferred in children due to the relatively large amount of wasted ventilation in the circuit. Most of the current non-invasive ventilators were designed for adults and, therefore, are often not triggered by very small or weak children with low inspiratory flow rates or with fast breathing rates.7
Techniques of Non-Invasive Positive Pressure Ventilation
Continuous Positive Airway Pressure (CPAP)
This is the simplest and by far the most common NIV mode. Positive pressure is delivered to the patient by continuous airflow and a pressure valve. This method maintains the airways patent throughout the respiratory cycle, improves functional residual capacity (FRC), and decreases the work of breathing. It also results in normalization of the pharyngeal dilator muscle activity during sleep in patients with SDB.8,12 CPAP requires a spontaneously breathing patient and is unable to support ventilation in the case of apnea.
Pressure requirements will vary among individuals, thus, mandating individual titration in the sleep laboratory.4 Parameters to program during titration include: expiratory positive airway pressure (EPAP) also named continuous positive airway pressure (CPAP), and FiO2 in specific ventilators capable of adjusting the concentration of delivered oxygen.
Automatically Titrated Positive Airway Pressure (APAP)
In this method, the ventilator continuously adjusts pressure as needed to eliminate respiratory events during sleep. Usually, it serves for titrating the optimal pressure for CPAP treatment (see titrating section). Its use for long-standing treatment of OSAS was studied in adults. At present, there is no evidence of superiority for APAP over CPAP for the treatment of OSAS.13
Bi-Level Positive Airway Pressure Ventilation (BIPAP)
BIPAP is often used for patients who fail a trial of CPAP for OSAS and in the treatment of central apnea/chronic hypoventilation in the pediatric population.14 Two levels of pressure are then delivered to the patient – a lower pressure during expiration (EPAP) and a higher pressure delivered during inspiration (inspiratory positive airway pressure; IPAP). Hence, this method requires triggering and synchronization with the patient’s spontaneous breathing.
Triggering the respiratory cycle is achieved by one of three modes:
Spontaneous (S) (or assist). All breath cycles are triggered by the patient without any background ventilation. The ventilator maintains CPAP and gives the patient pressure support during inspiration. The ventilator is programmed to sense inspiratory effort (by sensing either the negative inspiratory pressure or the inspiratory flow, the latter being more accurate) and to activate IPAP until a drop in the inspiratory flow reaches a threshold that terminates the inhalation phase (expiratory trigger).15
Machine settings include: CPAP and IPAP levels, inspiratory ramp slope (regulates the speed of gas flow into the lungs) and in some devices also the expiratory trigger level expressed as a percentage of the peak inspiratory flow (usually around 25%; however, in most modern ventilators, this variable is preset by the manufacturer). This may become a problem when a large leakage exists, since the ventilator will compensate with increased flow rates resulting in delayed termination of inspiratory phase. This problem may be avoided by increasing expiratory trigger to 40–70% of peak flow.16
Spontaneous/timed (S/T) (or assist-control). These are the same as S mode with a background breathing rate that guarantees a minimum rate and is used in cases of significant bradypnea or apnea (Figure 35-1).17
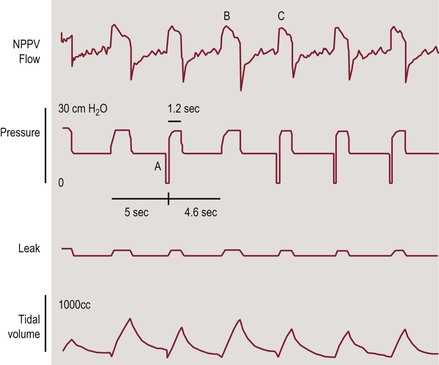
Figure 35-1 Tracing of NIPPV flow, pressure, leak, and tidal volume in a patient receiving BIPAP in the ST mode. Back-up rate is 12/min. When the ventilator did not sense patient’s inspiratory effort for 5 seconds, the machine triggered a breath (A). Spontaneous (B) and ventilator (C) triggered breaths have similar peak flows but different duration and tidal volume. Adapted with permission from ref. 17: Berry RB et al., NPPV Titration Task Force of the American Academy of Sleep Medicine. Best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation (NPPV) in stable chronic alveolar hypoventilation syndromes. J Clin Sleep Med. 2010; 6:491–509.
Average Volume Assured Pressure Support (AVAPS)
Combined Modes
This method, designed originally for conventional mechanical ventilation18 combines volume-controlled and pressure-controlled ventilation. BIPAP with fixed pressure support may not maintain adequate ventilation during the changes in pulmonary mechanics that occur during sleep. Hence, it has been suggested that hybrid modes that target a preset volume by adjustment of the supported pressure may be more effective.19 These novel modes estimate the expiratory tidal volume and respond by adjusting the inspiratory pressure (IPAP) accordingly to maintain ventilation.
Proportional Assisted Ventilation
By measuring the volume or flow that the patient generates during inspiration (with a pneumotachometer), the ventilator delivers inspiratory flow and pressure proportional to the patient’s spontaneous breathing effort.20 The respiratory pattern is dialed (normal, obstructive, restrictive or mixed) and all other machine variables are then automatically programmed (CPAP, maximum pressure, maximum tidal volume and percent assistance). Manual setting is optional.
Adaptive Servo-Ventilation (ASV)
ASV was developed to treat Cheyne–Stokes central sleep apnea in adult patients with congestive heart failure. It is also approved for use in patients with complex sleep apnea. The subject’s ventilation is controlled to equal a target ventilation of 90% of the long-term average ventilation by adjusting IPAP–EPAP difference.21
Expiratory Pressure Relief and Flexible Bi-Level Positive Airway Pressure
Exhaling against a positive airway pressure is uncomfortable and believed to be one of the reasons for low compliance to NIPPV treatment in patients with SDB. To address this problem, new ventilators equipped with pressure support algorithms that decrease mask pressure were developed. The main examples are the C-Flex® and the Bi-Flex® technologies (Respironics, Philips). The C-Flex pressure relief technology makes sleep therapy more comfortable by reducing pressure at the beginning of exhalation in order to minimize the back-pressure against which the patient exhales, returning to therapeutic pressure just before inhalation when upper airway narrowing and collapse are most likely. The level of pressure relief varies, based on the patient’s expiratory flow and which of the three C-Flex settings has been selected.22
Setting Parameters: EPAP and Gain
The bi-level flexible mode (Bi-Flex) results in pressure decrements during both late inspiration and expiration. These adjustments assume to optimize triggering and patient–ventilator synchrony. The magnitude of change of the IPAP and EPAP is proportional to patient effort (as in C-Flex).23 Parameters to be set are: Pbase (EPAP), gainins, maximal IPAP, gainexp and minimal PEEP. Experience with these modes of NIV in children with sleep-disordered breathing is emerging.
General Considerations
Leaks. A major issue in NIV is overcoming air leaks that result from mouth breathing and poor interface positioning. Ventilators compensate for leaks by increasing airflow.9 Leaks cause a decrease in tidal volume, and problems in triggering. For example, additional flow generated to balance leaks can delay the decrease in inspiratory flow and termination of inspiration, resulting in increased asynchrony between the patient and ventilator.
Humidification. High flow of air and unidirectional inspiratory nasal airflow due to mouth leaks can cause dryness of the nasal mucosa and increase the airway resistance.24 Humidification of inspired gas is essential to prevent these complications and also to maintain normal mucociliary function and improve gas delivery. Humidification is especially important in patients with chronic lung disease and in those with a history of chronic rhinitis. Types of humidifiers include: heat-and-moisture exchanger (HME), heated and non-heated pass-overs and pass-through devices. HME is less effective due to the high flows used in NIV. When using pressure-controlled machines only the pass-over humidifier is suitable since the other types may compromise pressure generating and triggering.9 Most NIPPV devices today have the option for an integrated heated humidifier which is recommended in general, especially for those with nasal symptoms.14
Oxygen supplementation. O2 may be added to NIV after adjustment of other ventilatory parameters (usually increasing CPAP and EPAP) has failed to correct hypoxemia or when the patient does not tolerate higher pressure. Another reason to add oxygen is lung disease.7 The FiO2 that is generated and reaches the patient by O2 supplementation to the ventilator circuit is generally unknown and potentially variable. There are many factors influencing FiO2 including the IPAP, EPAP, the O2 flow rate and the site where O2 is added to the circuit. One study in healthy volunteers found that during NIPPV, the closer the O2 was connected to the exhalation port (and not the patient) the higher was the FiO2 achieved.25 A study in a lung model found conflicting results where the most effective oxygen attachment site was on the mask itself.26 Due to the uncertainty regarding the FiO2 actually being delivered to the patient, it is recommended that children who require oxygen supplementation be monitored by pulse oximetry.
Response to Treatment
Adherence and Compliance
Empiric evidence regarding NIPPV adherence in pediatrics is relatively limited because, compared to adults, NIPPV by CPAP or IPAP modes has only more recently been used in children with OSA. Information from adults clearly shows a positive relationship between the level of adherence to NIPPV treatment and outcomes encompassing health, sleepiness, daytime functioning, neurobehavioral, cardiovascular and mortality measures. Hence, the importance of adherence cannot be underestimated. Although NIPPV use has become relatively common in children, there is a paucity of studies rigorously evaluating its use and outcomes. In particular, there have been very few studies evaluating NIPPV adherence in children using objective criteria.27,28 Although NIPPV in children has been shown to be highly effective in the laboratory situation, its use at home is significantly limited by suboptimal adherence.29,30 NIPPV is not approved by the Food and Drug Administration for children <7 years of age or weighing <40 lb (18 kg). This might have contributed to the limited experience and uncontrolled data in children. Nevertheless, the off-label use of NIPPV in infants and young children has not been shown to bear an increased risk.27–32 NIPPV might be the most practical or the only treatment for some infants and children with SDB.
A major determinant of the efficacy of NIPPV treatment is patient’s adherence. In general, adherence is measured by several variables: (1) the ratio of the number of hours the NIPPV is actually used (machine turned on, mask is applied and fitted and pressures are at required levels) to total sleep time per night; (2) number of nights NIPPV is used for a minimum number of hours a week; and (3) length of time (months/years) NIPPV is used.
Adherence studies are frequently skewed by difficulties in standardization for NIPPV usage and by using populations with average NIPPV application time that is only a little above half of the sleep time. Adult studies, for example, define regular use by at least 4 h of CPAP administered on 70% of the days monitored since only about half of the patients actually use CPAP for equal or greater than these rates.33–35 Hence, this definition results from practical reasons rather than from medical and outcome considerations. This imposition led to the erroneous assumption that CPAP use of 4 hours/night on 70% of nights is a clinically valid benchmark of CPAP adherence.36 Recent data show similar findings also in children.3 It has been shown that even in adherent children and families, the average usage time of NIPPV during sleep is only about 50%.29,30 The problem with such an approach is that it defines ‘outcome’ as the actual patient’s usage in real life and not as a short- and long-term medical and health consequence, hence, not representing the potential of NIPPV treatment since the device must be used consistently for all medical benefits to be evaluated and realized. We suggest that patients who do not comply with the treatment should not be considered only as adherence failure, but also as treatment failure.
Indeed, in many adherence studies, patients who did not use NIPPV or used it during a relatively short time of the total sleep time were excluded and were not included in the outcome analysis. Another problem with adherence is that many studies are based on patients’ or parental subjective reports.37 Objective data from both children and adults show that nCPAP usage is significantly lower compared to reported information.27,33 Data from adult patients confirm that the reported amount of CPAP use exceeded that recorded electronically.33,38,39 A recent report in children confirms these findings40 and highlights the importance of obtaining objective data for adherence in children since previous studies in children reported relatively good rates of subjective adherence on the basis of parental reports.
The application of new generations of NIPPV machines to children that record usage, application time, mode and pressures that are electronically downloaded to a built-in media allows one to objectively evaluate information of compliance and adherence.27–29 This is important both to allow the clinician to intervene at follow-up visits and for research purposes. Studies in adults have shown that factors that are often thought intuitively by clinicians to be important for NIPPV adherence, such as high pressures or interface issues,41,42 have not been borne out during scrutiny.28,43
CPAP adherence has been linked to multiple factors, such as disease characteristics and severity, patient’s characteristics, psychosocial factors, maternal education, titration procedure, mask style, nasal symptoms, pressure discomfort, perceived benefit and the ability to use the therapy. Lower maternal education was associated with lower CPAP use by children in one study.28 Full-face masks were associated with lower adherence than nasal masks.30 Conflicting results have been reported for the effect of age.28–31,37 Yet, most pediatric studies did not include infants and toddlers; hence, less is known about CPAP adherence in younger children. Nevertheless, no differences were found between infants and children who complied with NIPPV treatment and those who failed regarding gender, underlying diseases, OSAS severity (AHI, oxygen saturation), predisposing factors for OSA and mode of NIPPV delivery.36,37
Many children requiring NIPPV treatment have underlying chronic illnesses or developmental delays which further complicate efforts to improve long-term adherence.27–30,37 NIPPV can be particularly difficult for children with developmental delays, and for those with anxiety or behavioral problems. These children often verbally and physically resist caregivers’ efforts to get them to wear the mask, and may develop conditioned anxiety because of poorly fitting equipment and repeated association of the sight, sound, and sensation of NIPPV with discomfort from the mask, physiologic arousal from struggling, or both. They learn that physical and verbal resistance to PAP can cause the caregiver to delay or give up on the use of NIPPV.44
Similar findings have been reported from US and Canadian centers. A small cohort including 29 children from four US centers using objective data showed that one-third of children dropped out within 1–6 months of follow-up despite all patients receiving intensive support that included free equipment and continuing assistance.29 Of the 21 children for whom 6-month adherence data were recorded electronically, the mean nightly use was 5.3 ± 2.5 hours, i.e. only 50% of the sleep which is clinically suboptimal. Of concern is the fact that parental assessment of NIPPV use considerably overestimated actual uses (7.6 ± 2.6 h/night vs. 5.8 ± 2.4 h/night, respectively, P<0.001). This may further contribute to failure due to parental false assurance and avoidance of action to improve adherence. In a group of 52 children, the nightly mean use was 170 ± 145 minutes (range: 1–536 minutes).3 Over the first 60 nights of treatment, children applied NIPPV for only 60 ± 25 nights, i.e., the range of weekly usage was from only a couple of days to an entire week.3 Another recent small study showed that obese adolescents with OSA adhere poorly to NIPPV.45 Similar findings were reported in young patients with an average CPAP use of 3.35 h per night.40 Considering that adherence decreases with treatment time, these data are frustrating and raise concerns on how to significantly improve these unacceptably low adherence rates. More encouraging data from the UK reported that nCPAP was effective and tolerated by 86% of children. A period of acclimatization in the home environment was found to be a useful strategy to achieve success in 26% of patients who initially were intolerant to nCPAP.37
It has been suggested that early successful CPAP usage improves subsequent adherence30,46 with children who were less readily accepting of CPAP (i.e., >90 days to first use after CPAP titration polysomnogram) having also lower CPAP adherence. Probably the most reliable data for children come from a recent prospective study of NIPPV adherence where 56 children and their parents completed a series of psychosocial questionnaires prior to NIPPV initiation.28 Objective (electronically recorded) adherence data were obtained after 1 and 3 months of NIPPV use. In the first month, NIPPV was worn for an average of 22 ± 8 nights being used by only 78% of children for more than >50% of nights and for only 3 ± 3 hours/night. Adherence rates during the third month were slightly lower: 19 ± 9 nights/month and mean nightly use of 2.8 ± 2.7 hours, corresponding to an average use of only one-third of the sleep time for two-thirds of the nights. Lower maternal education was the strongest predictor of poor NIPPV adherence, implying that intensive training programs might improve adherence. For normally developing children, adherence correlated inversely with age. Adherence did not correlate with severity of apnea, pressure levels, or psychosocial parameters other than a correlation between family social support and nights of NIPPV use. As in adults, severity of baseline polysomnography and nasal symptoms at baseline did not affect adherence.28,42 Data from adults suggest that short- and long-term adherence are significantly affected by the severity of baseline symptoms, specifically being sleepy at baseline was associated with improved adherence.33,47–49 This pattern has not been observed in children. Hence, reinforcement from the clinician, regular assessment and motivation sessions are most important.
A major limitation of most studies is that adherence was assessed for only the first 3–6 months of treatment and in some studies children participated in intervention programs with particular efforts being invested to support adherence. Hence, data for long-term adherence in unselected groups cannot be ascertained. Only one recent study looked at adherence beyond the first 6 months of treatment and found an even lower adherence rate with CPAP usage with an average of only 3.5 hours/night.40 Thus, children demonstrate poor average nightly rates of CPAP use, ranging from 3.5 to 7 h per night. Given that children require between 9 and 12 hours of sleep per night depending on age, it is likely that overall NIPPV adherence in children is very poor.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


