Background
The clinical and financial burden from bladder infections is significant. Daily antibiotic use is the recommended strategy for recurrent urinary tract infection prevention. Increasing antibiotic resistance rates, however, require immediate identification of innovative alternative prophylactic therapies. This systematic review aims to provide guidance on gaps in evidence to guide future research.
Objective
The objective of this review was to provide current pooled estimates of randomized control trials comparing the effects of nitrofurantoin vs other agents in reducing recurrent urinary tract infections in adult, nonpregnant women and assess relative adverse side effects.
Data Sources
Data sources included the following: MEDLINE, Jan. 1, 1946, to Jan. 31, 2015; Cochrane Central Register of Controlled Trials the Cochrane Database of Systematic Reviews, and web sites of the National Institute for Clinical Excellence, and the National Guideline Clearinghouse from 2000 to 2015. Randomized control trials of women with recurrent urinary tract infections comparing nitrofurantoin with any other treatment were included.
Study Design
A protocol for the study was developed a priori. Published guidance was followed for assessment of study quality. All meta-analyses were performed using random-effects models with Stats Direct Software. Dual review was used for all decisions and data abstraction.
Results
Twelve randomized control trials involving 1063 patients were included. One study that had a serious flaw was rated poor in quality, one study rated good, and the remainder fair. No significant differences in prophylactic antibiotic treatment with nitrofurantoin and norfloxacin, trimethoprim, sulfamethoxazole/trimethoprim, methamine hippurate, estriol, or cefaclor were found in clinical or microbiological cure in adult nonpregnant women with recurrent urinary tract infections (9 randomized control trials, 673 patients, relative risk ratio, 1.06; 95% confidence interval, 0.89–1.27; I 2 , 65%; and 12 randomized control trials, 1063 patients, relative risk ratio, 1.06; 95% confidence interval, 0.90–1.26; I 2 , 76%, respectively). Duration of prophylaxis also did not have a significant impact on outcomes. There was a statistically significant difference in overall adverse effects, with nitrofurantoin resulting in greater risk than other prophylactic treatments (10 randomized control trials, 948 patients, relative risk ratio, 2.17; 95% confidence interval, 1.34–3.50; I 2 , 61%). Overall, the majority of nitrofurantoin adverse effects were gastrointestinal, with a significant difference for withdrawals (12 randomized control trials, 1063 patients, relative risk ratio, 2.14; 95% confidence interval, 1.28–3.56; I 2 , 8%).
Conclusion
Nitrofurantoin had similar efficacy but a greater risk of adverse events than other prophylactic treatments. Balancing the risks of adverse events, particularly gastrointestinal symptoms, with potential benefits of decreasing collateral ecological damage should be considered if selecting nitrofurantoin.
Accounting for more than $2.6 billion in annual costs in the United States alone, the clinical and financial burden from bladder infections is vast. More than 8.1 million visits to health care providers related to bladder infections occur each year. It is one of the most common bacterial infections in women and one of the most common diseases seen in general practice.
Forty to 50% of women have at least one episode of uncomplicated urinary tract infection during their lifetime, and 20–30% will have a recurrent episode. Moreover, evidence shows that between 20% and 50% of initial episodes have subsequent infections within 6 months. The urinary tract infection incidence peaks during ages 18 and 24 years at 17.5% but is still a substantial 9% for women of age 50 years and older; approximately 10% of those women who are postmenopausal report having had a urinary tract infection during the past year.
Although strategies for managing recurrent urinary tract infections are limited, daily antibiotic use is the currently recommended strategy for recurrent urinary tract infection prevention. The current guidelines from the American College of Obstetricians and Gynecologists recommend continuous once-daily prophylaxis with nitrofurantoin, norfloxacin, ciprofloxacin, trimethoprim, trimethoprim/sulfamethoxazole, levofloxacin, gatifloxacin, or fosfomycin tromethamine for 6–12 months.
In a review by Geerlings et al, nonantimicrobial options include cranberry products and lactobacillus crispatus intravaginal suppository in premenopausal women, and in postmenopausal women, options include topical estrogen, oral capsules with Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14. There are new promising strategies on the horizon for management of recurrent urinary tract infections, which are prophylaxis with bacterial extracts such as the oral immunosstiulant OM-89 or the vaginal vaccine Urovac. However, more evaluation must be done before introduction into clinical practice.
The issues of antibiotic resistance and the ecological adverse effects of antimicrobial therapy are important motivators for better, more evidence-based, strategies. In the past decade, multiple key organizations, including the Infectious Diseases Society of America, the Centers for Disease Control and Prevention, the World Health Organization, and the World Economic Forum, have made antibiotic resistance the focus of conferences and action plans.
Nitrofurantoin is a well-known and studied drug with limited antibiotic resistance since its use. The focus of this review is to conduct a current evaluation of head to head comparisons of nitrofurantoin vs other drugs. Our hypothesis is that nitrofurantoin treatment response is statistically significantly better than other drugs because of its low incidence of bacterial resistance in today’s current multidrug resistant era.
The aim of this systematic review is to provide pooled estimates of randomized control trials comparing the effects of nitrofurantoin with other drugs in reducing urinary tract infections in women with recurrent urinary tract infections and to study the comparative adverse effects associated with their use with the goal of providing guidance on gaps in the evidence to guide future research.
Materials and Methods
A detailed description of the methods is available online in a protocol registered a priori with the PROSPERO registry ( www.crd.york.ac.uk/PROSPERO ; record number 23966). An investigational review board reviewed and exempted this study.
Information sources and search strategy
MEDLINE was searched from Jan. 1, 1946, to Jan. 31, 2015, and the Cochrane Central Register of Controlled Trials with no date restriction for randomized and nonrandomized comparative studies using the following search terms: (1) recurrent (recur*), (2) urine or urinary (urin*), (3) infectious or infection(s) (infectious, infection*), (4) prevent (prevent*), (5) prophylaxis (prophyl*), (6) chemoprophylaxis (chemoprophy*), (7) chemoprevent (chemoprevent*), (8) reduce (reduc* or low* or few* or less*) (9) risk (risk*), (10) episode (episode*), (11) chance (chance*), (12) incidence (inciden*), or (13) frequency (frequen*).
In addition, a search for systematic reviews was conducted in MEDLINE, the Cochrane Database of Systematic Reviews, and web sites of the National Institute for Clinical Excellence and the National Guideline Clearinghouse from 2000 to 2015 with general search terms, women, recurrent, urinary, and infections.
Conference proceeding searches included the American Urologic Association, Society of Urogynecology and Female Pelvic Medicine Society, International Urogynecologic Association, and American Urogynecologic Society. Experts in urinary tract infection prophylaxis were consulted. Grey (unpublished) literature studies were searched through databases such as the World Bank Documents and Reports ( http://www-wds.worldbank.org/ ) and the National Research Register Projects Database ( https://portal.nihr.ac.uk/ ) prior to its phased shutdown from Jan. 1, 2010, to June 20, 2015.
Study selection
Titles and abstracts from the search strategy were screened by one author to identify studies that potentially met the inclusion criteria, with decisions confirmed for accuracy by a second author. Differences were resolved through consensus with the review group. Abstract screening was conducted using Abstrackr ( http://abstrackr.cebm.brown.edu ). The full text of these potentially eligible studies was retrieved and independently assessed for eligibility by 2 review team members. Any disagreement between them over the eligibility of particular studies was resolved through discussion with a third reviewer.
Inclusion criteria were the following: (1) studies of women aged 18–85 years who are receiving care in an outpatient setting for a recurrent urinary tract infection, which is defined as either 3 or more symptomatic urinary tract infection episodes in the past year (including the index infection) or 2 such episodes in the past 6 months, which are considered to meet the case definition for recurrent urinary tract infection; (2) interventions included nitrofurantoin vs trimethoprim, cefaclor, sulfamethoxazole/trimethoprim, cefixime, vaginal estrogen, estrogen of all types, cranberry supplements, bladder instillations, or fosfomycin; (3) studies reporting the following primary outcomes: mean number of urinary tract infections per recurrent urinary tract infection patient or reduction of urinary tract infections, microbiological cure, or clinical cure; (4) study duration of at least 6 months of prophylaxis; or (5) study was a randomized control trial or nonrandomized comparative study for all outcomes. Observational studies (cohort and case-control studies) were included for the assessment of harms. Studies were included if they fulfilled all the criteria. The primary outcome was microbiological or clinical cure. Secondary outcomes were mean time to urinary tract infection recurrence between urinary tract infection and adverse events.
Studies with any of the following criteria were excluded: published in non-English language unless institution resources were available for transcription, not reporting primary data from original research, not reporting relevant outcomes, or having greater than 20% of subjects not women.
Data extraction and quality assessment
A standardized form from RevMan (a Review Manager software) was used to extract data from the included studies for the assessment of study quality and evidence synthesis. Two review authors extracted data and study characteristics independently, and discrepancies were identified and resolved through discussion (with a third author where necessary). Extracted information included study setting, study population and participant demographics and baseline characteristics, details of the intervention and control interventions, study methodology, recruitment and study completion rates, outcomes, and timing of measurement. Missing data were requested from study authors.
Quality of included studies was assessed using the methods of the Drug Effectiveness Review Project quality assessment of randomized trials. Three quality categories were used: good, fair, and poor.
Data synthesis and analysis
A table of the findings from the included studies is structured around the type of intervention, target population characteristics, type of outcome, and intervention content. Studies were synthesized by pooling data on similar outcomes across studies with similar design, methodology, and patient populations. If studies were not similar on these characteristics, we did not pool results. Sensitivity analysis was done if there were different outcomes within the same comparator agents to see whether there was an impact on overall primary outcome.
Statistical analysis
All meta-analyses were performed using StatsDirect statistical software (version 2.5.7; stats Direct Ltd, Cheshire, UK). For all dichotomous outcomes, we calculated the pooled relative risk ratio and 95% confidence interval, using the random-effects model (DerSimonian Laird). Comprehensive analyses of all included studies were done. Separate analyses of same comparators with nitrofurantoin were done.
Results
Description of included studies
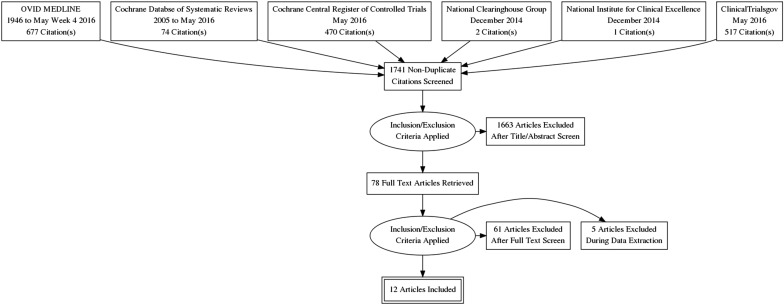
The literature search yielded 1061 articles, of which 78 were reviewed in full text ( Figure 1 ). Of these studies, 12 randomized control trials met the inclusion criteria. These trials included a total of 1063 patients, in whom 344 bacterial urinary tract infections were reported from 10 studies. The remaining studies reviewed in full text were excluded for the following reasons: studies without nitrofurantoin prophylaxis therapy, nitrofurantoin therapy with acute bacterial infections, other prophylactic therapies with other drugs, not original investigations, or duplicates of reports already identified. The characteristics and quality assessment of the included studies for the clinical trial studies are presented in Tables 1 and 2 .
| Authors, year | Study design, location, setting | Study population | Inclusion criteria | Exclusion criteria | Antibiotic group A (dosage, duration) | Compared group(s) (dosage, duration) | Follow-up after prophylaxis start |
|---|---|---|---|---|---|---|---|
| Brumfitt et al, 1991 | Single blind RCT Royal Free Hospital, United Kingdom, Urinary Infection Clinic | Women (mean age, group A, 38.9 y; group B, 37.2 y) | At least 4 UTI attacks in the preceding 12 mo with at least 1 documented urine culture 10 ˆ5 CFU | History of allergy to a quinolone, nitrofurantoin, or multiple agents; pregnancy; high possibility of death in the near future; acute hematopoietic disease; or chemotherapy for any malignancy; GFR <30 mL/min | Macrocrystalline nitrofuantoin (100 mg QHS, 12 mo) | Norfloxacin (200 mg QHS, 12 mo) | 18 mo |
| Kasanen et al, 1982 | RCT, Regional Hospital of Loimaa, Finland, Outpatient clinic, Turku University, Nephrological Outpatient Clinic | Women and men (mean age, group A 48.2 y; group B 49.7 y; group C 52.1 y; group D 51.1 y) Gender, %, female, 97% | Three or more UTIs during the last year | No UTI | Macrocrystalline nitrofurantoin (75 mg QHS, 12 mo) | Group B: methanamine (1 g QHS, 12 mo) Group C, trimethprim (100 mg QHS, 12 mo) Group D, placebo tablet (1 g QHS, 12 mo) | 12 mo |
| Brumfitt, 1980 | RCT, Royal Free Hospital, London, United Kingdom, Urinary Infection Clinic | Women (mean age, group A, 35.9 ± 16.7 y; group B, 31.3 ± 13.2 y) | At least 3 UTI attacks in the preceding 12 mo with at least 1 documented urine culture | No UTI | Nitrofurantoin (50 mg QHS, 12 mo) | Methanamine hippurate (1 g Q 12 h, 12 mo) | 12 mo |
| Brumfitt and Hamilton-Miller, 1995 | Double-blind RCT, Royal Free Hospital, London, United Kingdom, Urinary Infection Clinic | Women (median age, group A 45 (20-90 y); group B, 40 (18-89 y) | At least 4 UTI attacks in the preceding 12 mo with at least 1 of these episodes with growth of ≥10 4 CFU in the presence of pyuria and symptoms | Known allergy or intolerance to cephalosporin or nitrofurantoin or renal insufficiency (serum creatinine ≥133 μmol/L) | Macrocrystalline nitrofurantoin (50 mg QHS, 12 mo) | Cefaclor (250 mg QHS, 12 mo) | 12 mo |
| Brumfitt et al, 1985 | RCT, Royal Free Hospital, London, United Kingdom, Urinary Infection Clinic Department of Medical Microbiology, Royal Free Hospital School of Medicine, United Kingdom, | Women (mean age, group A, 40.9 ± 18.5 y; group B,, 37.6 ± 18.2 y) | History of at least 3 attacks of urinary infection in the previous 12 mo. At least 1 episode required unequivocal laboratory confirmation | No UTI | Macrocrystalline nitrofurantoin (100 mg QHS, 12 mo) | Trimethoprim (100 mg QHS, 12 mo) | 12 mo |
| Scherwin and Holm, 1977 | CT, Department of Geriatrics Clinic, Aarhus Kommune Hospital, Aarhus, Denmark | Women and men (median age, 82 y, range, 50-96 y) Gender, %, female, 87% | History of 2 significant bacteriuria (>10 5 on 2 successive examinations of midstream urine-sensitive intervention) Current UTI | Current UTI Serum creatinine <2 mg, % | Nitrofurantoin (100 mg QID, 1 wk followed by 50 mg TID, 12 mo) | Sulphamethoxazole/trimethoprim (400 mg per 80 mg BID, 1 wk, followed by 400 mg per 80 QD, 12 mo) | 12 mo |
| Vahlensieck and Westenfelder, 1991 | RCT, Department of Urology Clinic, University of Munchen, Munchen, Germany | Women and men (median age, group A, 43 y, range, 21-75 y; group B, 35 y, range, 20-55 y) Gender, %, female, 95% | More than 3 UTIs every year | No UTI Known allergies to the test drugs, pregnancy, breast feeding, neuritis, liver insufficiency, renal insufficiency, porphyria, glucose-6-phosphate dehydrogenase deficiency, Stevens-Johnson syndrome, Lyell syndrome, exfoliative dermatitis, thrombocytopenia, leukopenia, anemia, morphological or functional alterations of the urinary tract, and no possibility for 6 mo compliance | Nitrofurantoin (50 mg QHS, 6 mo) | Trimethoprim (50 mg QHS, 6 mo) | 6 mo |
| Nunez and Solis, 1990 | Single-blind RCT, Hospital Arzobispo Loayza, Peru Outpatient clinic | Women (mean age, group A, 44.7 ± 12.1 y; group B, 45.6 ± 11.2 y) | History of at least 2 UTIs during the previous 12 mo (verified by medical records) Child-bearing subjects on contraception for 6 mo Current UTI | Antimicrobial treatment during the previous 48 h, pyelonephritis, urogenital obstructive disease, venereal disease, associated illness that might interfere with the evaluation of the study medications, hypersensitivity to study drugs, liver disease, impaired renal function, nursing | Macrocrystalline nitrofurantoin (100 mg QID × 10 d followed by 100 mg QHS, 6 mo) | Norfloxacin (400 mg BID × 10 d followed by 400 mg QHS, 6 mo) | 6 mo |
| Raz and Boger, 1991 | RCT, Zamenhoff, Israel, Outpatient Clinic | Women (mean age, group A, 54.6 y; group B, 51.2 y) | History of 3 or more documented episodes of UTI during the last 6 mo | Pregnant women or planning pregnancy | Nitrofurantoin (50 mg QHS, 6 mo) | Norfloxacin (200 mg QHS, 6 mo) | 6 mo |
| Raz, 2003 | RCT, Israel, Outpatient clinic | Postmenopausal Women (mean age, group A, 66.9 ± 7.9 y; group B, 68 ± SD 7.2) | History of recurrent UTI (defined as 3 or more confirmed symptomatic episodes of UTI in the last year or at least 2 in the last 6 mo) | History of any HRT in the previous year, sex hormone-dependent malignancy, current malignancy, vaginal bleeding, active or recent thromboembolic disease, indwelling catheter, known urinary retention (PVR >150 mL), long-term (2 wks duration) receipt of antibiotic therapy in the past 3 mo, functional or anatomic abnormality of the urogenital tract, DM, severe renal or liver failure, allergy to nitrofurantoin | Nitrofurantoin (100 mg QHS + placebo vaginal pessary daily for 2 wks, followed by placebo pessary every 2 wks, 9 mo) | Estriol (estriol-containing [0.5 mg] vaginal pessary daily for 2 wks and then once every 2 wks for 9 mo together with oral placebo capsules QHS, 9 mo) | 9 mo |
| Stamey et al, 1977 | CT, United States, outpatient clinic | Women (median age 36 y, range, 19-67 y) | History of 3 or more UTIs in the preceding 12 mo were included | Current UTI | Macrocrystalline nitrofuranotin (UTI treatment for 10 d, followed by 100 mg QHS, 6 mo) | Trimethoprim-sulfamethoxazole (UTI treatment for 10 d, followed by 40 mg trimethoprim, 200 mg sulfamethaxole QHS, 6 mo) | 12 mo |
| Ruxer et al, 2007 | RCT, Poland, Diabetes Outpatient clinic | Women (median age, group A, 58.9 ± 6.8 y; Group B, 60.7 ± 9.0 y; Group C, 61.1 ± 8.6 y) | History of 3 UTI incidents in the last 12 mo and current bacturia (10 ∧ 5 CFU) sensitive to study agents | Current UTI not sensitive to study agents, serum creatinine greater than 1.5 mg/dL; the concentration of ALT and AST 2-fold exceeding the normal, bilirubin >1.3 mg/dL, kidney stones diagnosed by ultrasound, complications of late diabetes, hematological disease, alcoholism | Macrocrystalline nitrofurantoin (100 mg q 12 h × 7 d, then 100 mg QHS, 6 mo) | Group B, trimethoprim/sulfamethoxazole (80/400 every 12 h × 14 d, then QHS, 6 mo); group C, fosfomycin (3 g × 1, then 3 g q 30 d, 6 mo) | 9 mo |
| Study | Randomization | Concealment | Selection criteria | Group comparability | Assessors blinding | Outcomes intention to treat | Acceptable level of overall attrition (≤30%) Acceptable level of differential attrition (<10%) | Overall quality |
|---|---|---|---|---|---|---|---|---|
| Brumfitt et al, 1991 | Yes | Yes | Inclusion, yes Exclusion, yes | Yes | No | No | Overall, Yes Differential, Yes | Fair |
| Kasanen et al, 1982 | Yes | Not clear | Inclusion, yes Exclusion, yes | Yes | NR | Yes | Overall, not clear Differential, not clear | Fair to poor |
| Brumfitt et al, 1981 | Yes | Not clear | Inclusion, yes Exclusion, yes | Yes | NR | No | Overall, yes Differential, yes | Fair |
| Brumfitt and Hamilton-Miller, 1995 | Yes | Yes | Inclusion, yes Exclusion, yes | Yes | No | No | Overall, yes Differential, yes | Fair |
| Brumfitt et al, 1985 | Yes | Not clear | Inclusion, yes Exclusion, yes | Yes | No | No | Overall, yes Differential, yes | Fair |
| Scherwin and Holm, 1977 | No | Not clear | Inclusion, yes Exclusion, yes | Not clear | No | No | Overall, no Differential, no | Poor |
| Vahlensieckand Westenfelder, 1992 | Yes | Not clear | Inclusion, yes Exclusion, yes | Yes | NR | Yes | Overall, No Differential, no | Fair |
| Nunez and Solis, 1990 | Yes | Not clear | Inclusion, yes Exclusion, yes | Yes | No | Yes | Overall, yes Differential, no | Fair |
| Raz and Boger, 1991 | Yes | No | Inclusion, yes Exclusion, yes | Yes | NR | No | Overall, yes Differential, yes | Fair |
| Raz, 2003 | Yes | Yes | Inclusion, yes Exclusion, yes | Yes | Yes | Yes | Overall, yes Differential, yes | Good |
| Stamey et al, 1977 | No | No | Inclusion, yes Exclusion, yes | Not clear | NR | No | Overall, yes Differential, not clear | Poor |
| Ruxer et al, 2007 | Yes | No | Inclusion, yes Exclusion, yes | Yes | NR | No | Overall, yes Differential, yes | Fair |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


