Nevus and Melanoma
Terminology and Classification of Skin Lesions
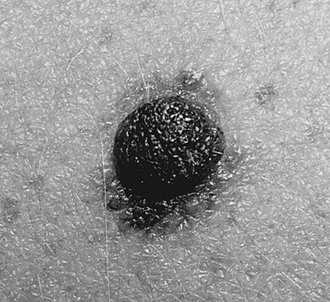
The terminology used for pigmented and nonpigmented cutaneous lesions is often confusing with multiple designations. The correct dermatologic terms are essential for clear communication (Table 71-1). An understanding of the epithelial embryology, anatomy, and physiology is also important for the clinical identification and correct classification of these lesions. As a reference, Figures 71-1 and 71-2 depict the basic anatomy of the dermis and epidermis.
TABLE 71-1
| Term | Definition |
| Nevus | Proliferation of cells within their tissue of origin |
| Macule | Circumscribed, pigmented lesion, less than 5 mm in diameter without elevation or depression from the surrounding skin |
| Patch | Lesion with larger area of involvement without palpable characteristics |
| Papules | Solid elevated lesions less than 5 mm in diameter and plaques are raised lesions larger than 5 mm |
| Nodules | Lesions that arise from the dermis or subcutaneous tissues |
| Dermal melanoses | Pigmented lesions resulting from the deposit of melanin in melanophages, free melanin in the dermis or in dermal melanocytes |
| Melanocytosis | Lesion associated with increased number of melanocytes |
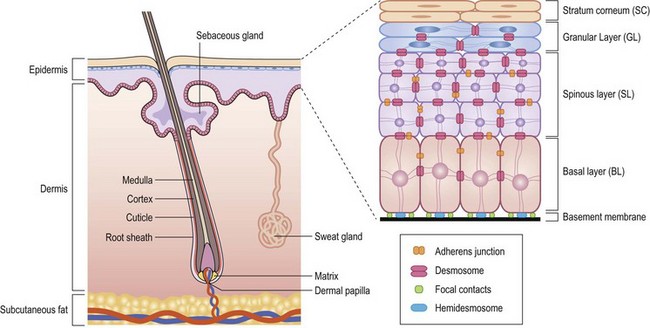
FIGURE 71-1 Structural organization of the epidermis and dermis. (Redrawn from Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet 2002;3:199–209.)
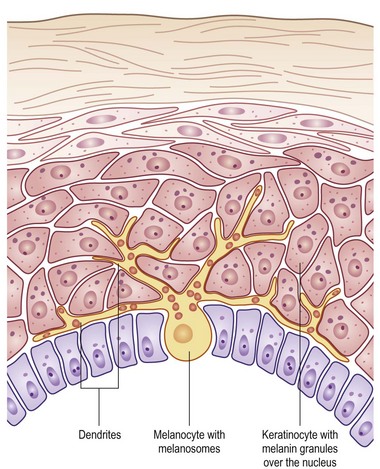
FIGURE 71-2 The melanocyte rests within the basal layer of the epidermis and is associated with a group of keratinocytes to which it delivers the melanosomes. (Redrawn from Gray J. The World of Skin Care. Available at http://www.pg.com/science/skincare/Skin_tws_16.htm.)
Anatomy of the Dermis and Epidermis
The skin is a morphologically and functionally complex tissue providing protection against physical elements by its anatomic structure and potential for immune responsiveness. The outermost layer is the stratum corneum or horny layer, which is composed of devitalized keratinocytes (see Fig 71-1). While the dermal layer is a derivative of the paraxial mesoderm, embryologically the epidermis is of ectodermal origin.
The basal layer of the epidermis is the site of mitotic activity leading to the proliferation of keratinocytes. This basal layer consists of columnar cells anchored to the basement membrane via hemidesmosomes and anchored to the dermis via penetrating fibrils (see Fig. 71-1). Interspersed between the basal cells reside melanocytes that are dendritic cells and are the source of melanin (see Fig. 71-2). Melanocytes are not secured via desmosomes or tonofilaments. Functional units (epidermal melanin units) are composed of a melanocyte and the keratinocytes to which it is responsible for the transfer of melanin. Melanosomes are the cytoplasmic organelles that result from the fusion of vesicles containing tyrosinase and vesicles containing the structural melanosomal proteins, both of which are generated by the melanocyte’s endoplasmic reticulum. Differential skin pigmentation amongst individuals results from the variable production of melanosomes, their content and rate of degradation, and not on numbers of melanocytes. The absence of tyrosinase prevents formation of melanosomes and results in albinism. The melanosomes migrate from the perinuclear area along microtubules to the dendritic tips where these are phagocytized by the keratinocyte. As the squamous cells differentiate, the melanosomes are degraded by lysosomal enzymes.
Classification of Nevi
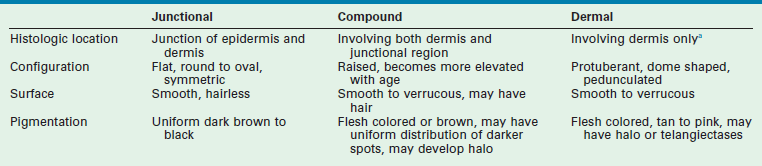
The most definitive classification is according to the cell of origin, and results in stratification between melanocytic and nonmelanocytic lesions. The melanocyte is a dendritic cell and neural crest derivative (Fig. 71-3). During development, the melanocyte precursors (melanoblasts) migrate to the dermis, hair follicles, leptomeninges, uveal tract, and retina. After the eighth week of development, they migrate from the dermis to the epidermis. Melanocytic nevi are derived from melanocytes or their precursors, and distinguish themselves by virtue of (1) the absence of dendrites; (2) the clustering of cells; (3) their variable location within the dermis and epidermis; and (4) their variable content of melanin (Table 71-2).
TABLE 71-2
Clinical and Histologic Features of Melanocytic Nevi
aOnly in the presence of ulceration or reticular dermal invasion.
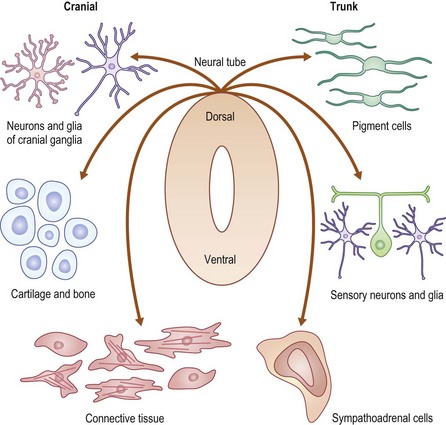
FIGURE 71-3 The neural crest derivation of these dendritic cells explains the association of melanocytic disorders with CNS, bony, and ophthalmic disorders. (Redrawn from www.nature.com/nrg/journal/v3/n6/images/nrg819-i1.jpg.)
The principal location of the nevus in the skin serves as a second potential descriptor. In contrast to the nonmelanocytic epidermal nevus, melanocytic nevi can be situated: (1) at the junction of the epidermis/dermis (junctional); (2) involve both regions (compound); or (3) be confined to the dermis (dermal). These are generic histological terms and do not imply biologic behavior.
The third dimension is related to the time of appearance. Melanocytic nevi can be classified according to whether or not they involve disorders of (1) melanocyte migration or (2) proliferation. These lesions are most commonly categorized based on age at presentation as either congenital or acquired (Fig. 71-4) Congenital melanocytic nevi (CMN) result from failure of embryologic migration of the melanocyte precursors and form hamartomatous lesions evident at birth, which may evolve over time. In addition, congenital nevi can occasionally present after birth up to age 6 months. Failure of migration also results in a variety of dermal melanoses, some of which are encountered most frequently as the ‘Mongolian’ spot over the dorsal sacral region of infants. In contrast to the congenital nevi, acquired nevi are proliferative lesions and represent benign neoplasms of the skin that are located within the epidermis, dermis, or both. Acquired melanocytic nevi are the result of benign proliferative disorders of nevi that have a tendency to increase in number during childhood, adolescence and early adulthood, but then spontaneously regress with age. Based on more specific clinical characteristics, including total numbers of lesions, family history, and specific appearance, subsets of nevi can be identified that will have unique risks related to melanoma.
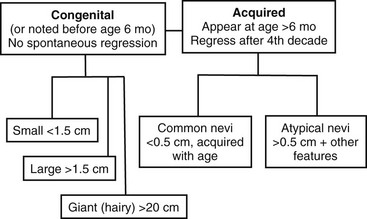
FIGURE 71-4 Pigmented lesions are classified based on their age at appearance and further stratified by size and other physical features to yield two subsets that require close monitoring of the patient for the development of melanoma.
Nonmelanocytic Nevi
Epidermal nevi are skin lesions not associated with proliferation of melanocytes. Instead, these represent proliferations of ectodermal origin that are classified based on their hyperplastic element. Keratinocytic lesions involve the epidermal layer only, whereas the organoid lesions involve the sebaceous, apocrine, eccrine, and follicular structures. These epidermal nevi are clinically distinct and appear as raised, pigmented, velvety to wart-like proliferations of the dermis along dermatomal distributions. Lesions are usually apparent in infancy, but some may appear in later childhood. The incidence in newborns is less than 1%. The most common sites involved are the scalp, face, trunk, and proximal extremities. Often a hairless patch is noted on the scalp or a raised linear yellow-tan to orange plaque is evident on the face or trunk during infancy. With age and the hormonal changes during puberty, these lesions change in appearance and become verrucous or develop more coarse hair growth. These progressive changes are what initiate the request for excision, along with the concern for potential malignant degeneration after puberty.
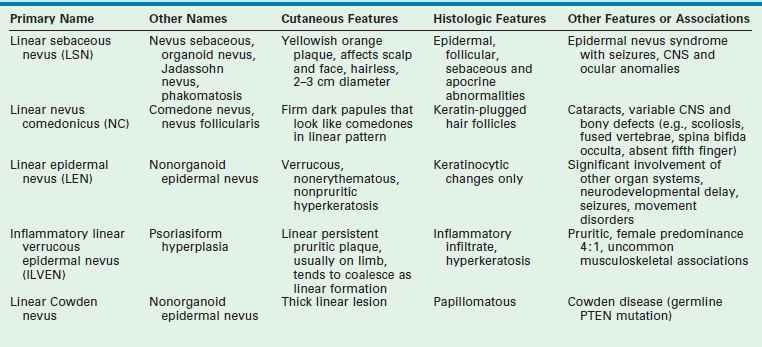
A number of syndromes are associated with these epidermal nevi, particularly when they are large or extensive. The epidermal nevus syndromes typically involve abnormalities of the central nervous system (CNS), ocular system, and skeletal system. While the classification of these lesions and syndromes is based on clinical findings, advances in molecular biology suggest that these disorders are based on genomic mosaicism and future biologic classification will become possible.1 The common variants of epidermal nevi include the (1) nevus sebaceous (Fig. 71-5) that represent 50% of the epidermal nevi; (2)keratinocytic nevi; (3) nevus comedonicus (Fig. 71-6); (4), inflammatory linear verrucous nevus; (5) linear Cowden nevus; and (6) Becker’s nevus (pigmented hairy epidermal nevus). Table 71-3 summarizes their clinical and histological features.
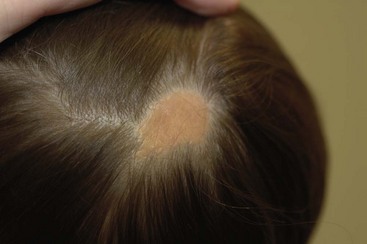
FIGURE 71-5 This young child has the classic appearance of a nevus sebaceous, which is a solitary yellow-orange plaque on the scalp. These lesions are well circumscribed, hairless, oval to round, and usually less than 2–3 cm in diameter.
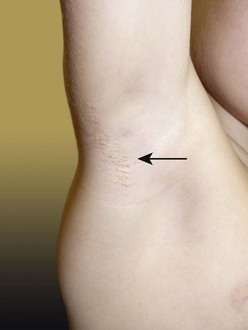
FIGURE 71-6 In the right axilla of this 5-year-old is a nevus comedonicus (arrow). This lesion is a well-circumscribed plaque that is composed of keratin-plugged hair follicles. It was present at birth and has been slowly enlarging.
These lesions are associated with a risk for transformation. The most common association is between nevus sebaceous and basal cell carcinoma (BCC), and this risk of transformation is believed to be based on shared deletions within the PTCH tumor suppressor gene. This natural history has formed the basis for current recommendations for excision prior to adolescence. A recent review by Rosen et al. suggests that the incidence of carcinoma may be less than previously reported as the demographic features of prior reports focused on predominantly adult populations.2 In this study, the mean age was 7.2 years (range 0.3–54 years). Six hundred fifty-one epidermal lesions in 631 patients were evaluated for the presence of a second intralesional diagnosis. Five patients had BCC (0.8%) and 1.1% had syringocystadenoma papilliferum. The mean age of BCC diagnosis was 12.5 years (range 9.7–17.4 years). On the basis of this finding of occasional prepubertal malignant degeneration, the authors advise individual counseling with patients and their families, informing them of the extent of resection and reconstruction techniques that may be required. The scalp pliability of the young child may also offer advantages and opportunities that must be taken into consideration when cosmetic concerns outweigh the concerns for malignant transformation. Certainly there is no scientific basis for suggesting an intervention to avoid malignant degeneration in infancy and early childhood.
Melanocytic Nevi
Congenital Nevi
CMN are pigmented lesions of variable size that develop during the first few months of life. They are the consequence of failure of melanocytic migration into the epidermis. These lesions grow proportionally with the infant and are generally categorized as small (<1.5 cm), medium (1.5–20 cm), and large (>20 cm). Those especially large lesions (>40 cm) are also referred to as a garment nevus, giant hairy nevus, or bathing trunk nevus since they tend to cover large truncal areas in confluence (Fig. 71-7). Although the pigmentation and surface are initially uniform and flat, the lesions evolve into thick, dark, and often hair-covered lesions. Histologically, the melanocytic cells in congenital nevi are located deeper than in acquired nevi (Fig. 71-8). The absence of superficial involvement may explain why transformation is often not noticed until late in its development. The incidence of transformation is greatest in the large or giant congenital nevi, and low in small to medium size lesions. The size of the congenital nevus therefore becomes the key element in discussing management.
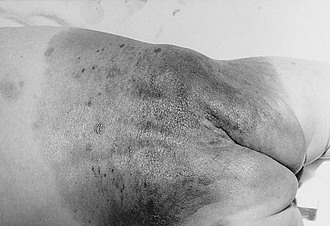
FIGURE 71-7 Giant congenital melanocytic nevus (garment nevus) of the back and buttock in a neonate.
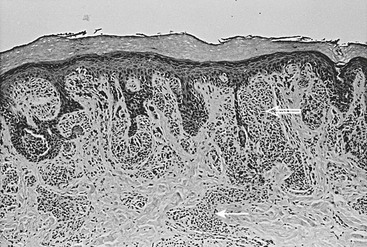
FIGURE 71-8 Congenital melanocytic nevus is characterized by nevus cells extending from the dermal–epidermal junction (open arrow) along skin appendage structures into the deep dermis (solid arrow).
The management of large or giant CMN is more controversial. The melanoma risk for these lesions is increased and estimated to be 5–10% over a lifetime with 50% of this risk during the first five years of life.3,4 The highest risk lesions are associated with the nevus that is situated over the posterior trunk, greater than 40 cm in maximal dimension, and associated with satellite nevi. Melanoma in these patients can arise from the deep dermal layer or even originate from sites distant from the skin such as the CNS (2.3% in one study5) or retroperitoneum.4 These patients are also at risk for neurocutaneous melanosis (7% in one study5) and CNS malformations such as Dandy–Walker malformation, defects of the vertebra, skull, and intraspinal lipomas. Screening MRI of the brain and spinal cord is recommended during first six months of life in these patients.5 Conversely, two-thirds of patients with neurocutaneous melanosis have giant CMN and the others have multiple nongiant lesions. The prognosis for patients with symptomatic neurocutaneous melanosis is extremely poor even in the absence of malignancy.6 The increased routine surveillance with MRI during infancy for children with large CMN has identified those with asymptomatic CNS involvement, and hopefully will impact the care and outcome of these patients in a positive manner. These patients are also at risk for other soft tissue malignancies such as rhabdomyosarcoma7 or neurofibrosarcoma (peripheral nerve sheath tumor).
Since operative resection of these extensive lesions is often not feasible and never completely eliminates the risk for melanoma, these patients need close medical surveillance. Areas of epidermal change within the nevus should be biopsied. However, caution must be exercised in interpreting the histological results, since the proliferative nodules may resemble melanoma, but behave in a benign manner.8 Features useful in differentiating a cellular nodule from melanoma include: (1) lack of high grade uniform cellular atypia; (2) lack of necrosis within the nodule; (3) rarity of mitoses: (4) evidence of maturation; (5) lack of pagetoid spread; and (6) no destructive expansile growth.9
When operative intervention is needed, collaboration with colleagues with added expertise in the use of tissue expanders, rotational flaps, and skin grafting may be helpful. In one study, the mean age for intervention was 5.1 years and took an average 1.3 years to complete resection and reconstruction in these patients with large and giant CMN.10 While the authors expressed a preference for earlier intervention, they acknowledged that the average age of the patients referred to them was 4.7 years, and many had already undergone an operative procedure. Over half of the cohort required more than one operative intervention and 22 of 40 patients needed skin grafts. Also, 18 patients benefitted from tissue expanders and autologous cultured skin replacements.
Acquired Nevi
Histologically, these acquired melanocytic nevi are divided into subtypes based on the location of the nests of nevus cells, and this feature corresponds with certain clinical findings (see Table 71-2). Junctional nevi tend to be flat with brown/black pigmentation and the nevus cells are located at the dermal–epidermal junction. When the nevus cells extend from the junction into the dermis, the lesion is described as a compound nevus. Clinically, this corresponds to a lesion that is slightly raised and pigmented brown/black. When the nevus cells migrate completely into the dermis, the lesion is an intradermal nevus, which is raised and typically not pigmented. In general, the deeper the nests of nevus cells, the more raised and less pigmented the lesion. (i.e., dark flat lesions vs raised tan lesions). The temporal evolutionary path of nevi was originally described by Stegmaier and explains the clinical observations of progressive change within individual nevi or their resolution.11 Newer nevi tend to be small and flat (junctional), and either develop a raised profile or disappear with time as a consequence of fibrosis. In one study, when the nevi on adolescents were followed over a 4-year period, there was a net increase of 50% in total number of nevi, despite a complete regression in 15%.12 This demonstrates the potential for active turnover in individual patients. Freckling and lighter colored skin are associated with increased numbers of nevi. In darker skinned individuals, nevi also develop, but are usually found on the palms and soles, unlike in the more fair complexioned individuals.
Sun exposure during childhood, especially when it is intense, intermittent, and not necessarily associated with sunburn, is a promoter for nevus development, and is the major environmental factor associated with the risk of melanoma. Recent evidence suggests that sunscreen use has led to a false sense of security in that it provides incomplete protection to UV rays.13 Sunscreen alone does not provide sufficient protection. Public health education must focus on the avoidance of midday sun and the use of physical barriers such as UV protectant clothing and hats.13–16
The role for excision of these acquired nevi is limited when they are clearly defined by their age at presentation and the features described in Table 71-2. The natural evolution and changes in these common lesions should not be mistaken for malignant progression.
Atypical Nevi
Atypical nevi, previously referred to as dysplastic nevi or Clark’s nevi, are common lesions found in 5% of the population, and may occur either sporadically or in a familial pattern (Fig. 71-9). The onset of their first appearance is usually during adolescence on the sun-exposed areas of skin, and they continue to increase in number and size with age. Unlike the common acquired nevi, these lesions are usually larger than 5 mm (up to 15 mm), and have an irregular surface ranging from completely flat to flat with a raised center resembling a fried egg. The pigmentation usually is dark and irregular, and the border of the lesion is often irregular as well. These lesions are most common in persons with light skin and hair color. Ultraviolet light has been implicated in the transformation of melanocytes in these nevi.
When a patient has multiple atypical nevi or moles, they may be diagnosed with the atypical mole syndrome. While it can be normal to have up to 10 to 20 nevi, people with this syndrome may have in excess of 100 moles. Individuals with 50–100 nevi and one or more first- or second-degree relatives with melanoma are considered to have the familial atypical mole and melanoma (FAMM) syndrome that identifies them at significantly increased risk (approaching 100%) for the development of melanoma. It is important to note that the melanoma may arise de novo on any part of the body and not necessarily from a preexisting atypical lesion. Simple excision of an atypical nevus therefore does not obviate the patient from close dermatologic follow-up. In families with the FAMM syndrome, nevi arising on the scalp can be an early predictor for this syndrome, although the scalp nevus itself may involute with time. In these individuals, the nevi tend to be similar in appearance, the corollary being that a new nevus that varies in appearance or location should be regarded with suspicion. The ability of clinical health care providers as well as nonclinicians to identify the ‘ugly duckling’ has been found to be reliable.17 Routine surveillance examinations with photo documentation are recommended with dermoscopic (epiluminescence microscopy) evaluation of those nevi undergoing significant change. Dermoscopy has been shown to improve sensitivity and specificity with regard to detecting melanoma.18
Specific Congenital or Acquired Melanocytic Lesions Requiring Distinction from Melanoma
Several melanocytic nevi are discussed separately due to their unique features, which frequently prompt their evaluation for malignancy. These lesions can be congenital or acquired, and are described in terms of their junctional, compound, or dermal location (Table 71-4). A recent review by Schaffer provides some practical advice regarding these specific lesions.13
Halo nevi (Sutton’s nevi) are unique lesions which appear between 6–15 years of age, and are typically located on the trunk or extremities. They appear as round or oval lesions, with a central area of pigmentation that may be tan to brown in color, and are surrounded by a rim of depigmentation (Fig. 71-10
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree