Urinary Tract Infections and Vesicoureteral Reflux
Urinary Tract Infections
Urinary tract infections (UTIs) are a common and significant source of morbidity in children. By 7 years of age, approximately 8% of girls and 2% of boys will have had at least one UTI.1,2 Children who have had at least one UTI are at risk for having a recurrence.3 The long-term sequelae include renal scarring, hypertension, chronic renal insufficiency, and pregnancy-related complications. Predisposing risk factors for UTIs include renal and bladder structural abnormalities as well as functional bladder and bowel dysfunction.4 Pediatric UTIs constitute a significant health burden and has been estimated to result in at least 13,000 hospital admissions with inpatient costs exceeding $180 million per year.5
Diagnosis
Localized clinical signs and symptoms are important clues in the diagnosis, but they are age dependent. Combinations of findings can be more useful than individual ones in identifying affected children.6 For example, neonates rarely present with symptoms specific to the urinary tract. Nonspecific symptoms of lethargy, irritability, temperature instability, anorexia, emesis, or jaundice predominate. Bacteremia is common with neonatal UTI, and a urine culture is an important aspect in the evaluation of neonatal sepsis.7 Confirmation of a UTI by microscopic examination and quantitative culture of a properly collected specimen is important. Older infants may present with nonspecific abdominal discomfort, emesis, diarrhea, poor weight gain including failure to thrive, and fever. Malodorous or cloudy urine may be reported by the parents.
Older children frequently present with dysuria and urinary frequency, urgency, and enuresis. Table 55-1 outlines the incidence of UTI symptoms as a function of age.8,9 As the symptoms can sometimes be obscure, it is important that care providers have a high index of suspicion in ill-appearing children. An unexplained high fever in an infant or toddler should prompt the clinician to obtain a urine sample.
TABLE 55-1
Presenting Symptoms in 200 Children with Urinary Tract Infection as a Function of Age
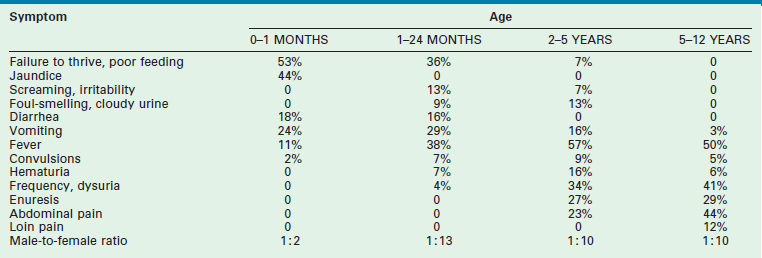
From Smellie JM, Hodson CJ, Edwards D, et al. Clinical and radiological features of urinary tract infection in childhood. BMJ 1964;2:1222; Bickerton MW, Duckett JW. Urinary tract infections in pediatric patients. AUA Update Service, Lesson 26, 1985;4:4.
Analysis of a properly collected urine sample is the cornerstone in the diagnosis of UTI.10 Errors in diagnosis most commonly result from failure to confirm a clinically suspected UTI by culture, or by reliance on a specimen that has been inadequately collected or mishandled. Specimens may be obtained by bag collection, clean catch, urethral catheterization, and suprapubic aspiration. Although invasive, urethral catheterization (or suprapubic aspiration) clearly offers the lowest risk of false-positive results.11 The results of a bag specimen or clean-catch specimen in a non-toilet-trained child are helpful only if negative.12 Bag specimens can be useful in an infant with a history of UTIs or structural abnormalities in whom a fever is present, but the suspicion for a UTI is otherwise low. Positive findings should be confirmed using a catheter or aspiration specimen unless the clinical presentation and laboratory findings are unequivocal. The accuracy of positive findings from a bag specimen in an infant has been estimated at 7.5%,13 whereas those of the midstream clean catch specimen varies with age: 42% under 18 months of age and 71% from 3 to 12 years of age.14 Specimens should be either analyzed and plated immediately, or placed on ice to minimize bacterial multiplication prior to testing.
The accepted gold standard for diagnosis remains the quantitative urine culture. The historically accepted criterion for diagnosis is greater than 105 colony-forming units per milliliter of a single bacterial species. The accuracy of such a positive finding on culture is estimated at 80% (single specimen) and 96% (confirmed by second culture).15 Table 55-2 outlines the probability of infection as a function of colony count and methods of collection that are used in children.16 One must avoid applying these criteria too strictly. The colony count varies as a function of hydration (dilution) and urinary frequency (bacterial multiplication time). One study of six untreated children with proven bacteriuria found colony counts to vary from 103 to 108 over a 24-hour period.17
TABLE 55-2
Criteria for Diagnosis of Urinary Tract Infections
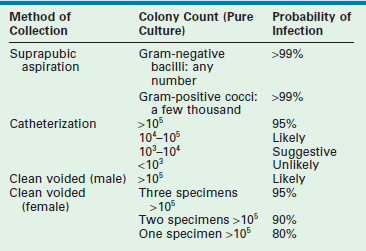
Modified from Hellerstein S. Recurrent urinary tract infection in children. Pediatr Infect Dis 1982;1:275.
Although clearly the most accurate laboratory test, urine culture results cannot provide an immediate diagnosis. As a result, initial treatment is generally guided by the urinalysis. Microscopic evaluation of a urine specimen should be done immediately on collection. This practice minimizes misleading ex vivo bacterial multiplication and deterioration of cellular elements. The identification of bacteria in an unspun urine specimen is very suggestive of significant bacteriuria.17 Pyuria (more than 10 leukocytes/mm3) is suggestive,18 but may also be seen in vaginitis, dehydration, calculi, trauma, chemical irritation, gastroenteritis, and viral immunization. Urinary Gram stain has been found to be reliable in detecting UTIs in young infants.19
A popular and indirect measurement of bacteriuria employs nitrite and leukocyte esterase analysis. Nitrate, normally present in urine, is converted to nitrite in the presence of bacteria. A positive nitrite reaction is indicative of bacteria with specificity and a positive predictive value approaching 100%.20 The nitrate-to-nitrite reaction requires a relatively long incubation period. Thus, urinary frequency and hydration may produce a false-negative result. Inadequate dietary nitrate and infection caused by nitrite-negative organisms may also cause false-negative reactions.21 The combination of nitrite and leukocyte esterase is more sensitive and specific than either alone.22 Overall, the combination of dipstick analysis and microscopic examination for bacteria have a sensitivity and negative predictive value approaching 100%.20
Classification
Laboratory studies designed to distinguish a lower tract from an upper tract UTI include antibody-coated bacteria assay, β2-microglobulin excretion, antibodies to Tamm–Horsfall protein, and urinary lactic dehydrogenase assay and procalcitonin.23,24 These tests are not sufficiently reliable for routine clinical use and thus are not typically employed. Direct culture by ureteral catheterization or percutaneous puncture is reliable, although cumbersome, and represents an option in complicated clinical situations. A quite useful study for localizing infection to the kidney is a radioisotope renal cortical scan (e.g., technetium-99m dimercaptosuccinic acid) during the initial presentation of the patient with a documented infection (Fig. 55-1).
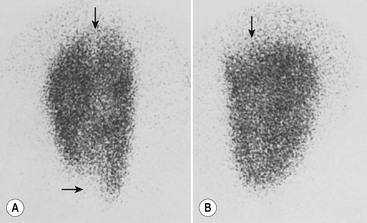
FIGURE 55-1 Technetium-99m dimercaptosuccinic acid (DMSA) scan. (A) The magnified view of the left kidney, seen by using a pinhole collimator, demonstrates defects in both poles that extend deep into the renal parenchyma (arrows), suggestive of acute pyelonephritis. (B) The right kidney has an upper pole defect (arrow) that may represent either acute or chronic pyelonephritis. (Courtesy of Michael J. Gelfand, MD.)
Epidemiology
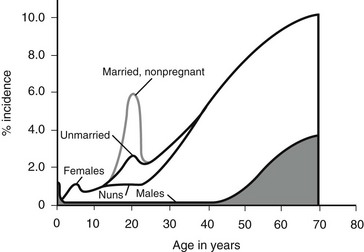
Figure 55-2 outlines the age- and gender-related incidence of UTIs. At all ages, with the exception of the neonatal period, the incidence of UTI is greater in females than in males. In both males and females, the incidence increases with advanced age. Although the male has one early peak in the newborn period, the female has two peaks, one at 3 to 6 years, and the other at the onset of sexual activity. The actual incidence of infection as a function of age and gender is difficult to determine from the literature. Table 55-3 summarizes the available data.16
TABLE 55-3
Incidence of Urinary Tract Infection as a Function of Age, Gender and Presence of Symptoms

a2.4–3.4% in premature infants.
Data compiled from multiple sources by Hellerstein S. Recurrent urinary tract infections in children. Pediatr Infect Dis 1982;1:271.
Pathophysiology
Host Factors
The establishment of clinical infection and its consequent injury to the urinary tract results from a complex interplay between host resistance and bacterial virulence. As a general rule, UTI-causing organisms originate from the feces of their host. Conceptually, four levels of defense are identifiable: periurethral, bladder, ureterovesical junction, and renal papillae.9 These concepts are illustrated in Figure 55-3.
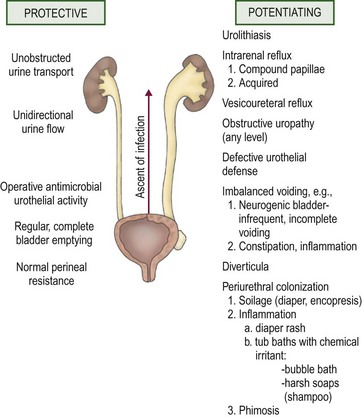
FIGURE 55-3 Host factors that protect the urinary tract from infection and abnormalities that potentiate the establishment of invasive bacterial infection.
Bacteria generally possess an ability to adhere to vaginal mucosal cells in order to readily establish infection.25 The resultant periurethral colonization then allows replication and migration, which ultimately lead to transurethral invasion to the bladder. Healthy girls have low bacterial colonizations of the periurethral region. Girls prone to UTIs experience greater colonization, especially prior to a new episode of UTI. Moreover, the cultured organism from the introital region belongs to the same strain as that from the urine during the UTI that ensues. Periurethral bacterial colonization is correspondingly low in UTI patients after resolution of recurrent UTIs.26 A similar mechanism may apply to bacterial adherence in the prepuce of males.27 This may explain why 92% of male infants less than 6 months old with a UTI are uncircumcised.28
A number of bladder defense mechanisms help maintain sterile urine. The most critical is the act of regular and complete voiding. The healthy bladder is capable of eliminating 99% of instilled bacteria and leaves a small residual urine that minimizes the inoculum at the onset of the following cycle.29 High intravesical pressure may also potentiate infection in children. In the absence of an elevated residual urine, uninhibited bladder contractions are associated with an increased risk of recurrent UTI, which may be lessened by anticholinergic therapy.30 Dysfunctional elimination syndrome with abnormal voiding habits and constipation can affect the development of UTI as well.31 The acidic pH of urine, as well as its osmolality, further discourages bacterial growth.32 The uroepithelial cells of healthy individuals suppress bacterial growth and are capable of killing bacteria. The uroepithelial cells secrete a mucopolysaccharide substance that, upon coating the surface of the uroepithelium, provides an additional barrier to uroepithelial adherence.29 Glycosaminoglycans are continuously shed and thus function to entrap and eliminate bacteria.
Abnormalities at the ureterovesical junction (UVJ) and altered ureteral peristalsis may allow vesicoureteral reflux (VUR), which potentiates but is not always necessary for upper tract invasion. Distortion of the pyramids allows renal parenchymal invasion, which results in irreversible renal injury. The anatomy of the renal papillae usually prevents intrarenal reflux (Fig. 55-4).9 Structural abnormalities that potentiate infection include phimosis, obstructive uropathy at any level (e.g., ureteropelvic and UVJ obstructions, posterior urethral valves [PUV]), VUR, bladder diverticula, urinary calculi or foreign bodies, and the renal papillary anatomy.
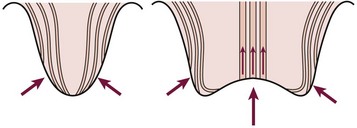
FIGURE 55-4 The normal oblique insertion of the collecting ducts onto the surface of simple papillae prevent intrarenal reflux (left). Collecting duct insertion onto the surface of compound papillae may allow intrarenal reflux (right). (From Ransley PG. Intrarenal reflux: Anatomic, dynamic and radiological studies. Urol Res 1977;5:61.)
Bacterial Factors
Several bacterial factors may potentiate a UTI and are outlined in Box 55-1.9,33 O antigens are lipopolysaccharides that are part of the cell wall. They are thought to be responsible for many of the systemic symptoms associated with infection. Of the more than 150 strains of Escherichia coli identified by O antigens, nine are responsible for the majority of UTIs.
K antigens are also polysaccharides, and their presence on Gram negative bacterial capsules is considered to be an important virulence factor. They are thought to protect against phagocytosis, to inhibit the induction of a specific immune response, and to facilitate bacterial adhesion. Bacterial strains causing UTI exhibit considerably more K antigen than those isolated from the feces. Urease, a virulence factor especially prominent with Proteus species, allows the breakdown of urea to ammonium. This process alkalinizes the urine and facilitates stone formation. Such bacteria are generally incorporated into the stone structure, making eradication extremely difficult. Mannose-resistant pili are important adherence factors. They promote adherence to uroepithelial cells as well as renal epithelial cells. This factor appears to counter the normal cleansing action of urine flow and allows tissue invasion and bacterial proliferation. That these factors truly are associated with virulence is shown in Figure 55-5.
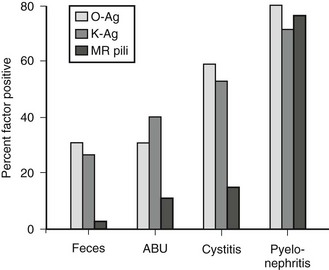
FIGURE 55-5 Presence of bacterial virulence factors as a function of the clinical setting. More invasive infections are associated with a high incidence of virulence factors, implicating these factors in pathogenesis. MR, mannose resistant; Ag, antigen; ABU, asymptomatic bacteriuria. (From Mannhardt W, Schofer O, Schulte-Wisserman H. Pathogenic factors in recurrent urinary tract infection and renal scar formation in children. Eur J Pediatr 1986;145:330.)
Increasingly invasive urinary infections are associated with bacteria with a high number of virulence factors. Figure 55-6 demonstrates the pathophysiologic changes of renal injury that can occur in the absence of significant host factors. Colonization of the feces with a virulent organism allows periurethral colonization and ultimately bladder entry. Uroepithelial adherence promotes bacterial proliferation and tissue invasion. This series of events is facilitated by the presence of one or more host factors (see Fig. 55-3).
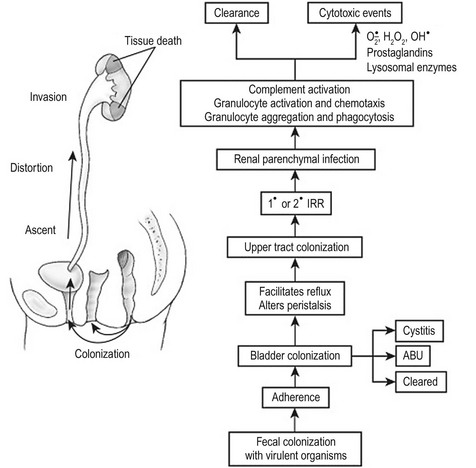
FIGURE 55-6 The pathogenesis of destructive infection is shown. The process is facilitated by, but does not require, defects in the host protective factors outlined in Fig. 55-3. IRR, intrarenal reflux; ABU, asymptomatic bacteriuria.
Investigation
Rationale for Radiographic Imaging
Although many patients with UTI do not develop serious illness, the pediatric caregiver must be cognizant of several important risks. Urinary abnormalities are found in approximately 50% of children up to the age of 12 years who present with UTI.34 VUR is found in up to 35% and obstructive lesions in 8%. Nonobstructive, nonrefluxing lesions are found in 7%.
While renal scars develop in about 13% of girls and 5% of boys with unspecified infection,35 they develop in up to 43% of kidneys involved in acute pyelonephritis.36 A recent meta-analysis assessing the prevalence of renal scarring in children after an initial episode of UTI found that 57% had acute renal cortical changes consistent with pyelonephritis in the acute phase and 15% had evidence of renal scarring on the follow-up Tc-99m dimercaptosuccinic acid (DMSA) scan.37 Pyelonephritic scarring is responsible for 11% of childhood hypertension cases38 and a majority of cases of severe hypertension.39 Although hypertension is most common with bilateral scarring, it is also seen with unilateral scarring. 40 Pyelonephritic scarring is also an important cause of end-stage renal failure in childhood and may require specific pretransplantation treatment, especially if associated with reflux.41 Additionally, approximately 50% of patients will suffer from recurrent UTI.33
Current Controversies
The standard of care for many years has been to perform imaging studies in children to assess for structural abnormalities that might lead to recurrent UTIs and renal scarring. It was felt that not only infants 42 but older children, particularly males, should be investigated after the initial infection.43 Several evidence-based guidelines have been published over the years.44 Controversy persists, however, with many researchers proposing even less aggressive approaches to the child with a UTI. Recent published guidelines from the American Academy of Pediatrics recommends deferring a voiding cystourethrography (VCUG) until after the second documented UTI in children ages 2 to 24 months if the ultrasound (US) is normal and the child has a complete clinical response to treatment.45 The urologic community has significant concerns about the paradigm shift made in these guidelines and the methodology employed in the meta-analysis upon which these recommendations were based.46
Imaging Studies
The initial radiographic evaluation ideally includes a fluoroscopic VCUG to precisely grade vesicoureteral reflux and assess for bladder and urethral abnormalities. Follow-up studies in females can be adequately performed with a radionuclear cystogram (RNC) that allows a somewhat lower radiation exposure to the ovaries. The exception is the girl with neurogenic bladder, ectopic ureter, bladder diverticulum, or ureterocele documented by ultrasound or previous cystograms. Radionuclide scanning using DMSA is readily available and can be used to diagnose acute pyelonephritis,47 detect renal scarring, and assess differential renal function.48
Treatment
Acute Phase
The treatment of an acute UTI is dependent on the clinical presentation. Ill-appearing children with pyelonephritis should be treated immediately and aggressively with broad-spectrum antimicrobial therapy and intravenous hydration. Initial empiric therapy should be initiated with broad-spectrum parenteral antibiotics until cultures return. Prompt, effective treatment is the most important factor in preventing permanent renal injury.49 Further therapy is dictated by culture and sensitivity findings. Indications for inpatient parenteral antibiotic therapy include very young age (<3 months), unusual or multi-resistant pathogens, persistent vomiting, concern for compliance with treatment, and/or significant urinary tract anomalies.
Once clinically stabilized and afebrile for 24–48 hours, patients with pyelonephritis and sensitive organisms may complete the course of parenteral culture-specific antibiotics on an outpatient basis, via a peripherally inserted central catheter (PICC) and a home-based nursing service. Some studies have shown that initial parenteral therapy followed by oral antibiotics can be as effective as a prolonged course of intravenous therapy in preventing renal scarring.50,51 Patients with obstruction or renal abscess who do not become afebrile or have persistent severe symptoms require repeat upper tract imaging. An abscess or obstructed kidney may require percutaneous, or very rarely, open drainage.
Initial empiric therapy in less toxic appearing children without vomiting can be performed with oral broad-spectrum antibiotics (e.g., cephalosporins) after obtaining a reliable urine culture. Short treatment courses appear to be insufficient for treatment of childhood UTI.52 Therefore, we prefer a 7–10-day course dictated by culture and sensitivity results.53 Retention by the toilet-trained patient due to fear of voiding or dysuria may be managed with phenazopyridine (Pyridium) and hydration, and allowing the child to void while sitting in a tub of warm clear bath water.
Prophylactic Antibiotics
Patients who have recurrent UTIs or those who are managed nonoperatively for VUR are often treated with long-term suppressive antibiotics. It is unclear whether the benefit outweighs the risks, particularly in low-grade VUR without nephropathy.45,54 The urothelial injury from an infection takes several months to fully recover. As a result, irritative voiding symptoms, such as dysuria, incontinence, and frequency, may persist despite the finding of sterile urine. A propensity for re-infection also exists.
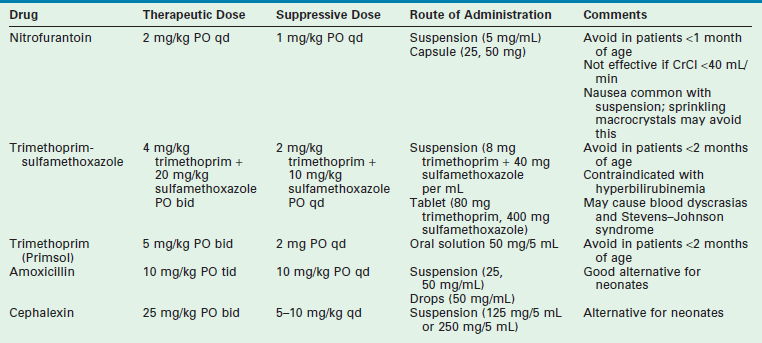
Patients without obvious urinary tract structural abnormalities should be evaluated for functional bowel and bladder dysfunction. Regular and complete elimination is mandatory to diminish the risk of recurrence. Along with aggressive treatment of constipation and voiding dysfunction, three months of antibiotic suppression therapy may help to break the cycle. Table 55-4 outlines the characteristics of the drugs that are most commonly used for suppression.
Vesicoureteral Reflux
VUR refers to the retrograde passage of urine from the bladder into the ureter. Although VUR was first discovered in the late 1800s, its clinical importance has only been recognized in the last five decades. Hutch’s studies, reported in the 1950s, demonstrated the pathophysiologic changes with VUR in children.55 These reports and Hodson’s observations in 1959 regarding the association between VUR, UTI, and pyelonephritic scarring set the stage for the modern era of reflux management.56
Although most commonly diagnosed during the evaluation for UTI, VUR may also be discovered during evaluations for hypertension, proteinuria, voiding dysfunction, or chronic renal insufficiency. In addition, VUR has been identified in asymptomatic patients with prenatally-detected hydronephrosis as well as through sibling screening.57
Pathophysiology
Figure 55-7 depicts the various anatomic components of the competent UVJ as well as the abnormalities most often implicated in the genesis of VUR. The normal UVJ is characterized by an oblique entry of the ureter into the bladder and a length of submucosal ureter providing a high ratio of tunnel length to ureteral diameter. This anatomic configuration provides a predominantly passive valve mechanism.58,59 As the bladder fills and the intravesical pressure rises, the resulting bladder wall tension is applied to the roof of the ureteral tunnel. This results in a compression of the ureter which prevents retrograde passage of urine. Intermittent increases in bladder pressure, such as the act of voiding, upright posture, activity, and coughing, are met with an equal and immediate increase in resistance to retrograde urine flow. This effect is supplemented by the active effects of ureterotrigonal muscle contraction and ureteral peristalsis. 59,60
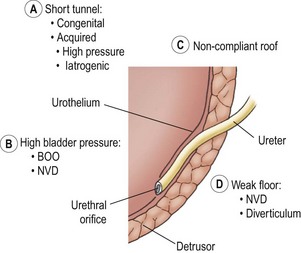
FIGURE 55-7 Components of the competent ureterovesical junction. Those abnormalities most often implicated in the etiology of vesicoureteral reflux are outlined. BOO, bladder outlet obstruction; NVD, neurovesical dysfunction.
Ureters with marginal tunnels can be made to reflux during infection due to UVJ distortion, loss of compliance of the valve roof, and intravesical hypertension. Excessively high intravesical pressure in neurovesical dysfunction (NVD) or bladder outlet obstruction (BOO) may also potentiate reflux as may a structurally weak detrusor floor (e.g., diverticulum or ureterocele). As the submucosal ureter tends to lengthen with age, the ratio of tunnel length to ureteral diameter increases, and the propensity for reflux may disappear.58,59
Of critical importance is the concept of intrarenal reflux (IRR), which has been demonstrated clinically61 as well as experimentally.62 The usually oblique entry of the papillary ducts onto the surface of simple papillae inhibits IRR. In contrast, the papillary duct entrance onto compound papillae facilitates IRR (see Fig. 55-4). The critical pressure for IRR is considered to be about 35 mmHg in compound papillae.62
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree