Chapter 49
Neurologic and Neuromuscular Disease
Angela M. Bader MD, MPH
Chapter Outline
MYOTONIA AND MYOTONIC DYSTROPHY
THE PHAKOMATOSES (NEUROCUTANEOUS SYNDROMES)
Cutaneous Angiomatosis with Central Nervous System Abnormalities
ACUTE IDIOPATHIC POLYNEURITIS (GUILLAIN-BARRÉ SYNDROME)
IDIOPATHIC INTRACRANIAL HYPERTENSION
MATERNAL HYDROCEPHALUS WITH SHUNT
ISOLATED MONONEUROPATHIES DURING PREGNANCY
The choice of anesthetic technique for pregnant women with neurologic disease requires knowledge of the pathophysiology of the disorder and an understanding of controversies involved in the diagnosis and management of the disease. If a patient’s neurologic condition deteriorates postpartum, the cause may be unclear and the anesthetic technique may be blamed unfairly. There are limited published data on specific neurologic and neuromuscular disorders in pregnant women. However, few of these disorders contraindicate the use of neuraxial anesthesia. In most cases, the obstetrician should obtain early antepartum consultation from an anesthesiologist. Early consultation allows accurate antepartum documentation of the extent and pattern of the neurologic deficit as well as discussion and formulation of the anesthetic plan with the patient, her obstetrician, and a neurologist or neurosurgeon.
Because patients with a wide variety of neurologic disorders will present for preoperative evaluation, the following thought process will assist the clinician with completing a proper evaluation and formulating an anesthetic plan.
What is the basic pathophysiology of the particular neurologic disorder? Neurologic disorders may be stable, progressive, or relapsing/recurrent. It is important to understand the common disease patterns. The potential for progression of the disease after delivery will depend on the pattern of progression and underlying pathophysiology and on the effect of pregnancy on disease progression.
What is the patient’s history and current findings after neurologic examination? A history should include the onset date and current course of the disorder. Symptoms related to neurologic issues should be documented (e.g., seizure type and frequency, deficits after cerebrovascular events, cognitive deficits). A basic physical examination should be conducted to document existing deficit patterns, including cognitive dysfunction (e.g., ability to understand and cooperate), deficits involving vision, hearing, speech, and swallowing; respiratory symptoms; and weakness and sensory deficits in the head and neck, trunk, and extremities. Motor and sensory deficits are classified as mild, moderate, or severe, with a description of the affected area. Special attention should be directed to limitations in ambulatory ability (e.g., bed-bound, wheelchair, walking with assistance) or positioning.
What are the current treatments and what testing results are available? Documentation of medical and nonmedical therapies is essential. For some disorders (e.g., myasthenia gravis), documentation of the timing of treatment is also critical. In most cases, specific laboratory testing will not influence management and outcome. However, pulmonary function testing should be considered in patients with neurologic disorders that result in significant respiratory compromise; the findings may assist the anesthesiologist in making decisions about anesthetic management.
What is the impact of the neurologic disorder on other organ systems (e.g., cardiac, respiratory, airway)? The patient’s neurologic disease may affect organ systems that are relevant to the anesthetic plan. For example, central core disease is associated with a risk for malignant hyperthermia. In addition, progressive neurologic disorders may significantly compromise the patient’s respiratory status, thereby increasing the risks associated with neuraxial and general anesthesia.
What are the potential impacts, risks, or benefits of particular anesthetic options based on the disease’s pathophysiology, symptoms, and treatment? Can treatment be initiated antepartum or before delivery that will improve outcome? For most rare neurologic disorders there is limited evidence on which to base decisions about anesthetic management. In these cases, the anesthesiologist should consider the disease’s basic pathophysiology and its possible direct and indirect interactions with specific anesthetic techniques. Encouraging the obstetrician to send these patients for early antepartum consultation will enable the anesthesiologist to obtain formal input from a neurologist or other consultant if necessary. A multidisciplinary discussion that includes the patient may be necessary to weigh the risks and benefits of specific obstetric and anesthesia plans.
In all cases, accurate documentation of the responses to the previous questions will greatly assist the team providing analgesic or anesthetic care for these patients. Some of the more common neurologic conditions are addressed in this chapter, and the existing literature is surveyed relative to the peripartum management of these patients. This knowledge allows the anesthesiologist an opportunity to formulate a safe and rational anesthetic plan as well as enable an appropriate discussion with the patient regarding the risks and benefits of particular anesthetic options.
Multiple Sclerosis
Multiple sclerosis is a major cause of neurologic disability in young adults. The prevalence of the disorder varies with the population. Recent data suggest that the prevalence of the disease is increasing, especially in females, and may be as high as 300 per 100,000 in some parts of North America.1 Both environmental and genetic factors appear to play a role in the incidence and prevalence of disease.
The disease is characterized by variable neurologic disabilities with two general patterns of presentation: (1) exacerbating remitting, which accounts for 85% of cases, in which attacks appear abruptly and resolve over several months, and (2) chronic progressive, in which continued deterioration occurs over time.2 The relapse rate varies significantly among patients, averaging approximately 0.4 attacks per year; this rate reflects the large proportion of patients with relapsing/remitting disease. The deficits tend to become more progressive and debilitating over time. Environmental factors (e.g., stress, infection, increased body temperature) may provoke a relapse. Most relapses reproduce previously experienced neurologic deficits, which can manifest as pyramidal, cerebellar, or brainstem symptoms.
The etiology remains unclear. There is a clinically significant heritable component, and alleles in the HLA locus have been identified as risk factors for multiple sclerosis.3 Pathologic findings include inflammation and loss of myelin in the central nervous system (CNS). It is possible that the disease results from a yet undetermined combination of genetic predisposition and exposure to specific environmental factors.
The more common symptoms include motor weakness, impaired vision, ataxia, bladder and bowel dysfunction, and emotional lability. Cerebrospinal fluid (CSF) immunoglobulin and lymphocyte concentrations are increased, and magnetic resonance imaging (MRI) studies demonstrate white matter plaques. Lesions may be documented by the demonstration of prolonged evoked potentials in areas of involvement.
There is no cure. Immunosuppressive therapies may hasten recovery from a relapse, but no evidence suggests that these agents influence the progressive course of the disease. Administration of interferon-beta may significantly reduce the relapse rate and retard disability; however, an increased risk for fetal loss and low birth weight (LBW) has been observed with the use of this therapy during the first trimester of pregnancy.4 In contrast, administration of intravenous immunoglobulin may reduce the risk for relapse and has no known adverse effects on pregnancy outcome.5 Acute relapses during pregnancy can be treated with intravenous corticosteroids, although their use may be associated with maternal glucose intolerance and neonatal adrenal suppression.6
Interaction with Pregnancy
Evidence regarding the effect of multiple sclerosis on pregnancy is conflicting. In one cohort study that compared 198 affected women with 1584 healthy women, the number of maternal complications was not higher in women with multiple sclerosis.7 However, infants delivered of women with multiple sclerosis appear to be at greater risk for meconium aspiration, even though the presence of moderate to heavy meconium is not significantly increased.7 This finding may reflect an intrauterine environment in patients with multiple sclerosis that is more susceptible to acute hypoxic events.7 A subsequent cohort study of 649 pregnancies in women with multiple sclerosis concluded that infants of these women were more likely to be small for gestational age; this outcome was also attributed to a suboptimal intrauterine environment.8 Moreover, this study found that mothers with multiple sclerosis were more likely to undergo induction of labor and operative delivery, possibly as a result of neuromuscular weakness and spasticity.
In a 2011 meta-analysis of reports of pregnant women with multiple sclerosis, the relapse rate was lower during pregnancy than before or after pregnancy.9 It is unclear whether the prevalence of cesarean deliveries, spontaneous abortions, preterm births, and LBW neonates is higher in women with multiple sclerosis than in healthy women, although the rates did not reach levels that would warrant great concern.
Data from prospective studies suggest that the rate of relapse increases during the first 3 months postpartum in comparison with the year before pregnancy.10 Relapses during this period were more likely in women who had higher relapse rates in the year before pregnancy or during pregnancy. Stress, exhaustion, infection, the loss of antenatal immunosuppression, and the postpartum decline in concentrations of reproductive hormones may account for the higher postpartum relapse rate. Treatment with immunologically active agents (e.g., interferon-beta) may result in a decreased postpartum relapse rate, but data are limited.10
Pregnancy does not negatively affect the long-term outcome of multiple sclerosis. Rather, at least one study has suggested that parturition may have a slightly favorable effect on long-term disease activity.11 Data are conflicting as to whether exclusive breast-feeding is associated with a lower risk for relapse than partial or no breast-feeding.12,13
Anesthetic Management
The anesthesiologist should assess the patient’s level of compromise, document the pattern of deficits, and give special attention to respiratory involvement. Historically, the optimal route of anesthesia in patients with multiple sclerosis has been controversial. Most anesthesia providers have considered general anesthesia to be safe, although published data are limited.14,15 Many anesthesia providers have been reluctant to administer neuraxial anesthesia because the effect of local anesthetic drugs on the course of the disease is unclear. Some anesthesiologists have expressed concern that neuraxial anesthesia may expose demyelinated areas of the spinal cord to potentially neurotoxic effects of local anesthetic agents. Several animal studies have investigated the histologic effects of local anesthetic agents on the normal spinal cord. In one study, subarachnoid injection of small doses of a local anesthetic agent produced no histologic changes in the spinal cord or meninges.16 Injection of very large doses caused reversible inflammatory and degenerative changes, but all changes resolved within 14 days of injection.
Diagnostic lumbar puncture is not associated with a higher rate of relapse.17 Two small reports have implicated spinal anesthesia in the exacerbation of multiple sclerosis.15,18 Bamford et al.15 described one case of relapse after the administration of spinal anesthesia in 9 patients, and Stenuit and Marchand18 identified two cases of relapse after the administration of spinal anesthesia in 19 patients. The relationship of these relapses to spinal anesthesia or other postoperative conditions (e.g., stress, infection, hyperpyrexia) known to exacerbate multiple sclerosis is unclear.
There are few published data on the use of epidural anesthesia in patients with multiple sclerosis. Warren et al.19 reported minor exacerbations after the administration of epidural anesthesia for two separate vaginal deliveries in one patient. Crawford et al.20 reported one postoperative relapse in 50 nonobstetric and 7 obstetric patients who received epidural analgesia. Confavreux et al.21 reported a study of 269 pregnancies in 254 women with multiple sclerosis, of whom 42 received epidural analgesia. They noted that epidural analgesia did not have an adverse effect on the rate of relapse or on the progression of disability in these patients. Bader et al.22 retrospectively evaluated 32 pregnancies in women with multiple sclerosis; they observed that women who received epidural anesthesia for vaginal delivery did not have a higher incidence of relapse than those who received only local infiltration anesthesia. In a prospective study of 227 women who had multiple sclerosis for at least 1 year before conception, of whom 42 received epidural analgesia during labor, no adverse effect of epidural analgesia on the rate of relapse or the progression of disability was identified.10
Bader et al.22 observed that all of the women who experienced a relapse after epidural anesthesia had received a concentration of bupivacaine greater than 0.25%. The concentration of local anesthetic in the CSF progressively increases during prolonged administration of epidural anesthesia, and the authors suggested that the higher concentration may overwhelm the protective effect of dilution within the CSF. An alternative explanation is that women who require a higher concentration of neuraxial local anesthetic may have more stressful labor. However, these observations suggest that anesthesia providers should use a dilute solution of local anesthetic for epidural analgesia during labor, when possible.
The addition of an opioid reduces the total dose of local anesthetic required for epidural analgesia during labor. Berger and Ontell23 reported the administration of intrathecal morphine, which was added to low-dose tetracaine for surgical anesthesia and postoperative analgesia, and observed no exacerbation of multiple sclerosis at 1 and 6 months after surgery. Leigh et al.24 described the successful use of intrathecal diamorphine for postoperative analgesia in a patient with multiple sclerosis who underwent a laparotomy.
The administration of neuraxial anesthesia for cesarean delivery is controversial. Because the operation is of limited duration, multiple doses of local anesthetic are typically not needed, so a progressive increase in CSF concentration of local anesthetic over time is less likely. In light of the significant benefits of neuraxial techniques for intraoperative anesthesia and postoperative analgesia, either spinal or epidural anesthesia is the principal anesthetic technique used for cesarean delivery in patients with multiple sclerosis in many institutions, including my own.
In summary, published data do not contraindicate the use of neuraxial anesthetic techniques for labor analgesia or operative anesthesia. The patient should be aware that there is a higher incidence of relapse during the postpartum period, even without the use of neuraxial analgesia or anesthesia. In addition, when anesthetic techniques are used, the type of anesthesia selected does not appear to influence the relapse rate. Neither pregnancy nor anesthesia appears to have a negative influence on the long-term course of the disease. The willingness of anesthesiologists to use neuraxial techniques in pregnant patients with multiple sclerosis is reflected in a survey of obstetric anesthesiologists published in 2006.25 The majority (91%) of respondents had seen fewer than 10 cases of multiple sclerosis in the past 10 years; 79% and 98% of anesthesiologists indicated they would perform a neuraxial anesthetic technique for labor and elective cesarean delivery, respectively.
Headache during Pregnancy
Headaches are among the most frequently observed neurologic symptoms during pregnancy (Table 49-1). Tension headaches, migraine headaches, and headaches associated with hypertension in pregnancy, including preeclampsia, are commonly observed during pregnancy. A pregnant patient with a history of chronic headaches who reports new or different symptoms should be closely evaluated to exclude serious etiologies such as preeclampsia, tumor, or intracranial vascular malformation. Symptoms of concern include sudden onset, intense severity, altered mental status, meningeal signs, fever, vomiting, and any localizing or lateralizing abnormality.
Tension Headache
Tension or muscle contraction headaches are the most common type of headache observed during pregnancy.26 The symptoms typically consist of dull, persistent pain that extends over the entire head. The onset is usually gradual, but the symptoms may persist for long periods. Although the etiology is unknown, this type of headache is believed to be associated with stress rather than hormonal changes. These headaches are more common in women, are frequently associated with anxiety, and may be a symptom of postpartum depression.27
Treatment
In the nonpregnant patient, treatment of tension headaches may involve acetaminophen, aspirin, opioids, tricyclic antidepressants, and benzodiazepines. In the pregnant patient, acetaminophen should be used as a first-line analgesic. Caffeine may be contained in combination analgesic products (e.g., Fioricet, Fiorinal). The American College of Obstetricians and Gynecologists (ACOG) has stated that, at the current time, there is no clear evidence that caffeine exposure increases the risk for fetal growth restriction (also known as intrauterine growth restriction).28 Because a final conclusion regarding risk of high caffeine intake and miscarriage cannot be made, the ACOG recommends moderate caffeine intake (< 200 mg/d) during pregnancy. Limited data suggest that butalbital is not associated with congenital anomalies.29 Ergot alkaloids (e.g., ergotamine) are contraindicated during pregnancy; these agents may cause marked increases in uterine tone, which may compromise placental perfusion and fetal oxygenation.29 Use of nonsteroidal anti-inflammatory drugs (NSAIDs) should be limited during the third trimester because of concerns about their association with premature closure of the fetal ductus arteriosus and prolongation of pregnancy. Although a 2013 review did not find evidence that first-trimester exposure to benzodiazepines is associated with an increased risk for congenital malformations,30 these drugs are not usually used to treat headache during pregnancy. Opioids and tricyclic antidepressants have a long record of safe use during pregnancy; one study suggested that tricyclic antidepressants do not have detrimental effects on the neurodevelopment of children exposed in utero.31
Obstetric and Anesthetic Management
Pregnancy is not likely to reduce the frequency or severity of tension headaches because they are not hormonally mediated. Obstetric and anesthetic management are rarely affected by the presence of tension headaches, although a history of chronic tension headaches has been associated with an increased risk for placental abruption (adjusted odds ratio, 1.60).32
Migraine Headache
Migraine headaches are classically described as unilateral, throbbing headaches sometimes accompanied by nausea and vomiting. The duration varies from hours to days. Visual disturbances (e.g., scotomata) typically precede the onset of these headaches, and focal neurologic symptoms (e.g., aphasia, hemiplegia) may also occur. Most investigators favor neurovascular vasospasm, followed by cerebral vasodilation, as a cause of these headaches; a primary vascular disorder or a disturbance in the noradrenergic nervous system also may be involved. Patients appear to be more susceptible to symptoms when serotonin levels are low.
The 1-year period prevalence of migraine headache in the United States is 3.9% for men and 5.1% for women.33 Prevalence is higher in middle life, between the ages of 30 and 59 years. Hormonal influences have a strong association with these headaches; estrogen withdrawal is associated with an exacerbation of symptoms.34 After delivery, the reduction in hormonal concentrations coincides with an increase in migraine symptoms.35 In a prospective study of 208 Japanese women,36 85% of women had headache regression during pregnancy; no patient had worsening of headache symptoms during pregnancy. More than 50% of women experienced recurrence of migraine headache in the first postpartum month; breastfeeding was protective against the recurrence of headache.
Treatment
In nonpregnant patients, therapy often involves ergotamine tartrate, typically in combination with caffeine (e.g., Cafergot, Migergot). However, ergot alkaloids are contraindicated during pregnancy because of associated uterotonic effects and possible (but unproven) teratogenic effects.29,35,37 In general, acetaminophen is the first-line treatment during pregnancy. Combination therapy with agents containing caffeine and/or butalbital can be used with caution; the caffeine component should be limited to a dose less than 200 mg/day (see earlier discussion). Use of NSAIDs should be limited during the third trimester because of concerns about their association with premature closure of the ductus arteriosus, oligohydramnios, and prolongation of pregnancy. Beta-adrenergic receptor antagonists (e.g., propranolol) may be used for prophylaxis; however, owing to their ability to cross the placenta, these agents should be used only when a patient’s symptoms are severe. Occasionally, calcium entry–blocking agents are used. The use of sumatriptan or other selective serotonin agonists is controversial. A higher incidence of congenital anomalies has been observed after administration of high doses of sumatriptan in animals37; however, in a review of human studies, no evidence of any specific adverse effect of sumatriptan on pregnancy outcome was found.38
Obstetric and Anesthetic Management
Women with a history of migraines have a higher risk for developing gestational hypertension or preeclampsia (adjusted odds ratio, 2.85).37,39 In addition, patients with a lifetime history of migraine have been reported to have a twofold increased risk for placental abruption.
Cerebral ischemia has been reported after the administration of terbutaline in pregnant patients with migraine. Rosene et al.40 recommended that physicians avoid the administration of terbutaline in pregnant women with a history of vascular headache.
There are no published data on the relationship between intrapartum anesthesia and postpartum migraine headaches.
Spinal Cord Injury
Worldwide, there are large geographic differences in the incidence, prevalence, and lethality of spinal cord injuries.41 In the United States, traumatic spinal cord injuries occur with an incidence of 23.7 to 77.0 per million population per year; the prevalence per million inhabitants is 473 to 1800.41 Improved handling and stabilization of victims at the site of an accident and the availability of extensive rehabilitation services have resulted in a higher number of women who present for obstetric care after spinal cord injury than in the past.
Patient disability and residual function depend on the anatomic location of the injury.42 Cord injuries below S2 involve mainly bladder, bowel, and sexual functions. Affected patients have relaxed perineal muscles, and women with such injuries experience pain during labor. Women with a lesion above T10 do not experience labor pain. Patients with a lesion above T6 have varying levels of respiratory compromise and are at risk for autonomic hyperreflexia (see later discussion).
Spinal shock, defined as transient sensorimotor dysfunction resolving in less than 24 hours, may develop in about half of spinal cord–injured patients.43 Neurogenic shock consists of hemodynamic and sensorimotor abnormalities and is characterized by flaccid paralysis with loss of tendon and autonomic reflexes for weeks to months.43 Patients with neurogenic shock lose vasomotor tone, temperature regulation, sweating, and piloerection in the parts of the body below the lesion. Pulmonary edema, hemodynamic instability, and circulatory collapse can develop in the absence of brainstem regulation of vasomotor tone. Patients are at risk for aspiration, infection, and other pulmonary complications. Paraplegic patients may have a compensatory tachycardia, whereas quadriplegic patients may have bradycardia due to unopposed vagal tone.
After a variable period, the patient progresses to a chronic stage in which reflex activity is regained. In most cases, this return of reflex activity occurs within 1 to 6 weeks after the injury; rarely, return of reflex activity may take several months. This stage is characterized by disuse atrophy, flexor spasms, and an exaggeration of reflexes. The mass motor reflex results from the absence of central inhibitory mechanisms. A stimulus that normally would cause the contraction of a few muscle units leads to the widespread spasm of entire muscle groups. The mass motor reflex can occur with any level of spinal cord injury. It may occur with autonomic hyperreflexia in a patient with a lesion above T6.44
Approximately 85% of patients with chronic spinal cord injuries at or above T6 experience the syndrome of autonomic hyperreflexia.43 This is a life-threatening complication that results from the absence of central inhibition on the sympathetic neurons in the cord below the injury. Noxious stimuli, bladder or bowel distention, and uterine contractions result in afferent transmission by means of the dorsal spinal root (Figure 49-1).45 These afferent neurons synapse with sympathetic neurons, and the impulse is propagated both cephalad and caudad in the sympathetic chain, without central inhibition. The propagation results in extreme sympathetic hyperactivity and severe systemic hypertension secondary to vasoconstriction below the level of the lesion. In response, the reflex arcs involving the baroreceptors of the aortic and carotid bodies lead to bradycardia and vasodilation above the level of the lesion. In patients with lesions of T6 and above, these compensatory mechanisms are insufficient to compensate for the severe hypertension. Intracranial hemorrhage, arrhythmias, and myocardial infarction occur in some cases. A variety of agents have been used for control of the hypertension of autonomic hyperreflexia (Figure 49-2).
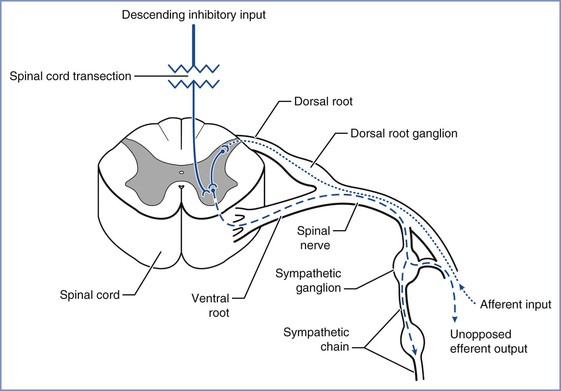
FIGURE 49-1 Noxious stimuli enter the dorsal horn of the spinal cord through the dorsal spinal root (dotted line). These afferent neurons synapse either directly or by means of interneurons (solid line) with sympathetic neurons in the intermediolateral columns of the lateral horns, which then project through the anterior roots to the paraspinal sympathetic chain (dashed line). The impulse is propagated peripherally at that spinal level and also travels both cephalad and caudad in the sympathetic chain, exiting at multiple thoracic and lumbar levels (dashed line) and resulting in sympathetic hyperactivity. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
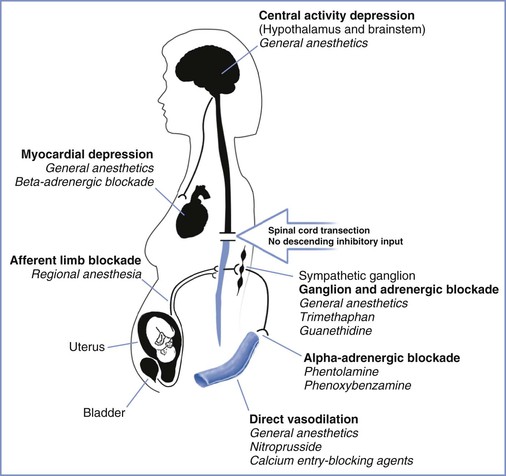
FIGURE 49-2 Sites of action for agents used in the control of hypertension associated with autonomic hyperreflexia. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
Obstetric Management
Pregnancy may aggravate many of the medical complications of spinal cord injury (Box 49-1).45 The loss of both functional residual capacity and expiratory reserve volume during pregnancy may increase the likelihood of respiratory compromise associated with spinal cord injury. Pregnancy increases the risks for thromboembolic phenomena and urinary tract infection. Loss of sympathetic tone below the level of the lesion renders pregnant patients with spinal cord injury particularly prone to orthostatic hypotension, which may result in a decrease in uteroplacental perfusion. Uterine contractions can stimulate autonomic hyperreflexia, and the resultant vasoconstriction can result in fetal hypoxia and bradycardia. In pregnant women, autonomic hyperreflexia occurs most commonly during labor.
Women with a lesion above T11 may have a higher risk for preterm labor.42 Because these women do not experience labor pain, obstetric management includes weekly cervical examinations during the third trimester. Vaginal delivery is preferred. The use of assisted vaginal delivery may be necessary because of the parturient’s inability to push.42 In a study of 52 pregnancies in spinal cord–injured women, 9 of 12 patients with lesions above T5 had symptoms of autonomic hyperreflexia. The cesarean delivery rate was 47% for women with lesions above T5 and 26% for women with lesions at T5 or below.46 Preterm delivery occurred in 19% of patients. Autonomic hyperreflexia may affect uteroplacental blood flow, necessitating careful monitoring of the fetal heart rate (FHR).
Anesthetic Management
Women with spinal cord lesions at or above T6 are at risk for autonomic hyperreflexia. This syndrome can be distinguished from other causes of intrapartum hypertension by the occurrence of cyclic hypertension (i.e., blood pressure increases during contractions and decreases between contractions). The ACOG47 recommends continuous hemodynamic monitoring during labor for all patients at risk for autonomic hyperreflexia.
Administration of neuraxial anesthesia is the most common method for prevention or treatment of autonomic hyperreflexia during labor and delivery. Spinal anesthesia has effectively controlled blood pressure in paraplegic patients undergoing general surgical procedures.48 Although some anesthesiologists contend that distortion of the vertebral column in paraplegic patients makes it more difficult to predict and control the level of spinal anesthesia, published data do not lend support to this argument.48 If spinal anesthesia is chosen, insertion of an intrathecal catheter and use of a continuous technique may be appropriate; this approach may allow careful titration of the resulting neuroblockade.
Most obstetric anesthesiologists prefer the use of epidural analgesia for the prevention or treatment of autonomic hyperreflexia during labor and delivery. Consideration also should be given to providing epidural analgesia after vaginal delivery to minimize the possibility of autonomic hyperreflexia, which has been reported to occur in response to pain as late as 5 days after delivery.49
Case reports have described the successful epidural administration of 0.25% or 0.5% bupivacaine or the administration of combined spinal-epidural (CSE) anesthesia for the mitigation of autonomic hyperreflexia.50–52 Baraka53 reported the successful use of epidural meperidine, an opioid with local anesthetic qualities, in avoiding the signs of autonomic hyperreflexia. Abouleish et al.54 observed that epidural fentanyl alone did not effectively treat the hypertension of autonomic hyperreflexia, but the addition of 0.25% bupivacaine led to a decrease in blood pressure to baseline levels. Maehama et al.55 described the successful use of magnesium sulfate for management of autonomic hyperreflexia during labor.
Patients with spinal cord injury often have a low baseline blood pressure and some hemodynamic instability. Placement of an intra-arterial catheter before induction of anesthesia allows the continuous assessment of blood pressure.
Positioning for neuraxial block may be difficult; the anesthesiologist should consider performing the block with the patient in a lateral position because the sitting position may cause hypotension from venous pooling in the lower body. Therapeutic doses of a local anesthetic agent should be administered cautiously with the understanding that the cephalad level of the sensory block can be fully assessed only if it is higher than the level of the spinal cord lesion. As a result, the typical epidural test dose may not identify unintentional subarachnoid injection in a patient with spinal cord injury. Neuraxial blockade can be partially assessed by evaluating segmental reflexes below the level of the lesion. For example, the anesthesiologist can lightly stroke each side of the abdomen above and below the umbilicus, looking for contraction of the abdominal muscles and deviation of the umbilicus toward the stimulus. Reflexes are absent below the level of the block. In some patients with spastic paresis at baseline, the level of anesthesia may be confirmed by the conversion of spastic paresis to flaccid paresis.50 A decline in blood pressure may also herald the onset of neuraxial blockade. Using a nerve stimulator connected to a saline-filled, wire-reinforced epidural catheter was found to be a reliable and relatively simple method of confirming catheter placement in the epidural space.56
Alternative means of treating autonomic hyperreflexia should be available if neuraxial anesthesia is not successful. Antihypertensive medications such as magnesium sulfate or arteriolar vasodilators may be effective, recognizing that hypotension can result in decreased uterine blood flow.46 Careful titration of nitroprusside, noting the potential for fetal/neonatal cyanide intoxication, or beta-adrenergic receptor blockade, may also be useful. The anesthesiologist should recognize that increased vagal activity during autonomic hyperreflexia can result in electrocardiographic changes including first- and second-degree atrioventricular block and sinus arrest.57
If cesarean delivery is necessary, epidural or spinal anesthesia can be administered. Spinal anesthesia is generally associated with a more rapid onset and a more unpredictable level of neuroblockade and can lead to significant hypotension.58 The effect of neuraxial blockade on respiratory function may be less severe with epidural anesthesia than with spinal anesthesia.
Severe respiratory insufficiency or technical difficulties with neuraxial anesthesia may necessitate the use of general anesthesia.59 If general anesthesia is required, a depolarizing muscle relaxant such as succinylcholine should not be given during the period of denervation injury. By a conservative definition, this period begins 24 hours after the injury and lasts for 1 year. The use of succinylcholine during this period of denervation injury may cause severe hyperkalemia60; therefore, a nondepolarizing muscle relaxant should be used to facilitate laryngoscopy and tracheal intubation.
Myasthenia Gravis
Myasthenia gravis is an autoimmune disorder characterized by episodes of muscle weakness that are made worse by activity. Its prevalence is 50 to 125 cases per million. Women are twice as likely to have the disease as men, and the onset is earlier (second or third decade in women versus the sixth or seventh decade in men).61 Myasthenia gravis has been classified according to severity as follows61:
II. Mild generalized myasthenia; may include ocular, oropharyngeal, and respiratory involvement
III. Moderate generalized disease
IV. Severe generalized weakness
V. Defined by requirement for tracheal intubation, with or without mechanical ventilation
Myasthenia gravis results from an abnormality in autoimmune regulation, which leads to the production of antibodies against the nicotinic acetylcholine receptor on the neuromuscular end plate of skeletal muscle. The result is receptor destruction as well as antibody-induced blockade of the remaining acetylcholine receptors.62 Smooth muscle and cardiac muscle are not affected. Thymic hyperplasia is common, and thymic tumors occur in approximately 10% of patients. There is an association between myasthenia gravis and other autoimmune disorders, such as rheumatoid arthritis and polymyositis. In general, an early age at onset and an extended duration of purely ocular myasthenia are good prognostic signs.
Medical Management
Treatment involves a thymectomy, administration of anticholinesterase medications and/or immunosuppressive agents, and plasmapheresis. A thymectomy improves the disease course in approximately 96% of patients; 46% of these patients undergo complete remission, and an additional 50% are asymptomatic or experience improvement with therapy.63 In addition, a thymectomy appears to exert a favorable influence on the outcome of pregnancy.64 One study noted decreased maternal and perinatal morbidity as well as less frequent clinical exacerbations in patients who had undergone thymectomy.64
Anticholinesterase drugs, which inhibit the breakdown of acetylcholine, are the mainstay of therapy. Decreased muscle weakness within minutes of administering an intravenous dose of edrophonium (10 mg) confirms the diagnosis of myasthenia gravis. Physostigmine crosses the blood-brain barrier and is not used for long-term therapy. Neostigmine and pyridostigmine are quaternary ammonium compounds that do not cross the blood-brain barrier. These drugs may be administered orally or intravenously. In general, pyridostigmine is preferred because it has less severe muscarinic side effects.65
Corticosteroids and azathioprine have been used with some success. Plasmapheresis can be especially helpful for patients in crisis. One study noted that preoperative plasmapheresis resulted in less need for mechanical ventilation and less time in the intensive care unit postoperatively.66
Myasthenia gravis can manifest in two types of crises. A cholinergic crisis results from an excess of the muscarinic effects of anticholinesterase medications combined with a poor response to anticholinesterase therapy. Symptoms include muscle weakness, respiratory difficulty or failure, increased sweating, salivation, bronchial secretions, and miosis. In contrast, a myasthenic crisis results from a worsening of the disease; its symptoms include more severe muscle weakness, including the respiratory muscles. These two crises can be distinguished by the administration of edrophonium. The symptoms do not improve if the crisis is cholinergic. In contrast, improvement indicates a myasthenic crisis and the need for a higher dose of anticholinesterase medication.
Many drugs can cause a worsening of myasthenic symptoms. These patients are extremely sensitive to drugs that potentiate muscle weakness.67 These agents include neuromuscular blocking agents, quinidine, propranolol, aminoglycoside antibiotics, and tocolytic agents such as magnesium sulfate68,69 and terbutaline. One case report noted worsened symptoms after the maternal administration of betamethasone.70
Obstetric Management
The course of myasthenia gravis during pregnancy varies. In general, approximately 29% of cases improve, 41% worsen, and 30% show no change.71 Approximately 30% of patients experience a relapse postpartum. The highest chance of exacerbations occurs in the first trimester and in the acute postpartum period.72
Myasthenia gravis increases the rates of pregnancy wastage, preterm labor, and maternal mortality and morbidity.72,73 In 1991 Plauché71 estimated that maternal mortality is approximately 40 per 1000 live births and perinatal mortality is approximately 68 per 1000 births. Maternal mortality risk is inversely proportional to the duration of myasthenia gravis, with the highest risk occurring in the first year; consequently, myasthenic women are sometimes counseled to delay childbirth for the first few years after diagnosis.73
The maternal physiologic changes of pregnancy, including alterations in drug absorption, increases in blood volume, and changes in renal clearance, may require adjustments in the doses of anticholinesterase drugs. However, in the presence of a myasthenic crisis, aggressive intravenous therapy is essential, even during labor. Anticholinesterase agents are quaternary ammonium compounds that undergo minimal placental transfer but have known uterotonic effects73; thus, uterine activity should be monitored during the administration of these drugs. Each patient should be monitored carefully for progressive respiratory compromise secondary to diaphragmatic elevation during pregnancy. Vital capacity can be measured to monitor fatigue during labor. The treatment of the myasthenic patient with preeclampsia or preterm labor is problematic because the use of magnesium sulfate for maternal seizure prophylaxis or fetal neuroprotection may be associated with a significant increase in maternal and fetal muscle weakness.72
The uterus consists of smooth muscle; therefore, myasthenia gravis should not affect the first stage of labor. However, the second stage of labor often requires the use of striated muscle and consequently an assisted (e.g., vacuum or forceps) vaginal delivery may be required.
Maternal antibodies to the acetylcholine receptor are transferred across the placenta. Neonatal myasthenia gravis occurs in approximately 16% of infants of mothers with myasthenia gravis.64,71 Physiologic variations in the levels of alpha-fetoprotein, which can block the binding of the antibody to the acetylcholine receptor, can alter the clinical course of myasthenia during pregnancy.74 The rapid decrease in alpha-fetoprotein concentrations in the neonate after birth may be responsible for transient symptoms of myasthenia (e.g., feeding problems, hypotonia, respiratory difficulty) within the first 4 days of life.72 The symptoms abate as the antibodies are metabolized, with resolution typically occurring within 2 to 4 weeks; however, anticholinesterase therapy may be required during the interim.
Anesthetic Management
Myasthenia gravis patients should undergo early antepartum consultation with an anesthesiologist. This evaluation should assess the extent of bulbar and respiratory involvement and overall baseline muscle strength. Pulmonary function testing should be performed in patients with evidence of respiratory compromise. In a study of surgical patients, the presence of bulbar symptoms, a preoperative serum level of antiacetylcholine receptor antibody greater than 100 nmol/L, and intraoperative blood loss greater than 1000 mL were risk factors for having a postoperative myasthenic crisis.75
Patients with respiratory compromise may be more susceptible to opioid-induced respiratory depression, and consideration should be given to minimizing or avoiding opioids when possible. Neuraxial techniques are the preferred method for labor analgesia in patients with myasthenia gravis, given their association with low pain scores and high maternal satisfaction, even without the addition of opioids.76 The use of anticholinesterase drugs may prolong the half-life of ester local anesthetic agents.
Neuraxial anesthetic techniques are preferred for cesarean delivery unless the patient has significant bulbar involvement or respiratory compromise. The use of bilevel positive airway pressure for ventilatory support in patients with moderate respiratory compromise may improve the safety of neuraxial anesthesia.77
In the patient with severe bulbar involvement or respiratory compromise, it may be prudent to secure the airway before surgery. Sodium thiopental, ketamine, and propofol have been used successfully for the induction of general anesthesia in patients with myasthenia gravis.73,77,78 Depolarizing muscle relaxants (e.g., succinylcholine) have an unpredictable effect in these patients, with affected and unaffected muscles being more sensitive and resistant to these agents, respectively.63 However, the commonly administered dose of succinylcholine (1 to 1.5 mg/kg), which is three to five times the effective dose in 95% of normal patients, will most likely provide adequate relaxation even for resistant muscles. Anticholinesterase agents and plasmapheresis cause decreases in the activity of plasma cholinesterase and may cause delays in succinylcholine hydrolysis.
Myasthenic patients are extremely sensitive to nondepolarizing muscle relaxants. If a nondepolarizing muscle relaxant must be given, the anesthesia provider should administer a small amount of an agent with a short half-life (e.g., rocuronium, atracurium, vecuronium).63 Mivacurium is metabolized via plasma pseudocholinesterase, which may be inhibited by pyridostigmine. In general, greater disease severity corresponds with enhanced sensitivity to nondepolarizing muscle relaxants, necessitating the use of clinical judgment and neuromuscular monitoring to determine the amount and timing of drug doses. Myasthenia may prevent a full-strength contraction with nerve stimulation; therefore, a control train-of-four stimulus test should be performed before paralysis for later comparison. For nondepolarizing agents, approximately 50% of the standard dose may be adequate, and a prolonged recovery should be anticipated. Small doses of neostigmine may be given cautiously for the reversal of neuromuscular blockade.
After delivery, fluid shifts and decreased maternal alpha-fetoprotein concentrations may necessitate an adjustment of anticholinesterase drug doses. Some patients who receive general anesthesia require postoperative ventilation. The following factors are predictive of an increased risk for postoperative ventilation: (1) female gender, (2) FEF25%-75% (forced expiratory flow during the middle half of the forced vital capacity) less than 3.3 L/sec and less than 85% of that predicted, (3) FVC (forced vital capacity) less than 2.6 L/sec and less than 78% of that predicted, and (4) MEF50% (maximal expiratory flow at 50% of expired vital capacity) less than 3.9 L/sec and less than 80% of that predicted.79
Epilepsy
Epilepsy is a condition characterized by recurrent seizure activity in the absence of metabolic disorders or acute brain disease. The classification scheme for epileptic seizures is evolving; however, most seizures are grouped into the two major types: partial and generalized.80,81 In partial seizures, the excess neuronal discharge is thought to originate in one region of the cerebral cortex; in generalized seizures, the discharge occurs bilaterally and involves the entire cortex.
Medical Management
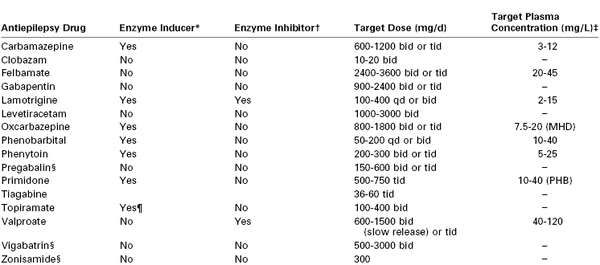
A variety of antiepileptic agents are used for seizure therapy, depending on the type of seizure and clinical response (Table 49-2). A variety of adverse effects have been reported with these agents, including early-onset events (e.g., somnolence, dizziness, hypersensitivity, rash, gastrointestinal symptoms) and late-onset events (e.g., depression, leukopenia, aplastic anemia, thrombocytopenia, megaloblastic anemia, hyponatremia).81
Prognosis for medical control of seizures is good for patients with generalized seizure disorders; as many as 2% to 40% of newly diagnosed epilepsy patients become seizure-free without or with minimal antiepileptic drug therapy.82 Two of three newly treated epilepsy patients will eventually enter long-term remission (5 years or more without a seizure).82 However, about one third of patients will have an intermittent pattern (early remission with late recurrence or late remission). Finally, the standard mortality ratio for patients with epilepsy (observed number of deaths in the study compared with the general population) is consistently increased in the first several years after the diagnosis of epilepsy.
Interaction with Pregnancy
Three to five births per thousand occur in women with epilepsy.83 A 2009 systematic review by the American Academy of Neurology and American Epilepsy Society concluded that there is insufficient evidence to determine whether seizure frequency changes during pregnancy.84 Women who are free of seizures for at least 9 months to 1 year before pregnancy have a probability of 84% to 92% of remaining seizure-free during pregnancy.84
Optimizing antiseizure therapy before pregnancy is critical. Because of the teratogenic aspects of many antiepileptic agents, some physicians consider withdrawal of these drugs after 2 years without seizures and recommend waiting at least 6 additional months after withdrawal before attempting to conceive. Substituting new antiepileptic agents after conception is not recommended.
A variety of causes have been proposed for the increase in seizure frequency observed in some pregnant women (Table 49-3). Higher estrogen concentrations in pregnancy lower the seizure threshold.85 Greater sodium and water retention, alkalosis secondary to hyperventilation, sleep deprivation, and increased stress and anxiety also have been suggested as mechanisms.86 In addition, anticonvulsant drug levels can decrease during pregnancy, often despite the administration of a larger dose87; this may be partially explained by the decreased plasma protein binding and greater drug clearance observed during pregnancy.88 The American Academy of Neurology and American Epilepsy Society concluded that monitoring of lamotrigine, carbamazepine, and phenytoin levels should be considered during pregnancy. Monitoring of levetiracetam and oxcarbazepine (and its active metabolite, monohydroxy derivative) may be considered, and monitoring of other antiepileptic agents should not be discouraged despite limited data regarding their pharmacokinetic behavior during pregnancy.89
TABLE 49-3
Possible Causes of Increased Seizure Frequency during Pregnancy
| Mechanism | Examples |
| Hormonal | Changes in levels of estrogen (proconvulsant) and progesterone (anticonvulsant) |
| Metabolic | Increased water and sodium retention |
| Psychological | Stress, sleep deprivation |
| Pharmacokinetics | Increase in liver metabolism, renal clearance, or volume of distribution |
| Physiologic | Decreased gastrointestinal absorption |
Maternal seizures can have devastating consequences. Hypoxia and acidosis that occur during a generalized seizure can result in fetal compromise or intrauterine fetal death. During the past three decades, the overall risk for obstetric complications in epileptic women has declined. Although some studies have suggested an increased risk for hypertension in pregnancy (including preeclampsia), bleeding, and preterm birth, a systematic review concluded that evidence is inconclusive.84 However, women with epilepsy may have a moderately increased risk for cesarean delivery.84
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree