Neonate and Infant with Cardiovascular Disease
David F. Teitel
OVERVIEW
Although there are over 7000 different congenital cardiac defects,1 there are only a very few ways that a newborn or infant presents with cardiac disease. Symptomatic heart disease occurs in about 40% of congenital lesions, so that many infants present without symptoms. For this reason, perhaps, many clinicians consider the presence of a murmur as the most definitive evidence of heart disease. Unfortunately, this is the source of a very large number of unnecessary referrals to the pediatric cardiologist—the vast majority of murmurs occur in patients with normal hearts, and most innocent murmurs are misdiag-nosed.2 Up to 50% of normal children present with an innocent murmur at some time, whereas only about 50% of newborns with symptomatic heart disease have murmurs on clinical presentation. Even assuming that all of the children without symptoms who have congenital heart disease have murmurs, this would make the presence of a murmur to be about 2% specific and 80% sensitive in the diagnosis of heart disease. There is no other sign in medicine that has such poor specificity and sensitivity yet is used with such certainty.
Rather than asymptomatic heart disease, it is of utmost importance for the pediatrician to be able to quickly diagnose symptomatic heart disease. The change from a fetal circulatory system to a transitional circulation occurs immediately at birth, and the mature circulation develops within weeks.3 Because of these dramatic alterations in blood flow and oxygen uptake patterns in the neonatal period, the fetus with a stable cardiovascular status can become a newborn with severe cardiovascular symptoms, potentially leading to death, soon after birth. On the other hand, advances over the last few decades have given the clinician the tools to rapidly stabilize these critically ill neonates, and subsequently correct or ameliorate even the most complex defects. Thus, it is essential that the pediatrician be able to recognize the infant with symptomatic heart disease quickly, so that the appropriate diagnostic and therapeutic interventions can be initiated as soon as possible. Many pediatricians are insecure in their ability to recognize symptoms of heart disease, and thus fall back on the murmur as their gold standard of diagnosis, which can lead to devastating consequences for those symptomatic infants without murmurs. It is possible to overcome this insecurity by recognizing that there are only 3 different, overlapping, modes of presentation of symptomatic heart disease in the newborn and infant, each very easy to recognize during a routine examination. These modes of presentation are cyanosis, decreased systemic perfusion (hypoperfusion), and respiratory distress/failure to thrive.
Prior to considering the modes of presentation of symptomatic heart disease in the newborn, it is worthwhile to review fetal physiology, to understand why symptomatic heart disease is rare in the fetus.
FETAL PHYSIOLOGY
Most of our knowledge of the fetal circulation presented in this section is derived from research in fetal sheep,4 but more recent studies in human fetuses using echocardiography and Doppler studies confirm those findings, with only modest differences in percentages of blood flow in the various vessels and chambers, primarily related to the significantly greater cerebral blood flow in the human.5 The fetal circulation is unlike the postnatal circulation because the 2 ventricles eject into the vascular beds that are not fully separated. Rather, there are three main connections, or shunts, between circulatory beds and cardiac chambers that allow for admixture of venous return to the ventricles and of their outputs to the arterial beds. These connections allow 1 ventricle to take over the work of the other when a congenital cardiac defect is present. Those shunts, along with the far lesser oxygen demand of the fetus compared to the newborn, are the main reasons that most forms of critical congenital heart disease do not present until after birth.
Although there is overlap in the function of the ventricles, they still primarily perform their postnatal tasks prior to birth. Postnatally, the right ventricle is the oxygen uptake ventricle, ejecting poorly oxygenated blood into the pulmonary circulation for oxygen uptake. The left ventricle is the oxygen delivery ventricle, ejecting highly oxygenated blood into the systemic circulation for oxygen utilization by the tissues. In order for the ventricles to perform these tasks in the fetus reasonably efficiently, the right ventricle should receive relatively deoxygenated blood and eject the majority of its output to the placenta, because that is the oxygen uptake organ, and the left ventricle should receive relatively highly oxygenated blood and eject the majority of its output to the most metabolically active tissues.
To understand how the fetal circulation supports the efficient function of the right and left ventricles, it is best to consider, in order, the components of venous return, the distribution of these components within the heart, and the various vascular beds into which the ventricles eject (Fig. 483-1). Because these venous and arterial components are not separated in the fetus, one cannot talk about “venous return” as representing systemic or pulmonary venous return, or “cardiac output” as representing pulmonary or systemic blood flow. The amount of blood that composes all of the venous components will be called the “combined venous return,” and the amount of blood that the ventricles together eject will be called the “combined ventricle output” (CVO). The latter is equivalent to 2 “cardiac outputs” of the normal postnatal circulation, as the sum of pulmonary and systemic blood flow.
There are 5 components of venous return in the fetus: the upper body systemic venous return via the superior vena cava (SVC); the lower body systemic venous return, via the inferior vena cava (IVC); the placental return, also via the IVC; the coronary venous return, primarily via the coronary sinus (CS); and the pulmonary venous return, via the pulmonary veins. The right atrium receives the vast majority of venous return in the fetus because the pulmonary venous circulation, the only one that drains directly into the left atrium, composes only about 8% of combined venous return (Fig. 483-2). Despite the fact that over 90% of combined venous return drains exclusively into the right atrium, flow patterns within the veins and the right atrium, in association with the foramen ovale, which shunts blood from the right atrium into the left, allow for the left ventricle to receive a large amount of relatively highly saturated blood and the right ventricle to receive primarily poorly oxygenated blood.
To understand this remarkable phenomenon, it is best to view blood flow patterns in Figure 483-3. The least saturated blood comes from the coronary and upper body circulations and preferentially flows to the right ventricle. The coronary sinus orifice is near the atrioventricular groove, just above the tricuspid valve, and below the foramen ovale. Thus, essentially all coronary venous return via the coronary sinus passes inferiorly via the tricuspid valve to the right ventricle. Similarly, the position of the eustachian valve and the superior vena cava ensures that well over 90% of SVC flow passes inferiorly and laterally, to the tricuspid valve and right ventricle. Unlike blood returning via the SVC and CS, that in the inferior vena cava is not well mixed just before it enters the right atrium but is composed of relatively separate streams of blood returning via the intrahepatic inferior vena cava, the ductus venosus, and the right and left hepatic veins. The intrahepatic inferior vena cava receives blood primarily from the lower limbs, the kidneys, and the adrenals, and that relatively poorly oxygenated blood is also directed laterally via the eustachian valve, across the tricuspid valve to the right ventricle. The 3 other venous streams have more complicated sources of blood flow, as shown in Figure 483-4. Umbilical venous blood first reaches the portal sinus, where the majority ascends via the ductus venosus to the medial aspect of the junction of the inferior vena cava and right atrium. This highly oxygenated ductus venosus blood primarily is directed through the foramen ovale, to the left atrium and ventricle. The remainder of the umbilical venous blood enters the left portal venous system, whereas the splanchnic venous blood, consisting of intestinal, stomach, and splenic venous return, goes via the portal sinus primarily to the right portal veins. The portal venous blood in each lobe mixes with a much smaller amount of hepatic arterial blood and returns to the inferior vena cava near the right atrium. The right hepatic veins, draining primarily the less oxygenated splanchnic blood, drain into the lateral wall of the inferior vena cava and flow, with the intrahepatic venous return, and pass primarily to the right ventricle, whereas the left hepatic venous blood, composed in large part of highly oxygenated umbilical venous blood, enters the medial aspect of the inferior vena cava and flows with the ductus venosus blood across the foramen ovale to the left atrium and ventricle.6 In the human, flow patterns in the central venous components and the atria lead to 55% of combined venous return entering the right ventricle, and 45% entering the left (Fig. 483-2). Although it has not been measured in the human, left ventricular blood is likely to be about 15% more saturated with oxygen than right ventricular blood, which would benefit its role as the oxygen delivery ventricle, and also has a higher glucose concentration, because it receives the vast majority of umbilical venous return, the source of substrate to the fetus. Thus, it is well set up to deliver oxygen and substrate to the highly metabolic tissues, as long as that is where its blood is delivered.
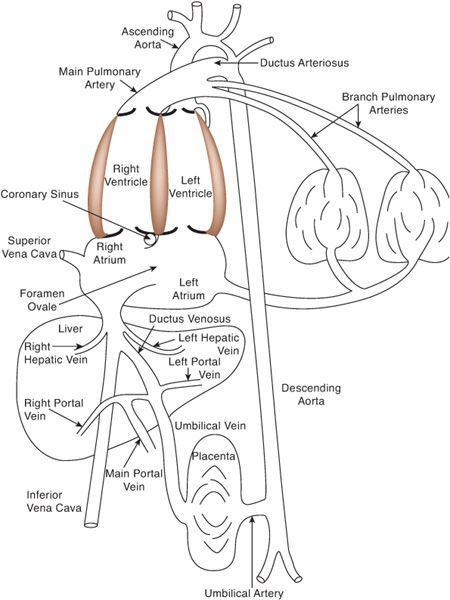
FIGURE 483-1. The fetal circulation, with direct communications existing between the right and left atria (foramen ovale), the aorta and pulmonary artery (ductus arteriosus), and the umbilical venous and systemic venous circulations (ductus venosus).
The organs that require the most nutrients, and thus blood flow, per gram are the heart, brain, and adrenal glands, though the latter are extremely small and thus are not of great consequence when considering blood flow distribution. The heart receives about 3% to 5% of CVO, and the brain receives about 32% of CVO (Fig. 483-2). Because both of these organs receive blood from vessels arising from the ascending aorta (Fig. 483-3), both receive blood entirely from the left ventricle in the normal fetus. Only a small amount of left ventricular blood, about 8% to 10% of CVO, is not delivered to the heart and upper body, but crosses the aortic arch and isthmus to the lower circulation (Fig. 483-2). About two thirds of the blood traveling down the descending aorta goes to the placenta for oxygen and substrate uptake, while the other third goes to the lower body of the fetus. Thus, only a small amount of left ventricular blood goes to the placenta. Conversely, the majority of right ventricular blood crosses the ductus arteriosus to the descending aorta, with only a minority going into the lungs, because of the high resistance in the pulmonary vascular bed. Over 80% of right ventricular output goes down the descending aorta, two thirds of which goes to the placenta. Thus, the majority of right ventricular blood indeed goes to the oxygen uptake circulation, whereas the majority of left ventricular blood goes to the highly metabolic tissues for oxygen and substrate utilization.
Although the ventricles perform their normal postnatal tasks relatively efficiently in the fetal environment, the intravascular and intracardiac shunts allow them to easily take over the other’s function in the presence of congenital cardiac defects, something that is not possible in the postnatal circulation. Alterations in gene expression in embryonic life alter cardiac and vascular structures, as described in the previous section, often leading to decreased blood flow through one ventricle or the other. These aberrations in structure lead to alterations in flow, which, although homeostasis is maintained, cause further changes in cardiac and vascular structures. The combination of primary molecular events and secondary flow events creates the specific congenital heart abnormality seen when the baby is born, but it is usual that we do not know which is the primary event and which is flow related. 
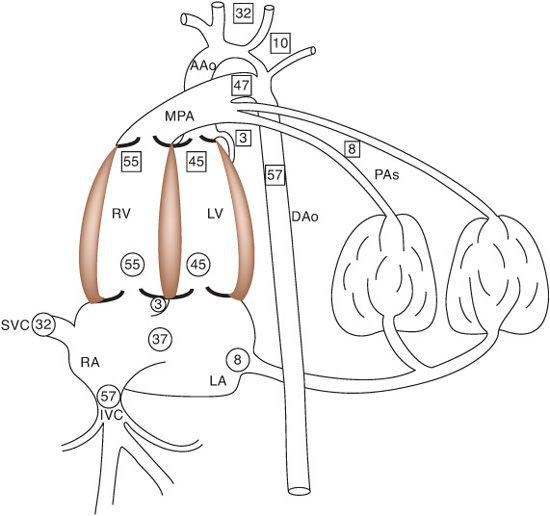
FIGURE 483-2. The central components of the fetal circulation, with the percentages of combined venous return presented in circles and of combined ventricular output in squares. IVC, inferior vena cava; SVC, superior vena cava; RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle; MPA, main pulmonary artery; AAo, ascending aorta; DAo, descending aorta; PAs, branch pulmonary arteries.
TRANSITIONAL CIRCULATION
At birth, dramatic changes in blood flow and cardiac function unfold, leading to a circulation that is more efficient at the uptake and delivery of oxygen but that depends on a nearly normal heart and circulation. The most dramatic changes occur within seconds and minutes of birth, followed by gradual changes, which lead to a mature circulatory pattern within weeks of birth. These changes are required for the healthy survival of infants who underwent normal cardiovascular development, but are potentially lethal for those with congenital cardiovascular defects.
The 3 most important changes at birth are the dramatic decrease in pulmonary vascular resistance, the abolition of the central vascular and cardiac shunts, and the increase in output of the two ventricles.7 Of the 3, the driving force toward the achievement of the normal postnatal circulation is the dramatic and remarkable fall in pulmonary vascular resistance.
Pulmonary vascular resistance is extremely high in the fetus, allowing only about 8% of combined ventricle output to enter the lungs until near term. The intense vasoconstriction occurs in the distal pulmonary arteries, which have a thick medial smooth muscle layer. Pulmonary vascular resistance actually begins to decrease substantially before birth, as a result of a large increase in vessels in late gestation, increasing the cross-sectional area of the vascular bed. They remain vasoconstricted, however, largely due to their hypoxic environment, the lack of vasodilating substances such as prostaglandins E2 and I2, and the presence of vasoconstrictors such as the leukotrienes. Endogenous nitric oxide (NO), an important dilator of these vessels, is thought to be present in low concentrations. Immediately at birth, there is an abrupt and large decrease in pulmonary vascular resistance, which leads to an enormous increase in pulmonary blood flow of 10-fold to 20-fold. Although increased oxygenation is thought to be a major contributor to this fall in resistance, ventilation with fetal gases has been shown to be capable of causing up to two-thirds of the fall that is seen, so that other mechanisms must be in play. The decrease in pulmonary vascular resistance induced by ventilation alone may in part be caused by a direct effect, as changes in surface tension at the alveolar air–liquid interface reduce perivascular tissue pressures, but likely is also caused by an increase in PGI2 induced by gaseous distension, and by an increase in endogenous NO, which may also mediate the oxygen responsiveness of the pulmonary arteries.8
The large increase in pulmonary blood flow is essential to the second major component of the transitional circulation, the abolition of the central shunts.9 These shunts are the foramen ovale, between the left and right atrium; the ductus arteriosus, between the main pulmonary artery and descending aorta; and the ductus venosus, between the portal sinus and inferior vena cava. In fetal life, the foramen ovale is like a windsock, directing blood from the ductus venosus and left hepatic veins toward the left atrium, and remains open because of the far greater right atrial venous return than that of the left. As pulmonary vascular resistance falls immediately at birth, pulmonary venous return to the left atrium increases dramatically, often exceeding that of the right atrium as the shunt through the ductus arteriosus reverses its direction, now going from the descending aorta to the main pulmonary artery. Once left atrial venous return and pressure exceed that of the right, the flap of the foramen, which is in the left atrium, presses against the floor of the fossa ovalis, abolishing the foraminal shunt. The shunt through the ductus venosus is maintained primarily by umbilical venous return from the placenta, which is just under 50% of combined venous return. This source of blood is instantly abolished when the umbilical cord is cut. Even before this, however, umbilical flow almost entirely ceases, due to the vasoconstrictor effects of oxygen and mechanical stretch on the umbilical artery. Ductus venosus flow ceases completely within 3 to 7 days after birth, likely due to the passive effects of diminished blood flow, although it has been shown to vasodilate with elevations in PGE1.
The shunt through the ductus arteriosus is reversed as pulmonary vascular resistance falls, as mentioned above. The ductus arteriosus has a large amount of medial smooth muscle and thus is a very reactive vessel. In the fetus, its patency is maintained in part by high levels of PGE2, which are about 4-fold higher than those after birth, but it is uncertain whether it is the circulating levels or local levels that exert the greater control. Use of nonsteroidal anti-inflammatory drugs by the mother before birth has been shown to lead to ductal constriction, which in turn can lead to progressive pulmonary hypertension and possible right ventricular failure, with hydrops. After birth, the ductus arteriosus is functionally closed in most term infants within 10 to 15 hours, and permanent closure, caused by thrombosis, intimal proliferation, and fibrosis, is seen usually within 3 weeks. The functional closure is likely caused by the elevated levels of oxygen seen at birth, and the decrease in levels of PGE2. Persistent patency of the ductus arteriosus in preterm infants is common and is likely caused by a lower responsiveness of the immature ductus to oxygen, and perhaps to persistently elevated PGE2 levels.
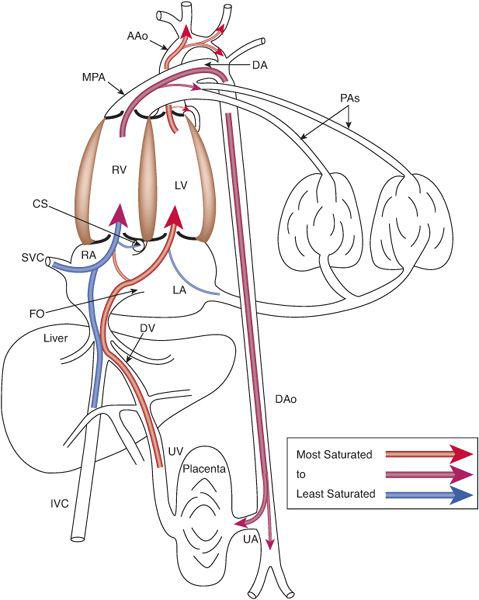
FIGURE 483-3. Blood flow distribution in the fetal circulation demonstrating the preferential flow of the less-saturated blood via the right ventricle to the placenta for oxygen uptake and of the more highly saturated blood via the left ventricle to the highly metabolic organs, the heart and brain. UV, umbilical vein; DV, ductus venosus; CS, coronary sinus; UA, umbilical artery; see Figure 483-2 for other abbreviations.
Last, there is a large, nearly 3-fold, increase in ventricular output at birth. The increase in left ventricular output exceeds that in the right because the right ventricle ejects more blood than the left in fetal life, whereas they eject the same amount (or the left may eject a little more than the right because of the early left-to-right shunt through the ductus arteriosus). This increase in output is driven by a similarly large increase in oxygen consumption, which has been shown to increase 3-fold in newborn lambs. The increase in oxygen consumption is likely driven by the need of the newborn to maintain thermoregulation and to breathe, neither of which consumes much oxygen in the fetal environment. 
SYMPTOMATIC HEART DISEASE IN THE NEWBORN AND INFANT
Most patients who eventually present with symptomatic heart disease do so during the neonatal period or early infancy, when there is a dramatic transition from the fetal to the transitional circulation (during the first days of life), a slower transition to the mature circulation as pulmonary vascular resistance continues to fall over the subsequent 6 weeks, and a period of physiologic anemia that occurs during the second and third months of life.
Symptomatic heart disease in the newborn and infant presents either primarily with cyanosis, inadequate systemic perfusion, or respiratory distress/failure to thrive (from excessive pulmonary blood flow, without either cyanosis or hypoperfusion). Cyanosis can be appreciated by careful visual inspection, hypoperfusion by examination of the extremities, and respiratory distress/failure to thrive by observing the respiratory rate and pattern, and by plotting the infant on the appropriate growth chart. A simple evaluation of the infant at each examination will undoubtedly uncover the possibility of congenital heart disease, and a thoughtful and rational approach will lead to the appropriate differential diagnosis and plan. Admittedly, this approach is imperfect; some lesions are complex with overlapping manifestations (eg, an infant with truncus arteriosus may present with cyanosis in the first hours of life as pulmonary vascular resistance is high, but becomes tachypneic without cyanosis over the next few hours and days). In addition, the differential diagnosis includes noncardiac disease, so that the initial evaluation points only to the possibility of heart disease, not to its definite presence. However, by focusing on these few signs of congenital heart disease and evaluating each infant throughout infancy, the pediatrician will not miss the diagnosis and will ensure that each infant is treated appropriately.
CYANOTIC HEART DISEASE
The newborn or infant who presents with cyanosis without significant respiratory distress almost always has structural congenital heart disease. The general approach to the cyanotic infant is detailed in Chapter 49. Because cyanosis can be diagnosed by inspection only, it is the most common manifestation of symptomatic congenital heart disease and may be associated with critically decreased oxygen delivery; therefore, it is important for the pediatrician to be able to quickly and accurately exclude its presence. The presentation, however, may be difficult to appreciate for a variety of reasons: the presence of a ductus arteriosus immediately at birth, which may not close during the short period of time that a newborn is in the hospital, may maintain adequate levels of systemic arterial oxygen saturations so that cyanosis is difficult to detect clinically; cyanosis may be masked by a decrease in blood hemoglobin concentration because it is not determined directly by the level of arterial oxygen saturation but by the amount of reduced hemoglobin in the blood; many cyanotic lesions are not associated with heart murmurs which, unfortunately, leads some clinicians away from the possibility of congenital heart disease; and cyanotic heart disease is diagnosed clinically by the presence of central cyanosis, and the few vascular beds that do not have significant vasomotor tone and thus reflect central oxygen saturation are not easy to evaluate. Because of these considerations, various investigators have evaluated the usefulness of routine pulse oximetry as part of the newborn evaluation and have subsequently proposed its adoption,10,11 though it has not been widely implemented as yet. Thus, the newborn with cyanotic heart disease may not be diagnosed prior to discharge from hospital, making it essential for the pediatrician to consider the possibility at each routine examination, at least through the first few months of life. Many infants have been diagnosed with complex cyanotic heart lesions several weeks after birth.
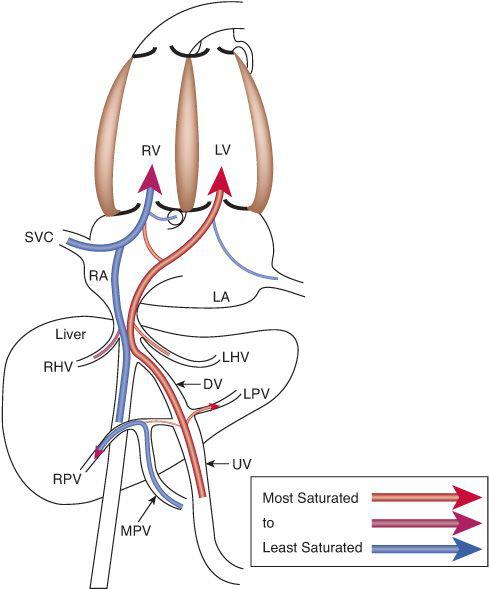
FIGURE 483-4. Venous return patterns demonstrate that the highly saturated blood from the placenta preferentially crosses via foramen ovale to the left atrium and left ventricle, and the less-saturated blood from the upper body, heart, lower body, and splanchnic bed preferentially flows across the tricuspid valve to the right ventricle. LPV, left portal vein; LHV, left hepatic vein; MPV, main portal vein; RPV, right portal vein; see Figures 483-2 and 483-3 for other abbreviations.
 HEMODYNAMIC CATEGORIES
HEMODYNAMIC CATEGORIES
As with the other forms of symptomatic congenital heart disease, it is best not to memorize the various lesions associated with cyanosis, but rather to understand the pathophysiologic processes that lead to the finding; moreover, as with each, cyanosis can be divided into 2 processes. Infants present with cyanosis due to heart disease either because the amount of blood going through the pulmonary vascular bed is decreased (decreased pulmonary blood flow) or the amount is normal or even increased, but that systemic venous blood is abnormally directed via the ventricle across the aortic valve (D-transposition complexes). It is useful to consider these 2 hemodynamic categories of cyanotic lesions separately, because lesions within each category tend to have similar presentations, associated findings in the fetus and after birth, and therapeutic approaches.
Decreased Pulmonary Blood Flow
Most lesions with decreased pulmonary blood flow have obstruction either to the inflow of blood to the right ventricle, or the ejection of blood from it. A much smaller number of lesions, occurring with much less frequency, are associated not with obstruction but with insufficiency of one of the right-sided valves, either of the inflow (tricuspid valve) or outflow (pulmonary valve). All of these lesions can be considered sequentially, along lines of blood flow (Table 483-1), and the lesions, when presented in the text. Systemic venous blood arrives in the right atrium and from there, crosses the tricuspid valve to enter the right ventricle. Thus, the first level of obstruction occurs at the tricuspid valve, which may be totally absent (tricuspid atresia) or narrowed (tricuspid stenosis, or hypoplasia). The former is almost always associated with a ventricular septal defect, whereas the latter is associated with hypoplasia of the right ventricle and secondary pulmonary valve atresia. The most common cause of insufficiency of the tricuspid valve is Ebstein anomaly, in which the septal leaflet is displayed inferiorly, toward the apex of the right ventricle, preventing coaptation of the leaflets and leading to severe valve insufficiency. Because the right ventricle cannot generate much pressure in the presence of severe insufficiency, there may be acquired pulmonary valve atresia, though often this is just functional (evidence that the valve is patent but cannot be opened by the right ventricle is the presence of pulmonary insufficiency on color Doppler echocardiography).
Table 483-1. Congenital Cardiovascular Defects Presenting with Cyanosis Caused by Decreased Pulmonary Blood Flow
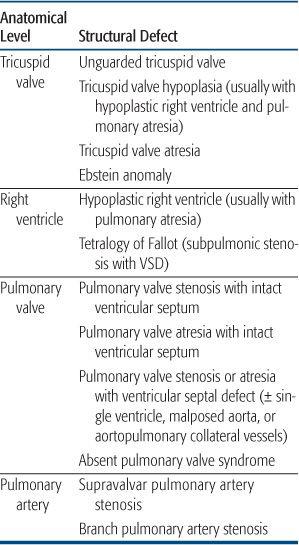
The next level of obstruction occurs within the right ventricle. Right ventricle hypoplasia, as mentioned above, is usually secondary to tricuspid valve hypoplasia and may include abnormalities in the volume of all 3 components of the right ventricle—the inflow, apex, and outflow. Outflow obstruction alone occurs most frequently when the outlet ventricular septum is malaligned anteriorly so that it does not meet the muscular and membranous septum, leading to an outlet ventricular septal defect. The association of anterior malalignment of the outlet ventricular septum, a ventricular septal defect, and outlet (infundibular) obstruction leading to a right-to-left shunt across the ventricular septal defect is called tetralogy of Fallot and is one of the most common forms of cyanotic congenital heart disease. Because tetralogy of Fallot involves abnormal embryonic movement of the outlet septum, it may be associated with a microdeletion of 22q11 (diGeorge syndrome, velocardiofacial syndrome, etc), which has many other manifestations thought primarily to be caused by abnormal migration of the cardiac neural crest tissue. This syndrome is particularly prevalent in tetralogy of Fallot when the aortic arch is right-sided, because this chromosomal abnormality affects arch development as well, causing other cardiovascular defects such as interrupted aortic arch. In the most severe form of tetralogy of Fallot, the pulmonary valve is atretic. In this situation, the branch pulmonary arteries may arise from a ductus arteriosus or may not form normally. If that occurs, the vascular segments of the lung are fed by major aortopulmonary collateral arteries, and surgical reconstitution of a normal vascular bed is very complex. 
Outflow obstruction without a ventricular septal defect rarely can occur, leading to cyanosis. There are two primary causes of outflow obstruction without an associated ventricular septal defect. The course of the moderator band, between the body and outflow of the right ventricle, can be anomalous and partially obstruct the outflow tract. Because there are high-pressure and low-pressure components to the right ventricle in this lesion, it is called double-chamber right ventricle. However, it usually occurs later in life, often in patients with a ventricular septal defect (which may have since closed), so that it is not commonly considered in the differential diagnosis of cyanosis in the infant and newborn. More commonly, right ventricular outflow tract obstruction without a ventricular septal defect can occur in hypertrophic cardiomyopathy of the newborn, often associated with maternal diabetes. In some cases, the massive septal hypertrophy can preferentially obstruct the right ventricular outflow tract, leading to a right-to-left atrial shunt and cyanosis.
The next level of obstruction is at the pulmonary valve, which may be stenotic or atretic. The diagnosis of critical valvar pulmonary stenosis is made when the systemic arterial saturation is under 92% in the absence of a ductus arteriosus, and requires neonatal intervention. When the pulmonary valve is atretic in the presence of right ventricular hypoplasia, it is generally thought to be a secondary phenomenon, with the primary embryological event being hypoplasia of the tricuspid valve. When there is a well-developed, tripartite (inflow, body, and outflow) ventricle, it may caused by a later, fetal event, perhaps a valvulitis, causing fusion of the commissures. The presence of well-developed ventricle allows for a transcatheter perforation and dilation of the valve, obviating the need for a surgical shunt (see Chapter 499, “Interventional Cardiology”). Above the pulmonary valve, supravalvar pulmonary stenosis may occur, usually in association with branch pulmonary artery stenosis, which are seen together in Williams syndrome, a genetic defect of the elastin gene, which has been mapped to chromosome 7.12 However, the arterial obstruction in Williams syndrome usually occurs over time, is rarely severe, and rarely presents with cyanosis in infancy. Branch pulmonary artery stenosis is also seen in Alagille syndrome, in which there is an associated paucity of bile ducts in the liver, leading to liver dysfunction. It also has a defined genetic basis,13 as about 88% of patients show a mutation of the JAG1 gene.
Finally obstruction can occur at the pulmonary arteriolar level. This is not cyanotic congenital heart disease, but is pulmonary hypertension of the newborn, in which the arterioles do not dilate normally at birth. It is discussed in a separate chapter. 
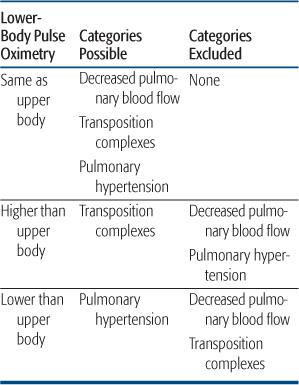
Findings determined by postnatal flow patterns help greatly with the clinical diagnosis and stabilization. In infants with decreased pulmonary blood flow, the decreased pressure and flow out the pulmonary valve implies that a ductal shunt, if present, must be left-to-right. Thus, upper- and lower-body pulse oximetry must be the same, whatever the lesion (Table 483-2). The presence of differential saturations excludes a lesion associated with decreased pulmonary blood flow, as does the presence of decreased lower body pulses, as mentioned above.
The time course of cyanosis can also lead the clinician to this category of lesions. In most lesions with decreased pulmonary blood flow, the ductus is widely patent at birth, supplying adequate flow for several hours or days. With the rapid fall in pulmonary vascular resistance, pulmonary blood flow may be 2 to 4 times systemic, causing saturations to be in the high 80s to low 90s, and preventing the appearance of cyanosis. Cyanosis may then progress gradually, over hours to days, or, as often happens, is first noticed when the newborn cries or is fed, both of which increase oxygen utilization and decrease pulmonary blood flow, by increasing pulmonary impedance in the former and decreasing systemic vascular resistance in the latter. This time course is very different than that seen in the transposition complexes, which will be presented below.
Last, blood flow patterns may also allow for the distinction of inflow lesions associated with decreased pulmonary blood flow and all other causes of cyanosis by simple physical findings. When the right ventricle does not fill appreciably, in tricuspid atresia or severe hypoplasia, with a hypoplastic right ventricle, it ejects a minimal amount of blood, and thus does not generate a parasternal impulse. In all other causes of cyanosis (decreased pulmonary blood flow with outflow obstruction, transposition complexes, and pulmonary hypertension of the newborn), the right ventricle ejects a reasonable amount of blood under high pressure, and thus there is a normal to increased parasternal impulse. Thus, the careful physical examination can lead to the rapid diagnosis of cyanosis secondary to decreased pulmonary blood flow caused by inflow obstruction to the right ventricle, and a simple electrocardiogram can usually differentiate the 2 possible lesions (Fig. 483-5).
Table 483-2. Hemodynamic Categories in Cyanotic Newborns Related to Upper and Lower Body Pulse Oximetry
Because blood flow patterns are similar in the fetus and newborn with most lesions causing decreased pulmonary blood flow, the means to stabilize the patient prior to definitive diagnosis and cardiac interventions are similar as well. The atrial septum is rarely restrictive, so that there is rarely a need for a cardiologist to perform a balloon atrial septostomy. Because pulmonary blood flow is usually maintained adequately when the ductus arteriosus is widely patent, these infants can almost always be stabilized by giving PGE1, as long as the side effects of that drug are properly considered and avoided. The ductus arteriosus may close even more rapidly than normal in these patients because it is often long and thin. Most importantly, care needs to be taken to ensure adequate ventilation, because apnea is a common occurrence, and the volume status and arterial perfusion pressure must be maintained, because PGE1 is also a potent systemic vasodilator.
D-Transposition of the Aorta
The second group of lesions associated with cyanosis in the newborn and infant can be considered together as defects in which the aorta is anteriorly and rightwardly displaced, committed to the systemic venous, or usually, right, ventricle. The aorta is transposed over the ventricular septum, and systemic venous rather than pulmonary venous blood preferentially flows across the valve to the body via the ascending aorta. Pulmonary blood flow may be normal, increased, or decreased in this group of lesions, depending on the associated lesions, but in most, is either normal or increased.
The classic and most common lesion in this group is d-transposition of the great arteries with intact ventricular septum, also called simple d-transposition of the great arteries. Details of the diagnosis and management of this lesion are provided in Chapter 484. In this lesion, the pulmonary artery is also malposed, sitting over the left ventricle. It is best to consider the various lesions in this group of patients along lines of flow as well, but in this group, that consideration relates to associated defects rather than the primary pathophysiology, which, in all lesions, is cyanosis due to preferential streaming of systemic venous flow across the aortic valve.
Valuable to the clinician is the timing of cyanosis in this group of lesions. In simple d-transposition of the great arteries, there is little mixing of the systemic and pulmonary venous circulations after birth, just as in the normal newborn. Unlike the normal newborn, though, this separation of venous returns causes the desaturated systemic venous blood to cross the aortic valve to the ascending aorta, leading to significant, often intense, cyanosis, immediately after birth. This is in contrast to the infant with decreased pulmonary blood flow in most of whom the ductus arteriosus is widely patent at birth, maintaining normal or increased pulmonary blood flow initially. Although the ductus arteriosus may close rapidly in such patients, cyanosis is often not appreciated in the first few hours of life. The earlier and more severe the cyanosis, the more likely the neonate has d-transposition of the aorta rather than decreased pulmonary blood flow. In addition, the presence of a ductus arteriosus and modestly elevated pulmonary vascular resistance in the first hours after birth may lead to somewhat higher saturations in the lower body in such an infant, which cannot happen in neonates either with decreased pulmonary blood flow or with persistent pulmonary hypertension (Table 483-2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


