Background
Preterm birth has staggering health implications, and yet the causes of most cases are still unknown. Placental features have been understudied as an etiology for preterm birth, and the association between placental pathologic lesions and neonatal outcomes are incompletely understood.
Objective
We sought to characterize births according to placental pathology and relate these to adverse neonatal outcomes.
Study Design
We studied 20,091 births (15,710 term and 4381 preterm) with placental evaluations. Births were classified according to the presence or absence of placental lesions consistent with malperfusion (vasculopathy, infarct, advanced villous maturation, perivillous fibrin, fibrin deposition) and intrauterine inflammation/infection (chorioamnionitis, funisitis, vasculitis). Outcomes were gestational week of delivery, birthweight z-score, neonatal respiratory distress syndrome, and intraventricular hemorrhage.
Results
Among all preterm births, evidence of placental malperfusion was identified more often than inflammation/infection (50.6% vs 27.3%, P < .0001). Placental malperfusion was associated with reduced fetal growth (adjusted birthweight z-score, –0.83, P < .0001) and lesions of inflammation/infection were associated with earlier delivery (adjusted difference –2.08 weeks, P < .0001) than those with no lesions. When both placental lesions were present, earlier delivery (adjusted difference –2.28 weeks, P < .0001) and reduced fetal growth (adjusted birthweight z-score difference, –0.24, P = .001) were observed more often than when neither lesion was present. Findings were similar when restricted to cases of spontaneous preterm birth. Intraventricular hemorrhage was higher in preterm births with malperfusion lesions than cases with no lesions (7.6% vs 3.4%; odds ratio, 1.98; confidence interval, 1.18–3.32), accounting for gestational age and other covariates.
Conclusion
Placental pathology provides important insight into subtypes of preterm birth with adverse neonatal outcomes. Co-occurrence of malperfusion and inflammation/infection, especially among spontaneous preterm births, may be a novel pattern of placental injury linked to severe adverse outcomes.
Introduction
Preterm birth has staggering health implications. It is the leading cause of infant morbidity and mortality, with impairments affecting the lifelong health of offspring. The cause of most preterm birth is still unknown. It is well established that preterm birth, defined as delivery <37 weeks, is a complex, heterogeneous condition and classification of subtypes according to underlying etiology is essential to identify causal pathways. It has been common to classify cases according to clinical presentation as spontaneous (either spontaneous preterm labor or preterm premature rupture of membranes) and medically indicated. There are several concerns with this approach. First, women may present with both contractions and ruptured membranes and clinical records may not be adequate to distinguish which came first. Second, medically indicated preterm births are influenced by clinical practice changes so this classification schema is not objective and not comparable across regions, countries, and time. Third, there is compelling evidence that the epidemiologic outcomes, metabolic changes, and abnormalities of implantation in indicated and spontaneous preterm births overlap.
Alternative classification schemas have been proposed; placental microscopic and histopathology features have been suggested as valuable and perhaps essential tools to align preterm birth classification with underlying etiology. Chorioamnionitis (intrauterine inflammation or infection detected in the placenta) is a precursor to many spontaneous preterm births, especially those occurring at early gestational ages. Indicated preterm births are dominantly related to clinical conditions such as preeclampsia and growth restriction, and placental evidence of maternal malperfusion is abundant in these cases. There is evidence, however, that the placental vascular lesions of maternal malperfusion also contribute to a third of spontaneous preterm birth cases. There are a few reports that co-occurrence of both malperfusion and inflammation/infection is linked to severe neonatal health consequences, but this has never been examined in a large cohort.
We utilized a clinical registry of 20,091 births delivered from 2008 through 2012 to classify subgroups of infants (15,710 delivered at term and 4381 delivered preterm) according to placental lesions indicating malperfusion or intrauterine inflammation/infection. We further examined the co-occurrence of these 2 lesion types, as comorbid pathologies may be of particular importance to neonatal health. We linked placental phenotypes to gestational age at delivery, fetal growth, respiratory distress syndrome, and intraventricular hemorrhage. These outcomes are highly relevant to immediate and long-term infant health, occur most often in preterm neonates, and are reliably available in medical records.
Materials and Methods
Delivery data were collected from the Magee Obstetric Medical and Infant database, which includes 300 variables for all deliveries at Magee-Womens Hospital in Pittsburgh, PA. Variables are derived from admitting services, International Classification of Diseases, Ninth Revision ( ICD-9 ) codes, medical record data abstraction, and ultrasound. Births occurring from 2008 through 2012 were selected for this study because, during this period, 2 placental pathologists (including W.T.P.) prepared all reports following a standardized protocol and used a uniform reporting approach and identical diagnostic criteria, provided in Supplemental Table 1 . The University of Pittsburgh Institutional Review Board approved the project, which did not require informed consent as all data were deidentified (institutional review board no. PRO13020275).
A broad set of indications, based on the College of American Pathologists guidelines, warranted referral of a placenta for pathology evaluation during this period ( Supplemental Table 1 ). In all, 44% of all deliveries and 92% of preterm births had placental pathology evaluation performed. Our placenta pathology matching and extraction process was described previously. Briefly, we identified 30,716 placenta pathology reports from 2008 through 2012. Data were reformatted into Extensible Markup Language and then linked to the Magee Obstetric Medical and Infant database. Together with a placental pathologist (W.T.P.), we identified key words to capture all possible descriptions and diagnoses. A validation study demonstrated excellent sensitivity and specificity for placental lesions when this automated abstraction process was compared to manual record abstraction. For the current analysis, we limited the study population to singleton live births, delivered between 20-42 completed weeks gestation, with a reported birthweight.
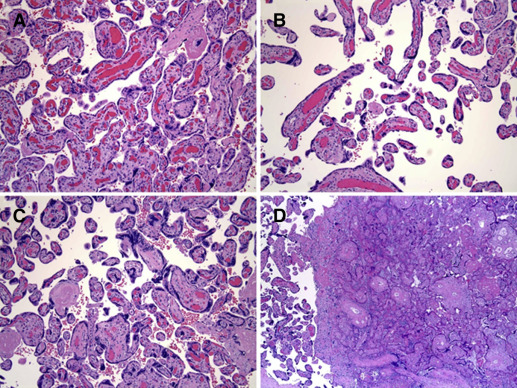
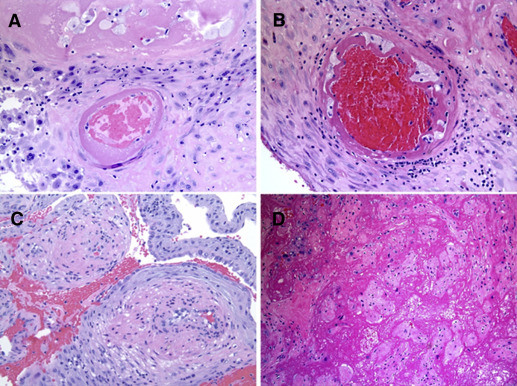
Presence of placental lesions was categorized according to proposed schemas as maternal malperfusion (decidual vasculopathy, villous infarction, advanced villous maturation, increased perivillous fibrin deposition, increased intervillous fibrin deposition) and/or intrauterine inflammation/infection (acute chorioamnionitis, acute funisitis, acute vasculitis) ( Figures 1-3 and Supplemental Table 2 ). Mild acute chorioamnionitis was defined as stage 1 (acute subchorionitis) with no accompanying chorionic plate vasculitis or umbilical cord vasculitis/funisitis. Severe acute chorioamnionitis was defined as higher stage acute chorioamnionitis (stage 2 or 3) and/or the presence of chorionic plate vasculitis or umbilical cord vasculitis/funisitis. A validation study (56 spontaneous, 19 medically indicated preterm, and 50 term births) compared the diagnosis of malperfusion and inflammation/infection on the clinical pathology reports to a review of the slides by a single pathologist blinded to all clinical information except gestational age (W.T.P.). There was excellent agreement (82%) between the clinical pathology reports and the review by the pathologist for inflammation/infection and good agreement (62%) for malperfusion lesions. Of note, the clinical report tended to underreport cases of malperfusion in both spontaneous and indicated preterm birth. Agreement was excellent for the malperfusion lesions among term births (82%). We also evaluated placental weight as <10th percentile or >90th percentile for gestational week based on reference ranges created at our hospital.

Gestational age in our data was based on the best clinical estimate at the time of delivery. Clinicians relied predominantly on first- and second-trimester ultrasound in conjunction with the last menstrual period. A validation study was conducted where a single obstetrician manually reviewed the medical records of 153 deliveries (n = 86 classified as term and n = 67 classified as preterm in our registry). The physician determined the best clinical estimate of gestational age at delivery based on date of last menstrual period, date of ultrasound, and gestational age at ultrasound. There was excellent agreement between the medical record and our registry (100% sensitivity for cases of preterm birth, and 96% specificity). For preterm birth <34 weeks, there was 100% sensitivity and 100% specificity.
Maternal race was self-reported as white, African American, Hispanic, Asian, and other. Numbers were too small to evaluate race/ethnicity groups other than white and African American, so all other groups were combined into 1 category. Absence of labor was identified via record abstraction. Covariates included maternal age at delivery, smoking during pregnancy, and primiparity. Maternal hypertension status was abstracted from the medical record using ICD-9 codes and reported as chronic (including cases of superimposed preeclampsia [642.0, 642.1, 642.2, 642.7]), preeclampsia (de novo hypertension with proteinuria [642.4, 642.5, 642.6]; diagnostic criteria provided in Supplemental Table 3 ), or gestational (de novo hypertension without proteinuria [642.3]). Gestational diabetes screening was universal (94% in our data) and gestational diabetes was defined according to the criteria of Carpenter and Coustan. Body mass index (BMI) (weight/height 2 ) was self-reported at the first prenatal visit and was available for 10,735 births (53%). We defined small for gestational age as fetal growth <10th percentile and large for gestational age as >90th percentile at each completed week of gestation, as recommended by the Global Reference Standard. This approach uses estimated fetal weights derived from ultrasound, accounts for race/ethnicity differences in mean birthweight, and identifies more fetal growth–related pathologies among preterm infants compared to birthweight-derived references. Birthweight z-scores were created using the mean and SD of births in our complete registry occurring at each gestational week. Cases of respiratory distress syndrome ( ICD-9 codes 769, 770.6, 518.82; including bronchopulmonary dysplasia [770.7]), and intraventricular hemorrhage (772.10, 772.11, 772.12, 772.13, 431) were identified in the neonatal medical record.
Statistical methods
Maternal characteristics were compared among women with and without placental data and according to gestational age groups, using χ 2 and t tests. The prevalence of specific placental lesions was compared according to preterm birth groups, as was the co-occurrence of both malperfusion and inflammation/infection. We used linear regression to model the gestational age of delivery and birthweight z-score related to placental lesion groups (malperfusion only, inflammation/infection only, co-occurrence of malperfusion and inflammation/infection). Births with neither lesion were the referent. We then stratified by preterm birth status given the evidence that some placental lesions such as infarcts or chorioamnionitis occur at term as part of normal placental aging or as a result of labor. In contrast, these lesions may be pathologic when they occur <37 weeks’ gestation. Models were also stratified according to clinical indication (spontaneous or indicated). Logistic regression models assessed the occurrence of neonatal respiratory distress syndrome or intraventricular hemorrhage according to placental lesion groups. Intraventricular hemorrhage was only evaluated in the preterm birth subset as 95% of cases occurred at preterm gestations. Covariates were selected a priori as race/ethnicity, maternal age, smoking, education, and prepregnancy BMI. Neonatal outcomes were then further adjusted for gestational age to determine if placental features may be on the pathway leading to earlier delivery and subsequent adverse neonatal outcomes.
Materials and Methods
Delivery data were collected from the Magee Obstetric Medical and Infant database, which includes 300 variables for all deliveries at Magee-Womens Hospital in Pittsburgh, PA. Variables are derived from admitting services, International Classification of Diseases, Ninth Revision ( ICD-9 ) codes, medical record data abstraction, and ultrasound. Births occurring from 2008 through 2012 were selected for this study because, during this period, 2 placental pathologists (including W.T.P.) prepared all reports following a standardized protocol and used a uniform reporting approach and identical diagnostic criteria, provided in Supplemental Table 1 . The University of Pittsburgh Institutional Review Board approved the project, which did not require informed consent as all data were deidentified (institutional review board no. PRO13020275).
A broad set of indications, based on the College of American Pathologists guidelines, warranted referral of a placenta for pathology evaluation during this period ( Supplemental Table 1 ). In all, 44% of all deliveries and 92% of preterm births had placental pathology evaluation performed. Our placenta pathology matching and extraction process was described previously. Briefly, we identified 30,716 placenta pathology reports from 2008 through 2012. Data were reformatted into Extensible Markup Language and then linked to the Magee Obstetric Medical and Infant database. Together with a placental pathologist (W.T.P.), we identified key words to capture all possible descriptions and diagnoses. A validation study demonstrated excellent sensitivity and specificity for placental lesions when this automated abstraction process was compared to manual record abstraction. For the current analysis, we limited the study population to singleton live births, delivered between 20-42 completed weeks gestation, with a reported birthweight.
Presence of placental lesions was categorized according to proposed schemas as maternal malperfusion (decidual vasculopathy, villous infarction, advanced villous maturation, increased perivillous fibrin deposition, increased intervillous fibrin deposition) and/or intrauterine inflammation/infection (acute chorioamnionitis, acute funisitis, acute vasculitis) ( Figures 1-3 and Supplemental Table 2 ). Mild acute chorioamnionitis was defined as stage 1 (acute subchorionitis) with no accompanying chorionic plate vasculitis or umbilical cord vasculitis/funisitis. Severe acute chorioamnionitis was defined as higher stage acute chorioamnionitis (stage 2 or 3) and/or the presence of chorionic plate vasculitis or umbilical cord vasculitis/funisitis. A validation study (56 spontaneous, 19 medically indicated preterm, and 50 term births) compared the diagnosis of malperfusion and inflammation/infection on the clinical pathology reports to a review of the slides by a single pathologist blinded to all clinical information except gestational age (W.T.P.). There was excellent agreement (82%) between the clinical pathology reports and the review by the pathologist for inflammation/infection and good agreement (62%) for malperfusion lesions. Of note, the clinical report tended to underreport cases of malperfusion in both spontaneous and indicated preterm birth. Agreement was excellent for the malperfusion lesions among term births (82%). We also evaluated placental weight as <10th percentile or >90th percentile for gestational week based on reference ranges created at our hospital.
Gestational age in our data was based on the best clinical estimate at the time of delivery. Clinicians relied predominantly on first- and second-trimester ultrasound in conjunction with the last menstrual period. A validation study was conducted where a single obstetrician manually reviewed the medical records of 153 deliveries (n = 86 classified as term and n = 67 classified as preterm in our registry). The physician determined the best clinical estimate of gestational age at delivery based on date of last menstrual period, date of ultrasound, and gestational age at ultrasound. There was excellent agreement between the medical record and our registry (100% sensitivity for cases of preterm birth, and 96% specificity). For preterm birth <34 weeks, there was 100% sensitivity and 100% specificity.
Maternal race was self-reported as white, African American, Hispanic, Asian, and other. Numbers were too small to evaluate race/ethnicity groups other than white and African American, so all other groups were combined into 1 category. Absence of labor was identified via record abstraction. Covariates included maternal age at delivery, smoking during pregnancy, and primiparity. Maternal hypertension status was abstracted from the medical record using ICD-9 codes and reported as chronic (including cases of superimposed preeclampsia [642.0, 642.1, 642.2, 642.7]), preeclampsia (de novo hypertension with proteinuria [642.4, 642.5, 642.6]; diagnostic criteria provided in Supplemental Table 3 ), or gestational (de novo hypertension without proteinuria [642.3]). Gestational diabetes screening was universal (94% in our data) and gestational diabetes was defined according to the criteria of Carpenter and Coustan. Body mass index (BMI) (weight/height 2 ) was self-reported at the first prenatal visit and was available for 10,735 births (53%). We defined small for gestational age as fetal growth <10th percentile and large for gestational age as >90th percentile at each completed week of gestation, as recommended by the Global Reference Standard. This approach uses estimated fetal weights derived from ultrasound, accounts for race/ethnicity differences in mean birthweight, and identifies more fetal growth–related pathologies among preterm infants compared to birthweight-derived references. Birthweight z-scores were created using the mean and SD of births in our complete registry occurring at each gestational week. Cases of respiratory distress syndrome ( ICD-9 codes 769, 770.6, 518.82; including bronchopulmonary dysplasia [770.7]), and intraventricular hemorrhage (772.10, 772.11, 772.12, 772.13, 431) were identified in the neonatal medical record.
Statistical methods
Maternal characteristics were compared among women with and without placental data and according to gestational age groups, using χ 2 and t tests. The prevalence of specific placental lesions was compared according to preterm birth groups, as was the co-occurrence of both malperfusion and inflammation/infection. We used linear regression to model the gestational age of delivery and birthweight z-score related to placental lesion groups (malperfusion only, inflammation/infection only, co-occurrence of malperfusion and inflammation/infection). Births with neither lesion were the referent. We then stratified by preterm birth status given the evidence that some placental lesions such as infarcts or chorioamnionitis occur at term as part of normal placental aging or as a result of labor. In contrast, these lesions may be pathologic when they occur <37 weeks’ gestation. Models were also stratified according to clinical indication (spontaneous or indicated). Logistic regression models assessed the occurrence of neonatal respiratory distress syndrome or intraventricular hemorrhage according to placental lesion groups. Intraventricular hemorrhage was only evaluated in the preterm birth subset as 95% of cases occurred at preterm gestations. Covariates were selected a priori as race/ethnicity, maternal age, smoking, education, and prepregnancy BMI. Neonatal outcomes were then further adjusted for gestational age to determine if placental features may be on the pathway leading to earlier delivery and subsequent adverse neonatal outcomes.
Results
There were 45,638 singleton, live births from 2008 through 2012; 92% of preterm and 39% of term deliveries had placental evaluation. Births to younger women, those of African American race/ethnicity, with less than a high school education, higher prepregnancy BMI, and who smoked were more likely to have placental data ( Supplemental Table 4 ). When limited to this group, as expected, those who delivered preterm were more likely to be African American, younger, to smoke, have a higher BMI, have less than a high school education, and have more pregnancy complications compared to term births ( Table 1 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


