Neonatal Oncology
Sheila Weitzman, Yaron Finkelstein, and Robert J. Arceci
Tumors are a rare but important cause of mortality and morbidity in the newborn. 
Newborn cancer makes up about 2% of malignancies in children.1 Tumors presenting after birth tend to be detected early, 50% on the first day of life and many others within the first 7 days.5,6 Fifteen to 20% are associated with congenital anomalies and syndromes.1
THE SPECTRUM OF NEONATAL TUMORS
Table 447-1 shows the distribution of newborn tumors from a large published series.7 Overall, teratoma is the most common neoplasm, whereas neuroblastoma is the most common malignancy in newborns and infants in Western countries,8,9 followed by leukemia, sarcoma, and brain tumors. 
Table 447-1. Spectrum of Newborn Tumors
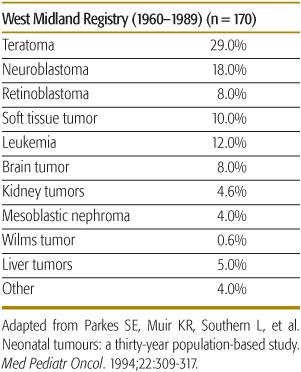
Mature (benign) teratoma is the most common germ cell tumor in the newborn, comprising 37% to 52% of congenital tumors.11 Malignant elements, usually endodermal sinus tumor (EST) are found in approximately 10% of teratomas in children under age 4 months.11 After age 4 months the malignancy rate of teratomas rises, reaching almost 100% at age 3 years.12 Mature teratomas are usually extragonadal and midline.13 The most common anatomic site of teratomas is sacrococcygeal, where these tumors may be primarily extrapelvic (type 1), extra- and intrapelvic (type 2), or entirely intrapelvic (type 3). Other common areas are intracranial, orbital, nasopharyngeal, neck, and anterior mediastinum.12 Extrapelvic sacrococcygeal teratomas may be very large, resulting in intrauterine rupture, bleeding, fetal death, or dystocia. Mortality is high, particularly if diagnosed before 30 weeks’ gestation.11 Caesarian section should be done as soon as lung maturity is deemed adequate. Inutero tumor debulking has been done in high-risk fetuses.14 Postnatal therapy consists of complete resection whenever possible. EST is the most common malignant germ cell tumor of fetus and newborn. Alpha feto-protein (AFP) levels are normally very high in newborn and young infants up to levels of 150,000 ng/ml or more and even higher in premature babies. AFP is cleared from the circulation with a half-life of 5 to 7 days. Mature neonatal teratoma may recur locally as mature or immature teratoma or, despite apparent complete resection, as pure EST up to 36 months later.11,12,16 The response to chemotherapy and survival of EST is excellent, even for those with metastases at the time of recurrence.19 All newborns with mature teratoma at any site should be carefully followed with AFP measurements at 3 monthly intervals until 36 months of age. 
Neuroblastoma (NB) is the most common malignancy in the neonate and infant. Forty percent of neuroblastomas present in the first year of life and 60% by age 2. There is a clear association between age, site, and extent of NB. Infants most commonly have localized disease arising equally from thoracic and abdominal sites; older children tend to have disseminated disease from an adrenal primary.20 Ninety percent of neonatal NB presents with stage 1 or 4S disease with a good survival,1 but the outlook may vary depending on tumor biology. Stage 4S NB is unique to the neonatal/infancy periods and is characterized by widely disseminated disease, often without an identifiable primary tumor, which usually regresses spontaneously. 
Spontaneous regression is frequent in infants. The routine use of fetal ultrasonography has resulted in increasing detection of an abdominal mass. Neuroblastoma (NB) is the second most common abdominal mass detected by prenatal ultrasound, once renal lesions are excluded. Differential diagnosis of a suprarenal mass in the newborn includes adrenal hemorrhage, other adrenal tumors, and subdiaphragmatic extrapulmonary lung sequestration. Most tumors detected prenatally or by screening are of favorable stage and have favorable biology.24 NB therapy depends on age, stage, and tumor biologic characteristics. 
Stage IVS neuroblastoma in the neonate is potentially life-threatening. The cause of death is often massive and rapidly progressive liver enlargement resulting in respiratory compromise, vena cava obstruction, and/or gastrointestinal compression. A scoring system to help in therapeutic decision making for such patients has been published.26 Any sign of respiratory distress or inferior vena cava (IVC) compression requires emergency therapy. Prompt referral to an oncology service is necessary. Therapy includes chemotherapy with or without radiation therapy, both of which may be life saving. This therapy is necessary to deal with immediate mechanical complications and does not necessarily have to be continued after resolution of the immediate problem. Therapy should include precautions against tumor lysis syndrome, which may occur in young infants with stage IVS NB. 
Congenital leukemia is most commonly of myeloid origin, with acute monocytic and myelomonocytic leukemia being most prevalent.28
Clinically, 50% of neonates with congenital leukemia present with cutaneous infiltration, a rare finding in older children.28 Hepatosplenomegaly, petechiae, and ecchymoses are common, and hyperleukocytosis, respiratory distress, and meningeal involvement occur more frequently in this age group. Approximately 10% of newborns with Down syndrome (DS) present with circulating blasts resulting from transient myeloproliferative disease (TMD). The blasts are clonal in origin and indistinguishable from those of acute megakaryoblastic leukemia (AMKL). Organ infiltration is commonly observed, particularly of the liver, spleen, heart, and skin. The blasts usually disappear spontaneously within the first 3 months of life, but 20% to 25% of these children with DS and TMD go on to develop true AMKL.30 The blasts of TMD and AMKL in DS are associated with somatic mutations in the gene, GATA1, which encodes a myeloid transcription factor, and an increased sensitivity to chemotherapeutic agents, such as cytarabine.31,32 Most patients with DS and TMD only require observation, but about 15% present with life-threatening complications including liver and cardiac failure, hyperleukocytosis, or respiratory impairment. These newborns usually require chemotherapy; low-dose cytarabine is commonly used.30
Congenital acute lymphoblastic leukemia (ALL) is less frequent than myeloid leukemia, but is biologically different and has a significantly poorer prognosis than ALL in older children. Neonatal ALL is usually B-lineage. Rearrangements of the MLL gene on 11q23 are observed in at least 80% of cases and are associated with very poor prognosis. As in neonatal AML, there is an increased frequency of poor prognostic features, such as hyperleukocytosis and central nervous system (CNS) disease.37
Many different chemotherapy regimens have been tried in neonatal ALL, but induction failure rate is high and toxic mortality is frequent. At present there is no evidence that myeloablative therapy with hematopoietic stem cell transplant (HSCT) improves the outcome in MLL-rearranged infant ALL. If cases of transient leukemia are excluded, the prognosis is extremely poor for most infants with congenital B-lineage or biphenotypic leukemia. By contrast, infants with AML have comparable outcomes to those seen in older children, if treated with the same intensive regimens.39
Brain tumors in the newborn comprise 5% to 12% of neonatal tumors.42,43 About half are detected prenatally. Postnatal clinical features include hydrocephalus, enlarged head circumference, and asymmetric skull growth. Associated congenital anomalies are common.45 Most frequent histologic diagnoses are intracranial teratoma, low- or high-grade astrocytoma, medulloblastoma, and primitive neuroectodermal tumor (PNET). The majority are supratentorial tumors. Benign brain tumors are common in the neonatal period and include choroid plexus papillomas (10–20% of infant brain tumors), craniopharyngioma, and hemangioma. Therapy of brain tumors includes surgical resection and chemotherapy. The poor prognosis is usually the result of the high surgical risk and the avoidance of radiation therapy at this vulnerable age. 
Retinoblastoma (RB) is the most common intraocular tumor of childhood. It presents in infants and young children and may be present in the newborn. All RB tumors show loss of function of the tumor suppressor gene, RB1. RB1 mutations occur in 60% of patients as somatic mutations, and in 40% as germ-line (inherited) mutations. Patients with germ-line mutations in RB1 are at a very high risk (90%) of a second mutation and bilateral RB. Of 46 neonates with RB in one study, 42 had bilateral (familial) RB.47
Early diagnosis is essential for survival and prevention of blindness. Early stage RB is treatable with cryo- or laser therapy. Later diagnosis may result in the need for enucleation sometimes with intensive chemotherapy and radiation therapy. All infants less than 2 months of age should undergo screening with a red pupil reflex test. The best chance for preservation of vision in patients with familial RB is making the diagnosis prenatally, to prevent development of large tumors.46
Nearly two-thirds of abdominal masses in the newborn arise from the kidneys. The majority of these masses are non-neoplastic, such as hydronephrosis, multicystic, dysplastic, and polycystic kidneys50 Neonatal renal tumors are uncommon and represent less than 10% of neonatal tumors. Benign congenital mesoblastic nephroma (CMN) comprises 78% of neonatal kidney tumors. Wilms tumor is much less common in this age group.52 An association of WT with congenital anomalies includes sporadic aniridia, hemihypertrophy, Beckwith-Wiedemann syndrome, and other overgrowth syndromes. Neonatal renal tumors usually present as asymptomatic abdominal masses detected by prenatal ultrasound or physical examination. Neonatal hypertension, hypercalcemia, maternal polyhydramnios, and hydrops fetalis may occur. Polyhydramnios is more common in CMN, which is also significantly associated with preterm labor and prematurity. CMN is treated by surgical removal and patients have an overall excellent chance of survival. 
Neonatal WT is treated with primary excision followed by chemotherapy at 50% of usual doses recommended for older children. The NWTS showed that children less than 24 months of age with small (less than 500 gm), stage I favorable histology WT had an 86.5% disease-free survival rate with resection alone.55 Neonates with nephrogenic rests in the contralateral normal kidney should have prolonged monitoring with ultrasonography at 3 monthly intervals to monitor for development of asynchronous tumors in the remaining kidney. 
Liver tumors in the neonate comprise approximately 5% of tumors in the perinatal period.56 The most common primary tumor is hemangioendothelioma (HE), followed by mesenchymal hamartoma, and hepatoblastoma (HB). Secondary tumors include metastatic neuroblastoma, renal tumors, and others such as endodermal sinus tumor (EST) and choriocarcinoma.1
Liver tumors may be detected prenatally and may be associated with anemia, hydramnios, or hydrops fetalis. Postnatal abdominal distention, abdominal mass, and respiratory compromise may also be seen.  HE may proliferate rapidly after birth followed by slow involution over several years. Five percent of unifocal and 49% of multifocal HE has associated cutaneous hemangiomas. Congestive cardiac failure and consumptive coagulopathy (Kasabach-Merritt syndrome) require urgent therapy, often with surgical resection, corticosteroids, low-dose vincristine, or interferon-α.56 However, interferon therapy has been associated with a significant risk of spastic paraplegia in young babies.
HE may proliferate rapidly after birth followed by slow involution over several years. Five percent of unifocal and 49% of multifocal HE has associated cutaneous hemangiomas. Congestive cardiac failure and consumptive coagulopathy (Kasabach-Merritt syndrome) require urgent therapy, often with surgical resection, corticosteroids, low-dose vincristine, or interferon-α.56 However, interferon therapy has been associated with a significant risk of spastic paraplegia in young babies.
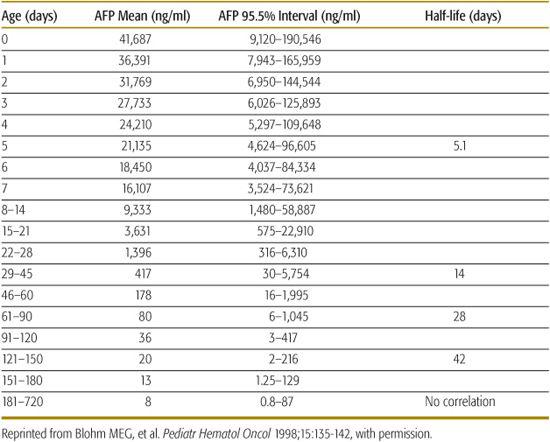
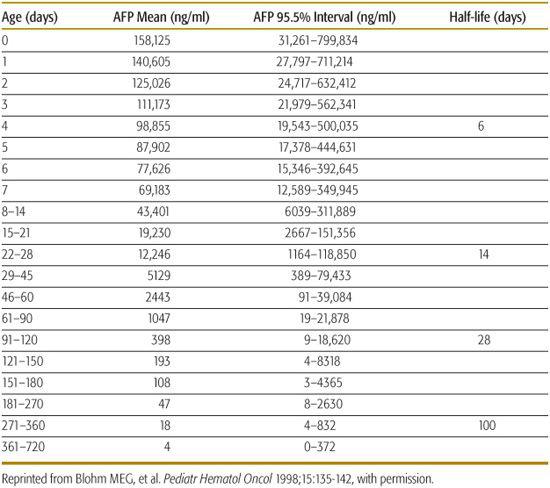
HB is the most common primary malignant liver tumor. Alpha feto-protein (AFP) is usually increased in HB but the high levels found normally in newborns can complicate the interpretation of this test in this age group (Tables 447-2 and 447-3). Therapy of HB consists of surgery and adjuvant chemotherapy; liver transplantation may be required for HB not amenable to surgical resection.58
Table 447-2. Serum AFP Values of Term Babies without Additional Factors Associates with Alpha Feto-Protein (AFP) Elevation
The most common presentation of mesenchymal hamartoma is with a multicystic or cystic and solid abdominal mass detected ultrasonographically or clinically. The treatment is surgical removal. Death may occur because of the rapid enlargement of a cystic mass causing respiratory distress and compression of intra-abdominal blood vessels. 
Table 447-3. Serum AFP Values in Prematures Babies without Additional Factors Associated with Alpha Feto-Protein (AFP) Elevation
SPECIAL CONSIDERATIONS IN CHEMOTHERAPY
In general, neonatal tumors manifest the same sensitivity to chemotherapeutic agents as tumors in older children. However, virtually all pharmacokinetic aspects of drug absorption, distribution, metabolism, and elimination in the neonate are different from those of older patients.59,60
 ABSORPTION
ABSORPTION
Gastric emptying is slow (6–8 hours) in neonates, and the rate at which most orally administrated drugs are absorbed is slower and therapeutic effect may be longer. 
The absorption of oral drugs is also affected by a decreased activity of gastrointestinal enzymes, which are low in infants younger than 4 months, whereas reduced bile acid metabolism results in prolonged clearance of chemotherapy agents and increased toxicity. 
 DISTRIBUTION
DISTRIBUTION
Once absorbed, the drug is distributed into various compartments of the body. 
At birth, total body water approximates 75% and the extracellular water about 40% of body weight. Water-soluble drugs have a larger volume of distribution in neonates and may be more accurately dosed using body surface area rather than weight. 
Protein binding is low and dose modifications of these drugs may be important. 
Neonates have relatively larger cerebrospinal fluid (CSF) volumes, which do not correlate with body surface area. In addition, there is an increased ability of drugs such as methotrexate and (ARA-C) to penetrate the newborn CNS. 
 METABOLISM
METABOLISM 
Delayed maturation of drug-metabolizing enzyme activity may account for lack of efficacy or increased toxicity of drugs in neonates. 
Neonatal levels of CYP 3A4/3A5, the major drug-metabolizing CYP isoenzymes, are less than 30% of adults.69 Cyclophosphamide, which is activated by CYP3A4 and CYP2D6, has lower transformation rates and biologic antineoplastic activity in the neonate.70 Drugs such as vincristine and daunorubicin, excreted via the biliary system, may have prolonged cytotoxic activity in this early age period. 
 ELIMINATION
ELIMINATION
The majority of drug elimination mechanisms are markedly reduced at birth.73,74 The glomerular filtration rate (GFR) is approximately 40 mL/min/1.73m2 in full-term neonates, approximately 30% of the healthy adult value. The GFR increases rapidly during the first 3 weeks, reaching adult levels by 3 to 5 months.75,76 Similarly, tubular secretion is reduced in the neonate and achieves adult capacity at about 1 year of age. Urinary pH is also lower in the newborn compared to the older child. Drugs that are more soluble in alkaline urine, such as methotrexate, are excreted at a slower rate.60,65,72 Chemotherapy drugs primarily eliminated by the kidneys should be individually tailored.
SUMMARY
Age-related physiologic changes should be taken into consideration in chemotherapy dosing in newborns. The lack of data regarding optimal dosing of chemotherapy for neonates have led to extrapolation of treatment regimes from dosages used in older children. Many neonatal and infant protocols suggest dose reduction of chemotherapy drugs by 50% of doses calculated on body surface area. For infants less than 6 to 10 kg, calculated doses are typically based on actual weight. Recent studies suggest that the simple weight-based model tends to give the best estimate of dose in infants.61,62
REFERENCES
See references on DVD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


