Neonatal Hypertension
INTRODUCTION
Over the past several decades, we have learned much about neonatal hypertension, resulting in an increased awareness in the modern neonatal intensive care unit (NICU). In healthy term infants, hypertension is exceedingly uncommon,1 with an incidence of approximately 0.2%. In critically ill infants admitted to the NICU, however, the incidence is higher, with reported rates2–4 ranging from 0.7% to 3.0%. The diagnosis of hypertension in neonates and infants can be challenging because their normal blood pressure (BP) range is dynamic, varying along with a number of factors, including gestational age, postnatal age, and weight. Despite this, a careful diagnostic evaluation should allow determination of the underlying cause of hypertension in most hypertensive neonates. There are numerous treatment options, and treatment decisions should be tailored individually based on the severity of the hypertension and concomitant disease states. Fortunately, in most infants, hypertension resolves over time, although a small number may have persistently elevated BPs throughout childhood.
DEFINING AND DIAGNOSING NEONATAL HYPERTENSION
Normative Neonatal Blood Pressures
One of the greatest challenges when diagnosing hypertension in neonates is the fact that the normative BP range is dynamic. BPs exhibit a variable pattern, and one must consider gestational age at birth, postnatal or postconceptual age, birth weight, and appropriateness of size for gestational age. In general, BP normally increases with increasing gestational and postconceptual age, as well as with increasing birth weight.5–9 Not surprisingly, a greater rate of increase is seen in preterm and infants small for gestational age, compared to term neonates. Fortunately, several recent studies have provided normative data that greatly facilitate the identification of neonates with elevated BPs.
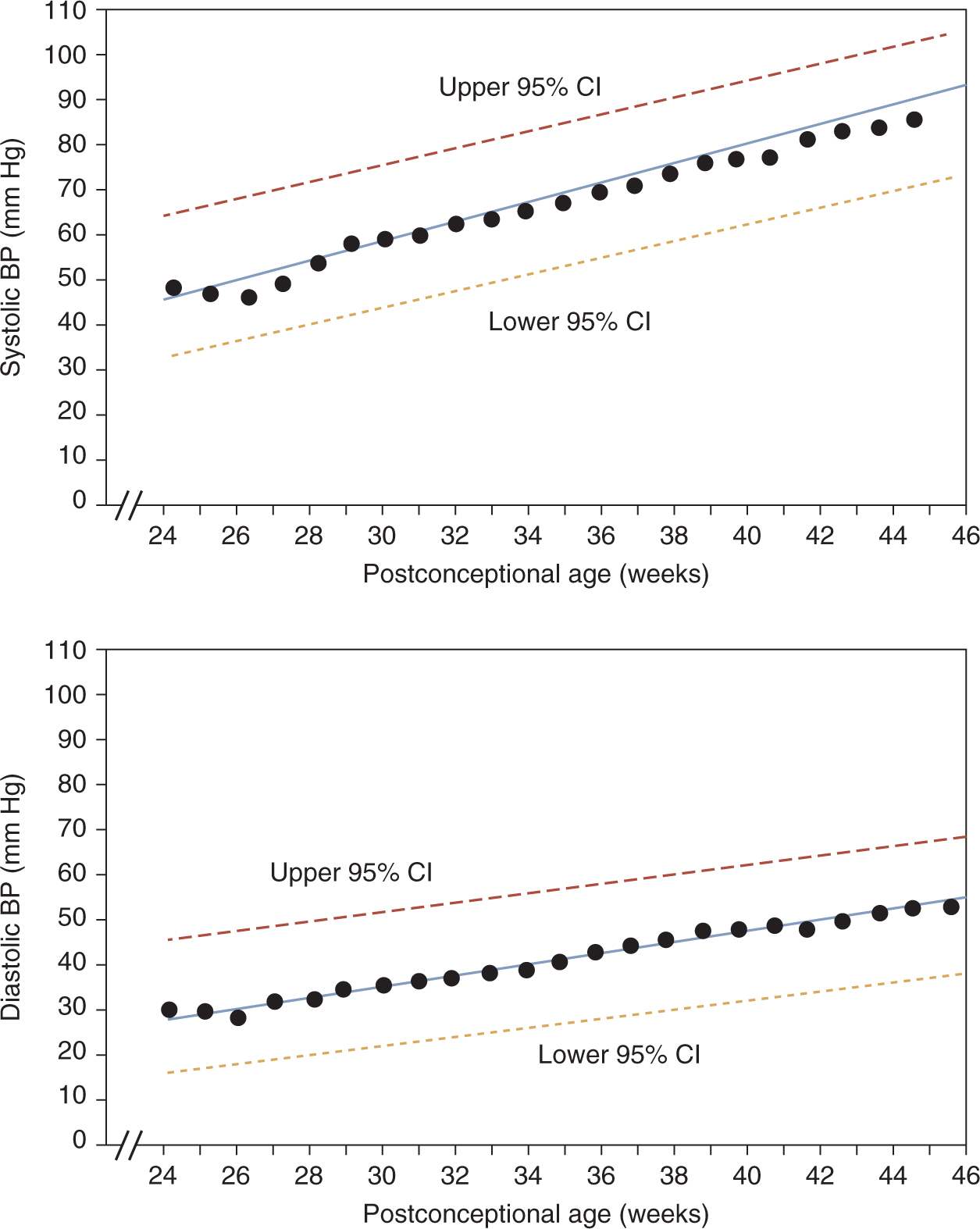
Zubrow et al defined mean and upper/lower 95% confidence limits for neonatal BPs based on prospective, serial oscillometric BP measurements from 608 neonates admitted to multiple NICUs.9 On day of life 1, systolic and diastolic BPs correlated strongly with birth weight and gestational age. BP progressively increased after birth, most rapidly during the first 5 days (1.6–2.7 mm Hg/d). This increase continued after the fifth day, albeit at more gradual increments (0.15–0.27 mm Hg/d). Most notably, in a multiple-regression analysis, the primary determinant of BP was postconceptual age (Figure 103-1).
FIGURE 103-1 Linear regression of mean systolic (top) and diastolic (bottom) blood pressures (BPs) by postconceptual age in weeks, with 95% confidence intervals (upper and lower dashed lines). CI, confidence interval. (Reproduced with permission from Zubrow et al.9)
In addition, Pejovic et al examined BPs measured by an oscillometric device in 373 hemodynamically stable premature and term neonates to evaluate the influence of gestational age, postnatal age, birth weight, gender, and sleep state on BP.8 Systolic and diastolic BPs progressively increased during the first month of life, with BP increasing more rapidly in preterm infants than in full-term infants. BPs on day 1 of life correlated with gestational age and birth weight. Multiple-regression analysis showed that mean BP during the first week and on the 30th day increased with gestational age. Furthermore, BPs were higher in the awake state than in the sleep state.
An Australian prospective study of oscillometric BP measurement in 406 term infants with a mean gestational age of 40 weeks showed no difference in BP on day 1 of life based on birth weight, length, or gestational age.5 In the first day of life, median systolic, diastolic, and mean arterial BPs were 65 (range 46 to 94), 45 (range 24 to 57), and 48 (range 31 to 63), respectively. BP measurements increased over the 4 successive days, albeit at a slower rate than seen in preterm infant studies, usually by 1–2 mm Hg/d; in addition, this study demonstrated that BPs stabilized earlier in term than in preterm infants.
Finally, Kent and colleagues studied BP measurements using oscillometric methods in 147 nonventilated, stable, premature neonates with gestational age ranging from 28 to 36 weeks. This study demonstrated that the period of rapid BP rise occurred over the first 2–3 weeks in infants born at 28–31 weeks’ gestation but only over the first week in infants born at greater than 32 weeks’ gestational age.7 Premature neonates were noted to stabilize their BP after 14 days of life; thereafter, they had BPs similar to those of age-matched term infants.5
These data highlight how challenging it is to establish normative values for neonatal BP, especially in preterm and critically ill infants, because of the effects of gestational age and maturation on BP values.
Diagnostic Criteria
Based on a review of published data, Dionne et al created a reference table of normal BP values after the first 2 weeks of life in infants with postconceptual ages between 26 and 44 weeks10 (Table 103-1). This table and the data shown in Figure 103-1 are the best currently available tools to identify infants with hypertension. Neonates who have BPs persistently above the 95th percentile should be considered hypertensive.
Table 103-1 Estimated Values for Blood Pressures After 2 Weeks of Age in Infants From 26 to 44 Weeks’ Postconceptual Age a,b
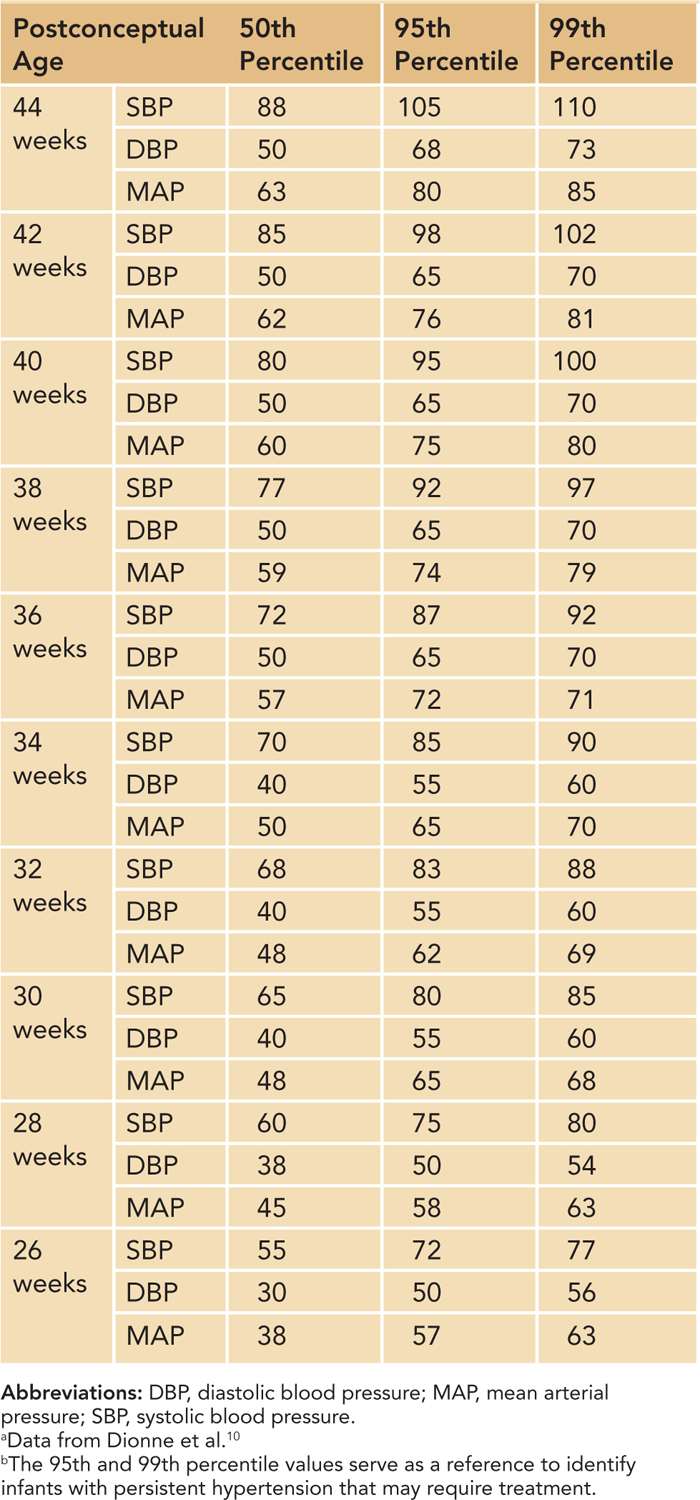
The 95th percentile curve in Figure 103-1 and the 95th/99th percentile values shown in Table 103-1 are intended to serve as a reference to identify infants with persistent systolic and diastolic hypertension that may require treatment. The data in Table 103-1 have the added benefit of mean arterial pressure (MAP) data. Some practitioners are more comfortable assessing neonatal BPs using a MAP value. MAP provides an assessment of the overall perfusion pressure, which may help guard against treatment of isolated systolic hypertension in infants with labile BPs.
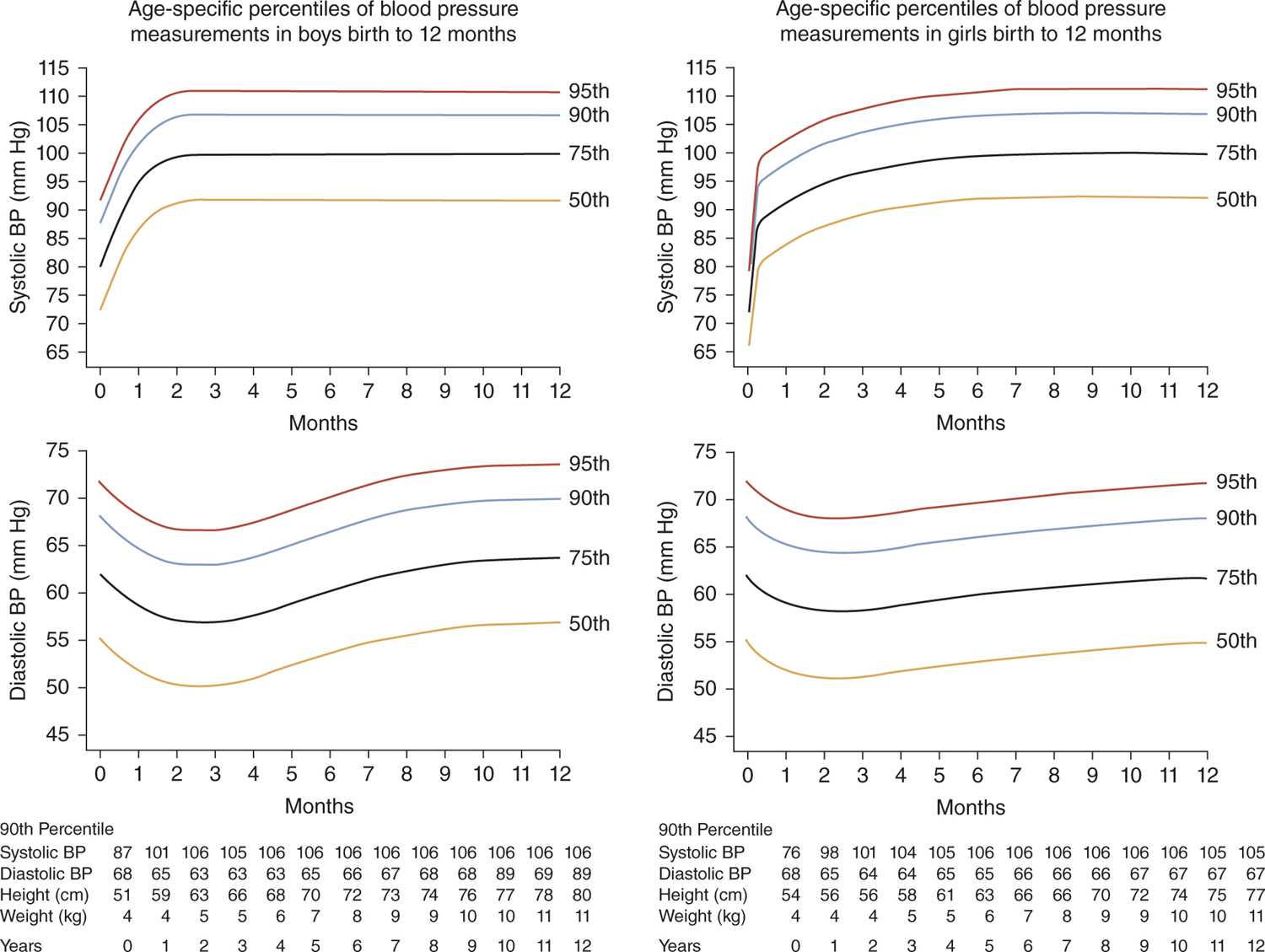
For older infants, the percentile curves reported by the Second Task Force of the National High BP Education Program (NHBPEP) Working Group (Figure 103-2)11 remain the most widely available reference values. These curves can be used similarly to those from Figure 103-1.
FIGURE 103-2 Age-specific percentiles for blood pressure (BP) in (a) boys and (b) girls during the first 12 months of life. (Reproduced with permission from Report of the Second Task Force on Blood Pressure Control in Children—1987. Task Force on Blood Pressure Control in Children. National Heart, Lung, and Blood Institute, Bethesda, Maryland. Pediatrics. 1987;79(1):1–25.)
MEASUREMENT OF BP IN NEONATES
Accurate BP measurement is the crucial first step in the diagnosis of neonatal hypertension. Crying, pain, feeding, and agitation all can increase BP in a neonate.12 BP is best measured by an oscillometric device, preferably 1.5 hours after feeding or a medical intervention when the infant is sleeping or resting. A critical component of noninvasive BP measurement is the use of an appropriate-size cuff,13 which is a cuff with an inflatable bladder width that is at least 40% of the arm circumference at a point midway between the olecranon and the acromion. In addition, the cuff bladder length should cover 80%–100% of the circumference of the arm; thereby, the bladder width-to-length ratio should be at least 1:2. Hence, in the term infant with a maximum arm circumference of 10 cm, the usual dimensions of an appropriate-size cuff bladder are 4 cm in width and 8 cm in length.13 BP usually is measured in an arm because most normative oscillometric data are based on right arm pressures; in addition, leg pressures tend to be higher in children. Because of this, it is important for the medical record to note the site of measurement. After cuff placement, the BP should be measured several minutes after the infant has settled into a calm state. Ideally, 3 successive BP readings should be obtained at 2-minute intervals.
In critically ill neonates, continuous, direct intra-arterial BP measurement through a catheter placed in the aorta or the radial artery is the most accurate technique. Although radial artery systolic BP measurements may be 20% to 30% higher than central values in adults,14 radial pressures appear to more closely mimic aortic pressures in newborns.15 Intra-arterial catheters should be discontinued as the neonate clinically improves to avoid possible risk of complications like infection and thrombosis.
ETIOLOGY OF NEONATAL HYPERTENSION
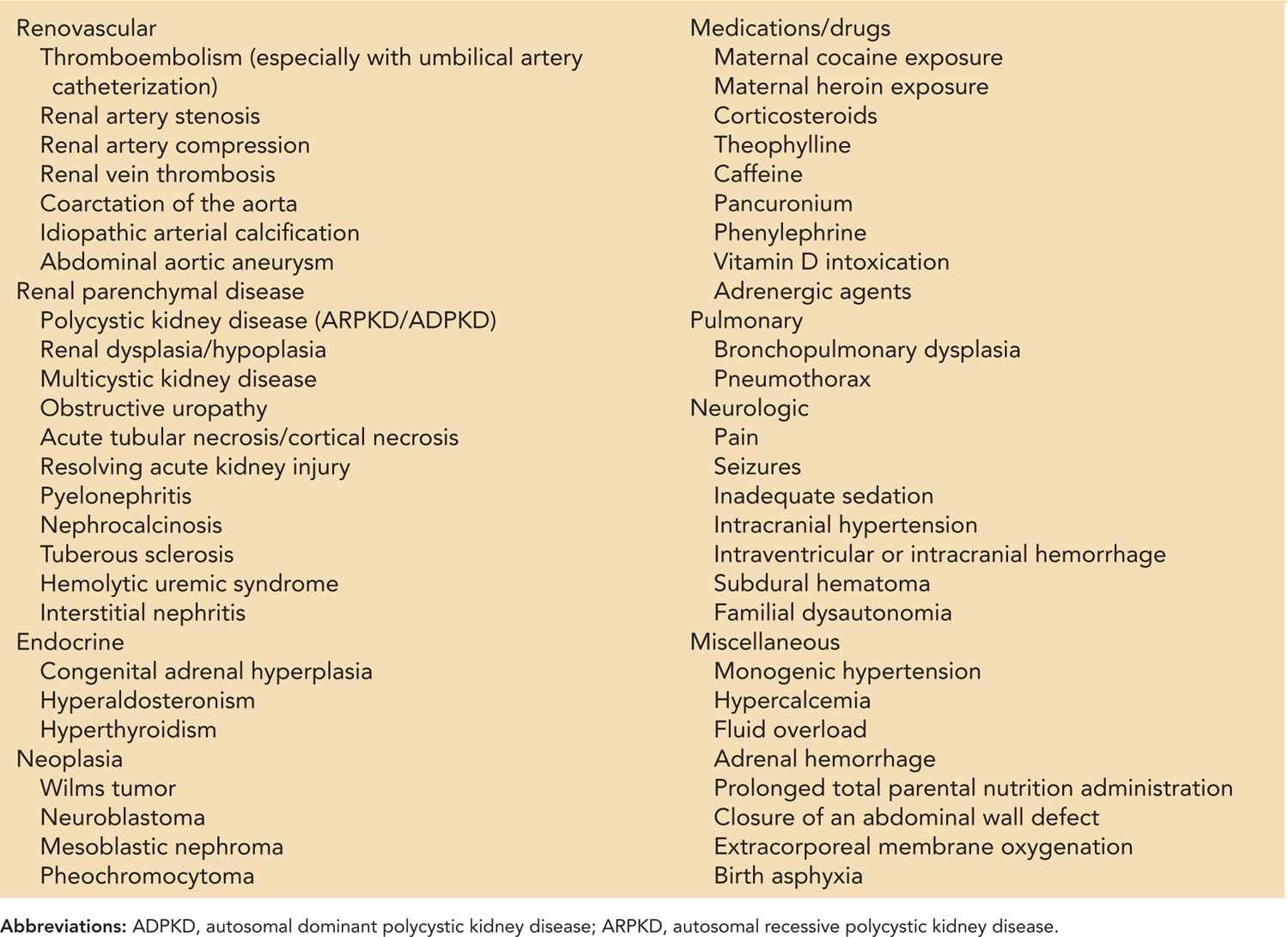
Numerous potential causes of neonatal hypertension have been identified (Table 103-2). However, the most common etiologies are umbilical artery catheter-associated thromboembolism, followed by chronic lung disease and renal parenchymal disease. Other common etiologies include fluid overload, medication-related side effects, and inadequate sedation for intubated patients. Although a thorough history and examination, coupled with screening diagnostics, usually allow identification of an etiology, at times no cause can be identified. In such cases, hypertension may be caused by the presence of an undetectable renovascular event. Table 103-2 offers a comprehensive list of possible etiologies; the more common etiologies are discussed next.
Table 103-2 Etiology of Neonatal Hypertension
Vascular Etiologies
The most common cause of hypertension in neonates is vascular occlusion caused by thromboembolic events related to umbilical artery catheterization.16 Low-lying catheters are just as likely to be associated with the development of hypertension as “high” catheters17; however, a longer duration of catheterization is associated with a higher rate of thrombus formation.18 Renal vein thrombosis, which classically presents with the triad of gross hematuria, thrombocytopenia, and a palpable renal mass, can also be associated with hypertension. It is often associated with hypercoagulable disorders, such as infants of diabetic mothers or factor V Leiden mutations.19,20 Aortic coarctation is a potential cause of neonatal hypertension that can be easily identified and treated.4 Uncommonly, hypertension in neonates may be related to renal artery stenosis or narrowing caused by fibromuscular dysplasia, congenital rubella infection, or mechanical compression from an abdominal mass.21–23
Renal Etiologies
Neonates with congenital renal parenchymal abnormalities comprise the next largest group of neonates with hypertension. Autosomal dominant and recessive polycystic kidney disease (PKD) may present in the newborn period with nephromegaly and hypertension.24 In addition, hypertension has been reported in neonates born with unilateral multicystic dysplastic kidneys.2,25 Urinary obstruction caused by congenital ureteropelvic junction obstruction, posterior urethral valves, or other intra-abdominal masses may be accompanied by hypertension, which is likely mediated by the renin-angiotensin system.2,26 Less commonly, hypertension can be related to acquired renal parenchymal disease, such as acute kidney injury and resolving acute tubular necrosis, interstitial nephritis, or cortical necrosis; in these situations, the hypertension is usually related to volume overload or hyperreninemia. Hemolytic uremic syndrome is rare but has been described in term and preterm infants,27 usually accompanied by severe hypertension.
Pulmonary Etiologies
Neonates with bronchopulmonary dysplasia (BPD) are clearly at increased risk for development of systemic hypertension. Commonly, the hypertension may not become manifest until late in the NICU course or even after discharge. Hypertension in these infants correlates with the severity of lung disease and a greater need for diuretics and bronchodilators.28 Moreover, those who show concurrent nephrocalcinosis are significantly more likely to develop late-onset hypertension.29 This emphasizes the importance of monitoring for the development of hypertension in “NICU graduates” as 5%–40% of neonates with BPD are hypertensive.28 The physiology of this association is not completely understood but may be related to increases in sympathetic activity and angiotensin II.30
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree