Neonatal Bacterial Meningitis
EPIDEMIOLOGY
Prior to the introduction of sulfonamides (late 1930s) and antibiotics (mid-1940s), a diagnosis of neonatal meningitis was almost always a death sentence. For the few who survived, severe neurologic sequelae were likely to occur. Subsequent to the introduction of antimicrobials, deaths were still common.
During the last 25 years, there has been an apparent decrease in both the incidence and the case fatality of neonatal bacterial meningitis in developed countries. The probable reason for this is a more aggressive approach to eradication of maternal colonization by those bacteria most commonly associated with early-onset (EO) meningitis. One of the most common organisms causing EO meningitis in the latter part of the 20th century was the group B streptococcus (GBS; also called Streptococcus agalactiae). However, in the 1990s, an aggressive approach to the elimination of GBS in both mother and infant was adopted to prevent neonatal sepsis and meningitis. Nevertheless, GBS and Escherichia coli (E. coli) remain the predominant organisms causing EO neonatal sepsis and meningitis. Recent figures from the USA (using the National Institute for Child Health and Human Development [NICHD] network) indicate that 43% of EO sepsis and meningitis were caused by GBS and 29% by E. coli. GBS occurred mostly in term infants (73%), whereas E. coli occurred predominantly in preterm infants (81%).1
It may be difficult to distinguish between sepsis and meningitis in the neonate, and they are often reported together. EO sepsis and meningitis are sometimes considered to be that which occurs within the first 3 days after birth (which may be considered as perinatal, with infection transmitted by the mother) and sometimes as that which occurs within the first week after birth (which might also include some cases of nosocomial infection, ie, hospital-acquired). There seems to be greater unanimity about the definition of late-onset (LO) meningitis, which is generally regarded as that which occurs more than 7 days after birth. At present, most cases of GBS meningitis are LO.
Although it may be difficult to distinguish clinically between sepsis and meningitis in the neonate, a diagnosis of bacterial meningitis usually requires a culture of cerebrospinal fluid (CSF) that is positive for pathogenic bacteria. However, if CSF cannot be obtained before administration of antibiotics, a diagnosis of meningitis may be supported by CSF pleocytosis and decreased CSF glucose (and increased CSF protein).
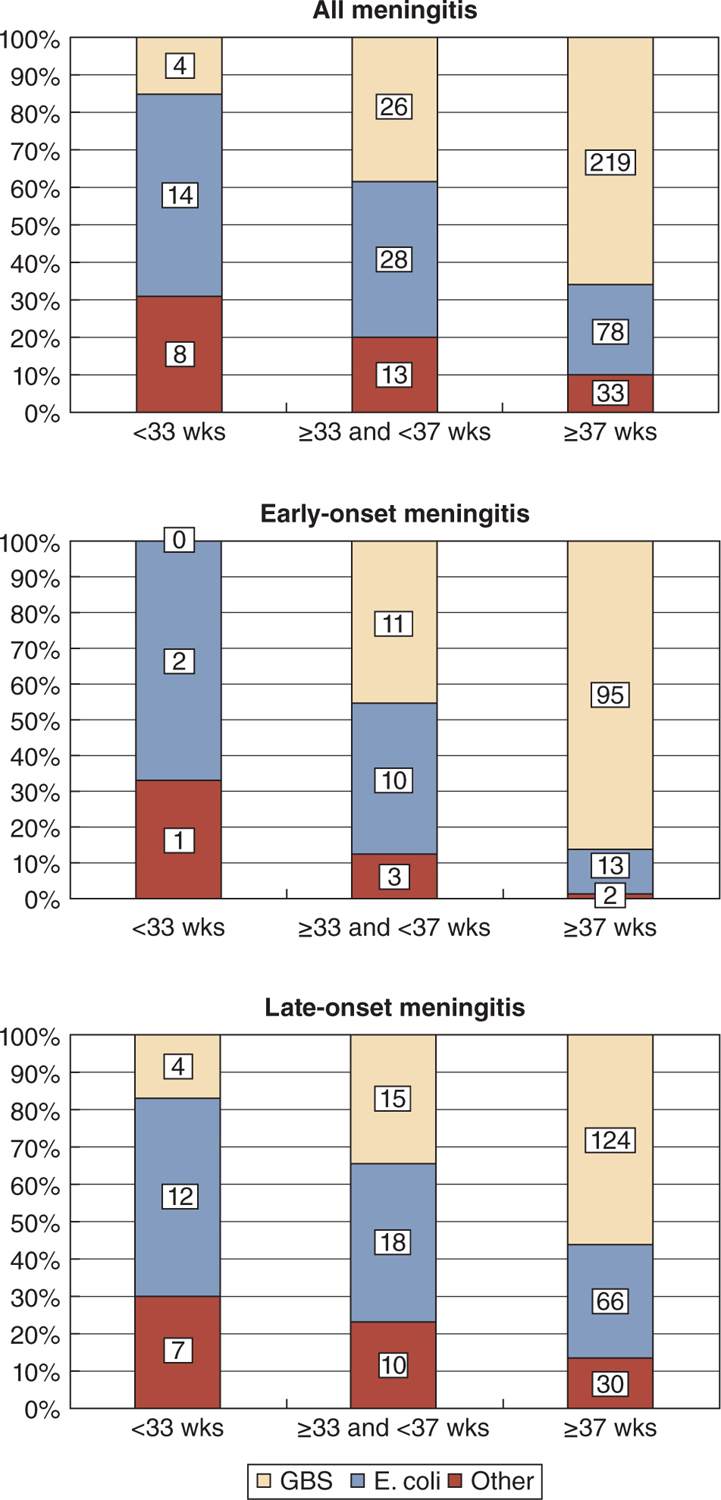
In addition to GBS, the other major organism associated with neonatal meningitis is E. coli, but Klebsiella species have also been implicated frequently in some countries, and Listeria monocytogenes accounts for a substantial number of LO cases in other countries. GBS and E. coli remain the predominant organisms causing both EO and LO meningitis in France. The distribution of organisms at different gestational ages, as well as different ages of onset, can be seen in Figure 16-1.2 Although only a handful of bacteria are commonly associated with neonatal meningitis, an enormous number of bacteria have been implicated during the past 50 years. Details can be found elsewhere.3 Especially in the very preterm infant, both Staphylococcus aureus and coagulase-negative staphylococci (mostly Staphylococcus epidermidis) have been implicated,4,5 and Citrobacter koseri (formerly Citrobacter diversus) deserves special mention because of the frequent association with brain abscesses.6
FIGURE 16-1 Distribution of all, early-onset, and late-onset neonatal meningitis according to gestational age at birth and causative organism. GBS, group B streptococci. (From Gaschinard et al.,2 Figure 2.)
Incidence
Because neonatal bacterial meningitis is comparatively infrequent, especially since the start of the new millennium, it is difficult to derive useful data from single centers. There have been a number of national studies over the years, which have provided useful information and allow incidence and case fatality rates (CFRs) to be derived.
Among developed countries, the lowest incidence of neonatal bacterial meningitis has been reported from Australia. In the 1990s, the incidence of neonatal meningitis in Australia was 0.15–0.17 per 1000 live births (LB), and a report from 2006, which also included New Zealand, documented an incidence of 0.10/1000 LB.7 Reports from England and Wales in the mid-1980s and mid-1990s revealed little change, with incidence figures of 0.32 and 0.31/1000 LB, respectively.8 In the Netherlands, the incidence was 0.23/1000 LB in the period 1976–1982.9
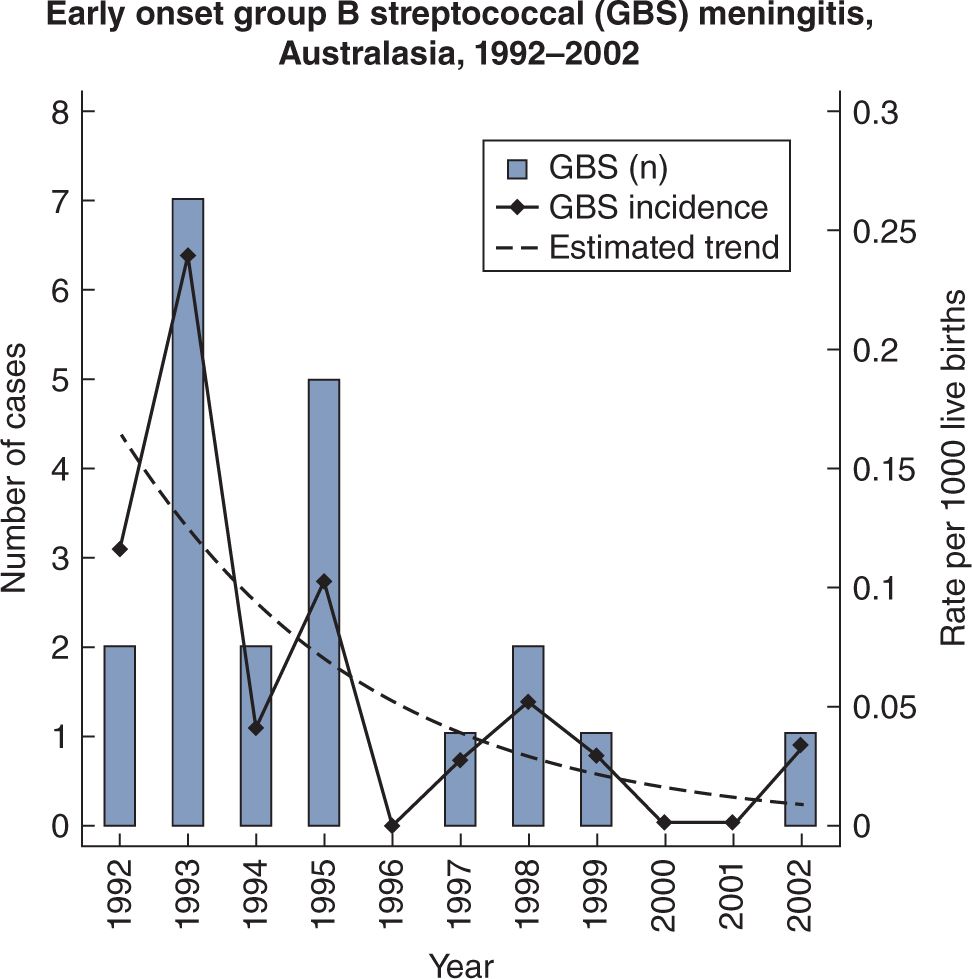
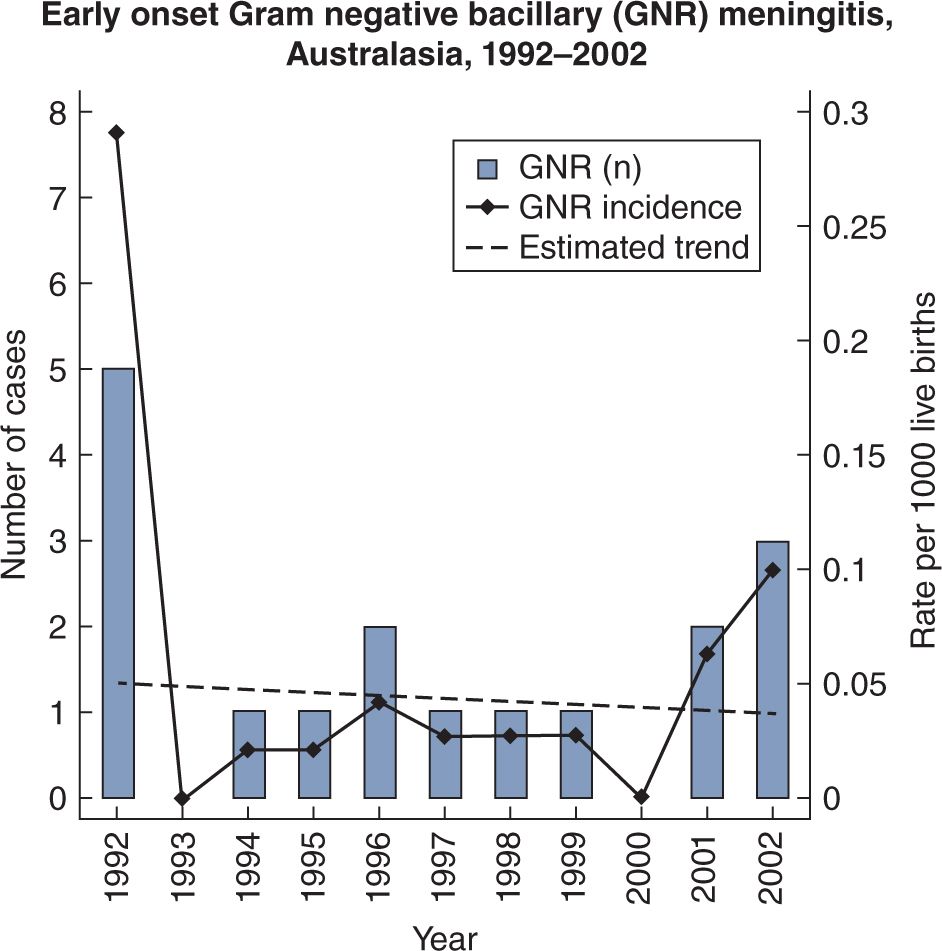
The introduction of widespread intrapartum chemoprophylaxis to prevent neonatal GBS sepsis and meningitis has resulted in a marked decrease in the incidence of GBS meningitis in some countries, whereas there was little change in the incidence of E. coli meningitis. A report from Australasia (Australia and New Zealand) in 2005 showed that EO GBS meningitis in 2002 fell from 0.24/1000 LB in 1993 to 0.03/1000 LB10 (see Figure 16-2). On the other hand, gram-negative bacilli did not obviously decrease (see Figure 16-3). In contrast, a report from the Netherlands in 2007 documented a reduction in EO GBS sepsis from 0.54/1000 LB in 1997–1998 to 0.36/1000 LB in 1999–2001, but a slight increase in GBS meningitis from 0.14 to 0.17/1000 LB for the 2 time periods, respectively.11 It is much more likely that GBS sepsis will be accompanied by meningitis after the first week of age. In the Netherlands study, babies investigated within 12 hours of birth who had GBS sepsis, had meningitis in only 6% of cases, whereas when investigated at greater than 7 days of age, 53% had accompanying meningitis.11
FIGURE 16-2 Decreasing incidence of early-onset group B streptococcal meningitis in Australasia, 1992–2002.
FIGURE 16-3 Minimal change in incidence of early-onset gram-negative bacillary meningitis in Australasia, 1992–2002.
Incidence in the 21st Century
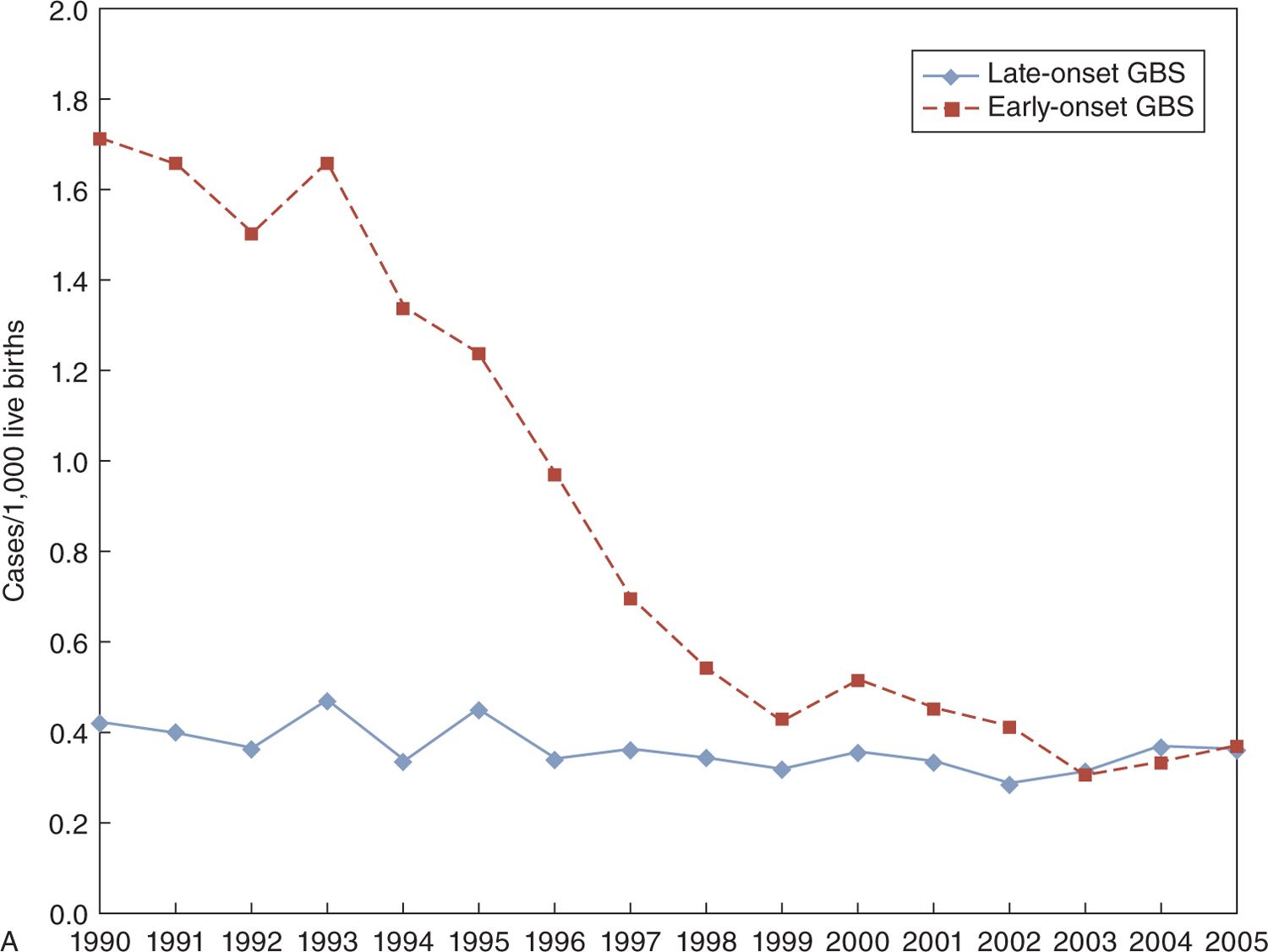
Further reductions in the incidence of bacterial meningitis have probably occurred in the last decade because there have been few reports dealing exclusively with neonatal meningitis, from single centers, during these years. The incidence of GBS sepsis has fallen substantially in the United States, with EO sepsis at 1.7/1000 LB in 1990 and 0.4/1000 LB in 2005, with a presumed reduction in EO meningitis12 (see Figure 16-4). However, LO GBS sepsis has remained constant at about 0.4/1000 LB, which suggests that LO GBS meningitis has also stayed about the same.12 In northern Italy, the incidence of EO GBS sepsis was 0.27/1000 LB, with only 2 cases of GBS meningitis in the years 2003–2005, whereas LO GBS sepsis was 0.23/1000 LB with 12/26 cases of sepsis accompanied by meningitis in the same time period.13
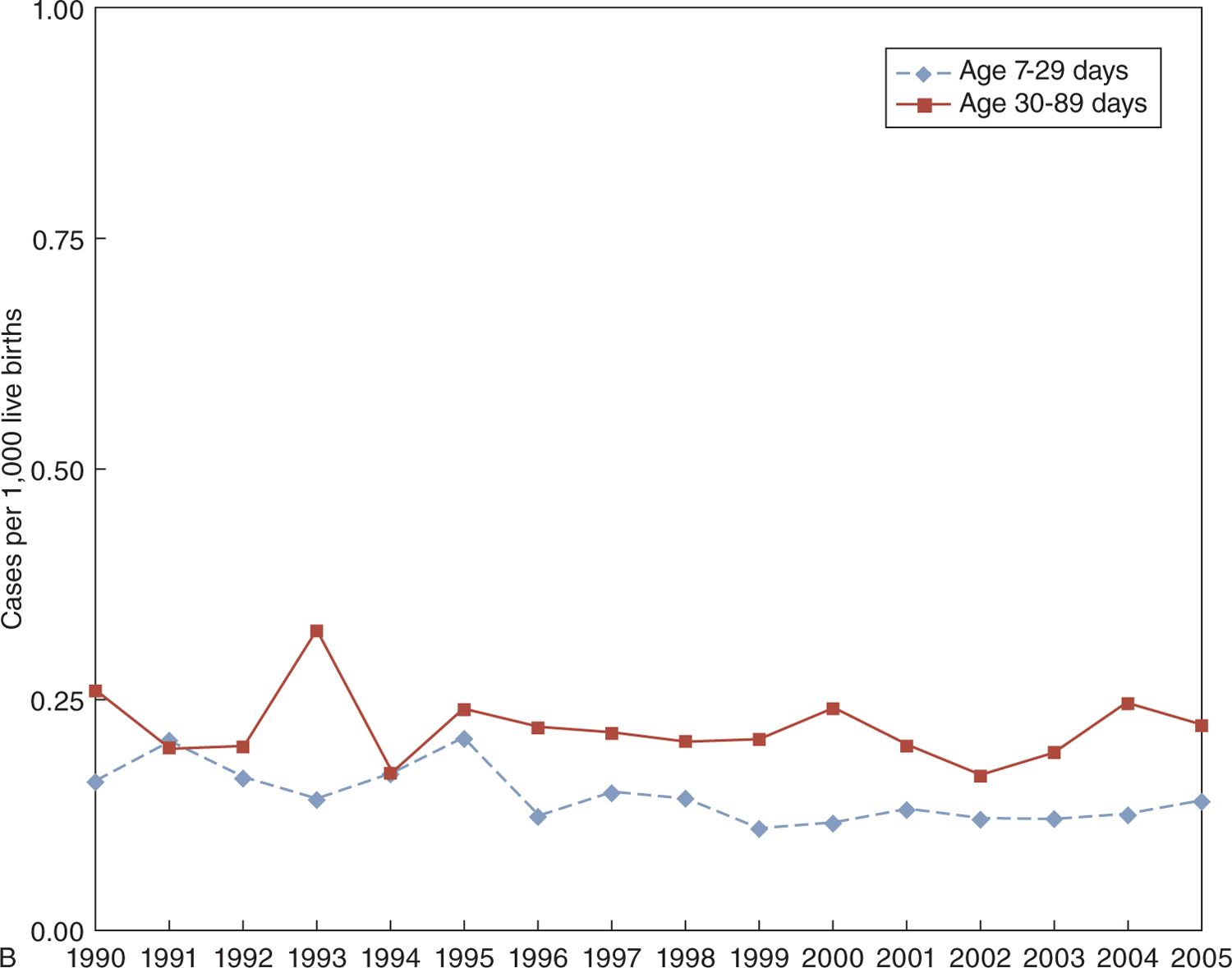
FIGURE 16-4 Marked decrease in incidence of early-onset group B streptococcal (GBS) disease in the United States, 1990–2005 (upper portion of panel A), with little change in the incidence of late-onset GBS disease (lower portion of panel A). Rates may have decreased slightly for late-onset meningitis in infants aged 7 to 29 days compared to those aged 30–89 days (panel B).
Using the Active Bacterial Core (ABC) surveillance system, a recent US national report documented rates of meningitis at less than 2 months of age as 0.65/1000 LB in 1998–2001 and 0.63/1000 LB in 2002–2007 and indicated that most cases were of LO.14
As with most forms of neonatal infection, the incidence of sepsis and meningitis is higher in low birth weight (<2500 g) infants and especially among very low birth weight (VLBW; <1500 g) infants.1 Most studies also reported higher incidence in males than females.
Case Fatality
In an earlier era, the CFRs for neonatal meningitis were high, but they seem to have decreased in recent years. Although it is comforting to think that this is the result of improved care, it could (in part) be because of the decreased virulence of the infecting bacteria. In England and Wales, among 450 cases seen in 1985–1987, the CFR was 29%; the CFR was 32% with gram-negative infections. In the years 1996–1997, a similar study of neonatal meningitis from England and Wales reported only a 10% CFR.8 In southern Taiwan, the CFR was 17% in 1986–1993 but decreased15 to 8% in 1994–2001. In less-well-developed countries,16 CFRs of 30% to 40% were still being reported in the late 1990s.
The CFR in Toronto, Canada,17 for the years 1979–1998 was 13%, and in a report from Greece in the year 2000, a remarkable 70 of 72 term infants with gram-negative bacterial meningitis survived to discharge with few sequelae.18 Recent information from the United States suggests a CFR of 11% in meningitis occurring in infants under the age of 2 months (mostly because of GBS), with a somewhat higher rate (18%) with L. monocytogenes.14 Rather similar data come from a national study in France, which reported on 444 cases seen between 2001 and 2007. In term infants (n = 330), the CFR was 10%, but in preterm infants, it was 26%. Also of interest is the fact that growth-restricted babies had a rate that was double (25%) that for appropriately grown infants (12%).2
Susceptibility
As mentioned, male infants appear to be more susceptible to neonatal infection than female infants. The reason for this is not clearly understood, but some protection seems to be conferred by the presence of the extra X chromosome in females. Increased susceptibility to infection in VLBW infants is caused (in part) by minimal transfer of protective antibodies from the mother until after 32 weeks’ gestation. Although twins have been reported to be at increased risk for GBS infection, this is probably related to a greater likelihood of twins being born preterm.
A specific susceptibility may occur in infants with galactosemia, who are at particular risk of developing infection with E. coli. During a 12-year period, in an era when GBS was predominant, 35 infants with galactosemia were detected on routine neonatal metabolic screening. Of this group, 10 developed systemic infection with E. coli. All 9 with bacteremia died, despite antibiotic therapy.19
PATHOPHYSIOLOGY
Meningitis refers to inflammation of the leptomeninges covering the surface of the brain. With bacterial infection, this usually results in a purulent exudate. In the neonate, there may be difficulty localizing infection and inflammation, with the result that the brain (cerebral hemispheres and cerebellum) may be involved in a process of meningoencephalitis.
One of the factors limiting the spread of infection in older infants and children is the blood-brain barrier. This barrier seems to be less effective in the neonate. An additional concern for the preterm infant is that considerable brain growth occurs during the third trimester of pregnancy, and meningoencephalitis in the preterm infant can interfere with the normal processes of brain growth, with resultant developmental deficits.
Acquisition of Bacteria
As might be surmised from the fact that neonatal septicemia (sepsis) and meningitis are difficult to separate clinically, bloodstream infection is probably the most important route of transmission of bacteria to the meninges. Nevertheless, other routes are possible.20 The most common route probably involves bacteria traversing placental blood vessels of the mother to infect the fetus. Nasopharyngeal colonization may occur, and access could possibly be gained by traversing the cribriform plate. Perhaps more important is intestinal colonization, with subsequent uptake into the bloodstream. This could result from contamination of the amniotic fluid, which the fetus is swallowing. Yet another portal of entry is a congenital dermal sinus, which needs to be looked for in the midline of the back; whenever an infant has a CSF shunt in place (eg, for posthemorrhagic hydrocephalus), there is a possibility for infection of the spinal fluid. It has also been reported that, later in the neonatal period, meningitis may frequently be preceded by a urinary tract infection.
In most cases of EO infection, it is difficult to know if bacteria are transmitted transplacentally or if infection is “ascending” secondary to infection of the amniotic fluid or colonization of the maternal genital tract with pathogenic organisms. In LO infection, acquisition of pathogenic bacteria may come from direct contact with colonized caregivers, the equipment surrounding or inserted into the infant (incubators, endotracheal tubes, ventilators, and intravenous catheters), as well as community-acquired sources.
Transfer of Maternal Antibody
There is a strong correlation between colonization of the mother and colonization of the infant. Although few infants who are colonized with pathogenic bacteria become infected, the more frequently mothers are colonized, the more likely is it that neonates will become infected. One of the key factors allowing neonates to become infected is the lack of specific antibody to a specific organism. Neonates born to mothers who have developed an antibody to certain bacteria are unlikely to become infected if they are born at term. This is less true in the preterm infant because the majority of maternal antibody transfer to the fetus occurs after 32 weeks’ gestation, although some protection may be provided by the small amounts that manage to be transferred prior to 32 weeks.
As mentioned, susceptibility to infection is related to absence of antibody to common bacteria. With regard to GBS infection, this was strikingly demonstrated some time ago by Vogel et al.21 They documented the lack of serospecifc antibody in neonates who acquired GBS infection. Only immunoglobulin G (IgG) usually traverses the placenta, by an active process, largely after 32 weeks’ gestation. Because immunoglobulin M (IgM) does not cross the placenta, this may explain to some extent why E. coli is prominent in neonatal infection because antibody to E. coli and other gram-negative bacteria is found predominantly in the IgM fraction. Nevertheless, a small amount of antibody is in the IgG fraction and can provide some protection even in very preterm infants.22
Bacterial Characteristics
The characteristics of certain bacteria seem to increase the likelihood that they will cause neonatal meningitis. Organisms that have the propensity to cause neonatal meningitis are usually protected from the neonate’s defense mechanisms (such as complement-mediated lysis and phagocytosis) by a polysaccharide capsule. One feature associated with this propensity is the presence of sialic acid in high concentrations in the capsule of some bacteria. Such is the case for the capsular polysaccharide of GBS serotype III, E. coli K1, and L. monocytogenes serotype IVb, the bacteria most commonly associated with neonatal meningitis. Another feature is the ability of bacteria to interact with neutrophils and affect their virulence. It has been shown that virulent clones of E. coli have impaired interaction with neutrophils, which makes them more likely to produce invasive infection.23
Although it has been known for many years24 that certain strains of GBS are more likely to produce meningitis, particularly serotype III (but also IA and V), more recently the biotype has received increasing attention when it is related to loss of catabolic function.25 Invasion of the central nervous system (CNS) of the neonate was 13 times more likely with biotypes B1 to B6 than with biotypes B7 to B15. Biotypes B1 to B6 were identified in 86% of 42 GBS strains associated with neonatal meningitis.25
Additional highly virulent clones of GBS have been noted in France, with ST-17 sequence type being important.26 Using polymerase chain reaction (PCR) techniques, 156 strains of GBS were evaluated, and 40 were positive for ST-17. Among neonates with LO GBS disease (with a high incidence of meningitis), 78% of strains were ST-17.
With E. coli meningitis, the predominant strains are those carrying the K1 capsular antigen. K1 strains have been demonstrated in approximately 75% of cases of neonatal meningitis caused by E. coli and in 1 report accounted for 88% of cases.9
Recently, the “hypervirulence” of the ST-17 strain of GBS has been attributed to a specific surface-anchored protein called hypervirulent GBS adhesion (HvgA) protein.26 The authors suggested that GBS that express HvgA adhere more efficiently to intestinal epithelial cells, choroid plexus epithelial cells, and microvascular epithelial cells, which constitute the blood-brain barrier. They also suggested that entry of bacteria may be from colonization of the intestine, followed by translocation across the intestinal barrier, as well as the blood-brain barrier, resulting in meningitis. In this study, evaluation of LO GBS meningitis revealed 88% of cases caused by serotype III and 83% had ST-17.27
There is also recent evidence concerning meningitis caused by E. coli. It is suggested that extracellular loops of E. coli (RS218) contained on the outer membrane protein A are important in bacterial survival in serum, as well as in entry into brain microvascular endothelial cells. Cellular invasion requires activation of host cytosolic phospholipase A2.28 Apparently, the K1 capsule does not play a specific role in the invasion of the blood-brain barrier but seems to be important in maintaining bacterial viability during the invasion.28
The ability of certain bacteria, such as E. coli K1, L. monocytogenes, and GBS type III, to breech the blood-brain barrier seems to be because of an increased capacity to penetrate the cerebrovascular endothelium while increasing blood-brain barrier permeability. There are receptors on various elements of the neurovascular unit that allow endotoxins and proinflammatory cytokines to bind to them.29
Basic Mechanisms in Pathophysiology
It may be useful to consider that the pathophysiology of neonatal meningitis consists of 3 main phases23: (1) infection (colonization and invasion), (2) bacterial multiplication and CNS inflammation, and (3) brain damage. The mechanisms are complex and beyond the scope of this discussion, but include initial stages of immune recognition, activating brain cells to produce cytokines (such as tumor necrosis factor α and interleukin [IL] 6) and chemokines, matrix metalloproteinases, and reactive oxygen species. These may all contribute to an increase in the number of cells in the CSF (pleocytosis), which is usually a characteristic feature of meningitis. However, as discussed in the section on diagnosis, the CSF cell count can be remarkably normal in the early stages of neonatal bacterial meningitis, although CSF culture may be positive for bacteria.
CLINICAL MANIFESTATIONS AND DIFFERENTIAL DIAGNOSIS
Although some clinical features of bacterial meningitis may be more or less pathognomonic, these are all findings that are seen late in the course of the infection. To make an early diagnosis, clinicians need to be aware of findings that may point toward systemic infection (sepsis or meningitis). Early diagnosis and treatment are likely to result in a good outcome, whereas delay in diagnosis is more likely to produce a bad outcome. Clearly, late findings are more likely to be associated with a poor prognosis.
There are 2 specific “clusters” of disorders that should be kept in mind in the differential diagnosis. These are inborn errors of metabolism30 and the more recently described autoinflammatory diseases in the neonate.31
The list of clinical features that should raise the suspicion of sepsis and meningitis is long. On the other hand, investigation of the neonate, soon after delivery, solely on the basis of maternal risk factors (such as prolonged rupture of the membranes or maternal fever) is unlikely to reveal meningitis. Beyond the first few hours (approximately 12 hours) after birth, investigation for sepsis will almost always be because of a number of clinical manifestations that are detailed in the following material. These findings include those presented in Table 16-1.
Table 16-1 Clinical Features of Meningitis
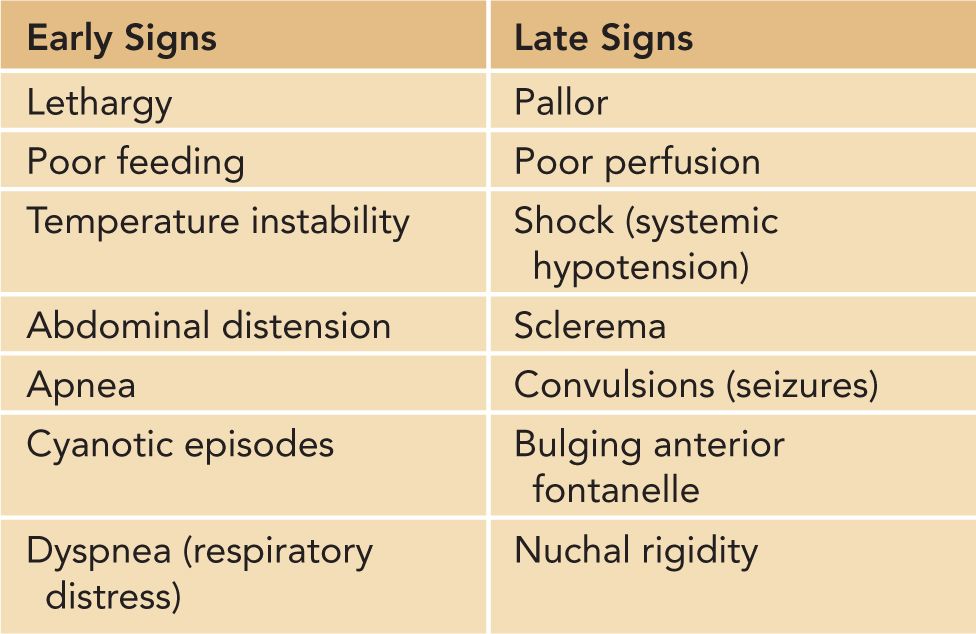
Early Signs
Lethargy
It is difficult to objectively define lethargy, but it is reasonable to think of a “lethargic” baby as one who is difficult to arouse at times when one would expect to be able to arouse him or her. For instance, a term baby who has recently been fed would not be considered lethargic, despite the difficulties one might encounter in arousing the baby from sleep. Similarly, the infant born following prolonged magnesium sulfate administration or following prolapse of the umbilical cord might be difficult to arouse because of hypermagnesemia or asphyxia, but a diagnosis of sepsis would not be entertained immediately. On the other hand, one of the earliest manifestations of sepsis/meningitis might be a “depressed” (poorly responsive) baby with low Apgar scores. Maternal administration of general anesthesia or analgesia, or fetal asphyxia, would need to be considered.
Poor Feeding
Similar difficulty to that of defining lethargy is encountered when trying to define poor feeding. The implication is that a baby who was formerly feeding (breast or bottle) quite vigorously now does so reluctantly, or not at all. Thus, an infant of less than 32 weeks’ gestation would not usually fit into this category because adequate coordination of sucking and swallowing does not occur until after 32 weeks’ gestation. However, “difficulty with feeding” could apply to preterm infants (or others) who are fed by intragastric tube (gavage), as increasing gastric residuals, or poor gastric emptying, may suggest an underlying infectious problem.
Temperature Instability
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree