Necrotizing Enterocolitis and Spontaneous Intestinal Perforation
INTRODUCTION
Necrotizing enterocolitis (NEC) remains one of the most devastating diagnoses in the neonatal intensive care unit. Primarily affecting preterm infants, it is a leading cause of death and lifelong gastrointestinal and neurodevelopmental morbidities in the very low birth weight (VLBW) (birth weight < 1500 g) population. Despite decades of research, the specific etiology for NEC remains unknown. NEC appears to be precipitated by an insult that leads the immature neonatal immune system to mount an abnormal and deleterious inflammatory response. Since many neonates die or suffer unfavorable outcomes despite maximal medical and surgical therapy, a preventive strategy is greatly needed. This chapter reviews the epidemiology, pathophysiology, diagnostic criteria, and supportive treatment of NEC, as well as promising novel, preventive strategies currently under investigation.
EPIDEMIOLOGY
Necrotizing enterocolitis is estimated to occur in 1 per 1000 US live births each year.1 Its annual rate in the highest-risk population, VLBW infants born prior to 29 weeks, is much higher (10%–15%). NEC frequency is inversely related to birth weight and gestational age in a nonlinear fashion, with a marked increase in NEC incidence as birth weight decreases below 1500 g.2 In the United States, the incidence rate in the VLBW population has remained stable at 11% in recent years.3 Despite this stability, the proportion of neonatal deaths secondary to NEC has actually increased over the past 2 decades, as preterm deaths from other causes have fallen due to advances in neonatal care.4
Although NEC is predominantly a disease of VLBW infants, it is occasionally seen in term infants following other illnesses. Concurrent diagnoses of congenital heart disease, bacterial sepsis, polycythemia, and hypotension are associated with NEC in term and late-preterm infants.5
Patient Demographics
Necrotizing enterocolitis is reported more frequently in male infants and non-Hispanic infants of African descent.1 Timing of onset is inversely related to gestational age, with a median onset of nearly 4 postnatal weeks in infants born at less than 25 weeks’ gestation, 2 weeks at 27–29 weeks’ gestation, and within the first week in term infants.6 Most NEC cases present sporadically, although temporal, nonseasonal clustering of NEC cases within neonatal intensive care units has been reported.7
Antenatal Risk Factors
The main antenatal risk factors for NEC are those associated with preterm birth, the leading risk factor for NEC.8 Thus, at birth it is nearly impossible to predict which VLBW infants will be at higher risk for NEC. Recent investigations have reported an association between antenatal indomethacin administration for tocolysis and NEC.9,10
Postnatal Risk Factors
Prematurity is the leading postnatal risk factor for NEC, which only rarely occurs in term infants. The association between preterm birth and NEC has been consistently verified over time in numerous investigations.1,3,4,8 Since NEC only affects a small fraction of preterm infants, clinical researchers continue to investigate other factors that may be associated with developing NEC.
Packed red blood cell (PRBC) transfusion in VLBW infants and empiric antibiotic use in infants with extremely low birth weight (ELBW) (<1000 g) have been associated with the development of NEC, although further research is needed before any causal relationship may be established.
In a retrospective cohort of VLBW infants, Paul et al found that those receiving PRBC transfusions had double the odds of NEC, even when transfusions after the diagnosis of NEC were excluded.11 Other authors have found that patients with NEC have an increased likelihood of having been transfused with PRBC within 48 hours of NEC onset.12,13 Infants who developed NEC after transfusion were older at presentation (mean age > 30 postnatal days) than those who were never transfused.11,12
In a retrospective cohort study of ELBW infants within the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) centers, Cotton et al found that empiric antibiotics started within the first 3 days of birth and continued for 5 days or greater with negative initial culture results were associated with an increased incidence of NEC.14 A case-control study by Alexander et al determined an association between duration of antibiotic exposure in neonates without a prior bloodstream infection and risk of NEC.15 A leading hypothesis for this association is that antibiotics disrupt normal bowel colonization and flora, thus predisposing the bowel to the inflammatory cascade associated with NEC. Normally, a commensal relationship exists between native microflora and the intestinal epithelium.16 Normal epithelial cell development appears to be dependent on commensal bacteria, and the presence of a diverse microflora has been associated with both increased digestive tolerance and weight gain in preterm infants.17
Other postnatal risk factors associated with development of necrotizing enterocolitis are the need for resuscitation with chest compressions or resuscitative drugs immediately following birth and being born to a teenaged mother.18 Recent case reporting led to a Food and Drug Administration (FDA) warning and voluntary recall of the use of a xanthan gum–based formula thickener (Simply Thick®) in preterm infants, and pending further investigation, the use of thickening in preterm infants should be avoided prior to 44 weeks’ adjusted gestational age.19,20
Spontaneous Intestinal Perforation
Spontaneous intestinal perforation (SIP), also known as focal intestinal perforation, has been used to describe the occurrence of an isolated perforation of the ileum without other signs of NEC occurring in a premature infant.21 SIP occurs in VLBW infants with a median gestational age of 25–26 weeks22,23 and usually presents within the first 2 weeks of life. There is an association between indomethacin and corticosteroid administration within the first 3 days of life and SIP.22 The diagnosis of SIP cannot be confirmed unless a laparotomy is performed and perforation in the absence of necrotic bowel is noted.
Debate exists regarding whether SIP is a mild variant of NEC or a unique illness. Proponents of classifying SIP as a distinct illness note that the pathology of SIP has been reported to differ from NEC. Instead of widespread inflammation and necrosis, histological examination reveals intact mucosal epithelium and absence of intestinal musculature limited to the focal area of perforation.24 A diagnosis of SIP is associated with decreased mortality and better neurodevelopmental outcomes compared to NEC.8,21
Despite these conflicting viewpoints, the 2 entities present in a similar manner: a premature infant with free intraperitoneal air requiring operation. Data from the NICHD NRN and others suggest that the absence of pneumatosis does not rule out widespread NEC.8 Necrosis is often found during laparotomy in infants with a preoperative diagnosis of SIP.8 All infants with intestinal perforation require either peritoneal drain placement or laparotomy, regardless of the extent of additional disease.
PATHOPHYSIOLOGY
The pathophysiology of necrotizing enterocolitis results from a hyperactive intestinal inflammatory response. This is thought to be a maladaptive reaction of the immature neonatal intestine to a specific trigger(s), which remain(s) elusive and unknown. Bowel immaturity appears to be a key factor in NEC since the disease is only found in infancy.
Gross and Histological Bowel
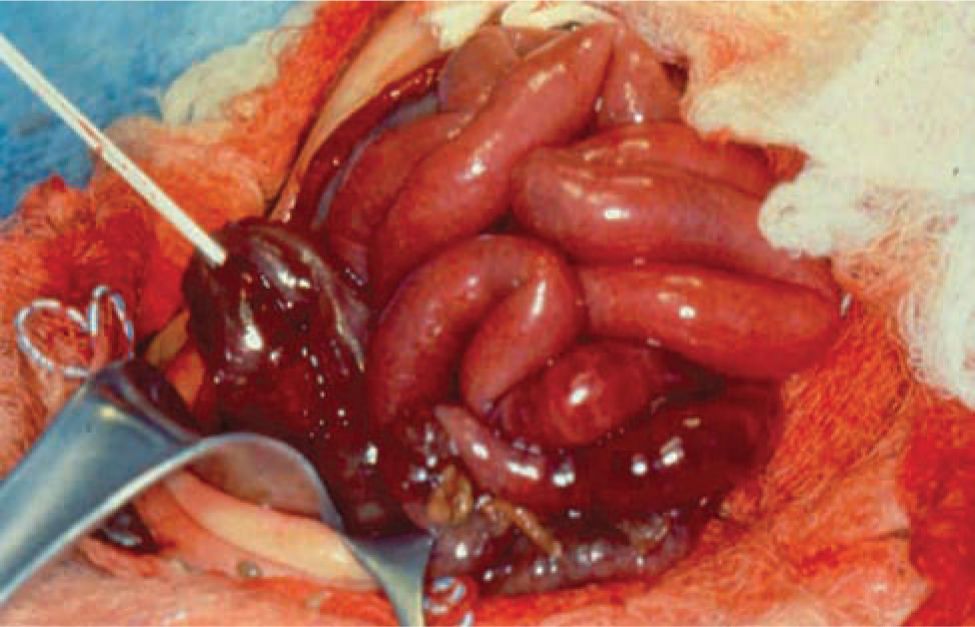
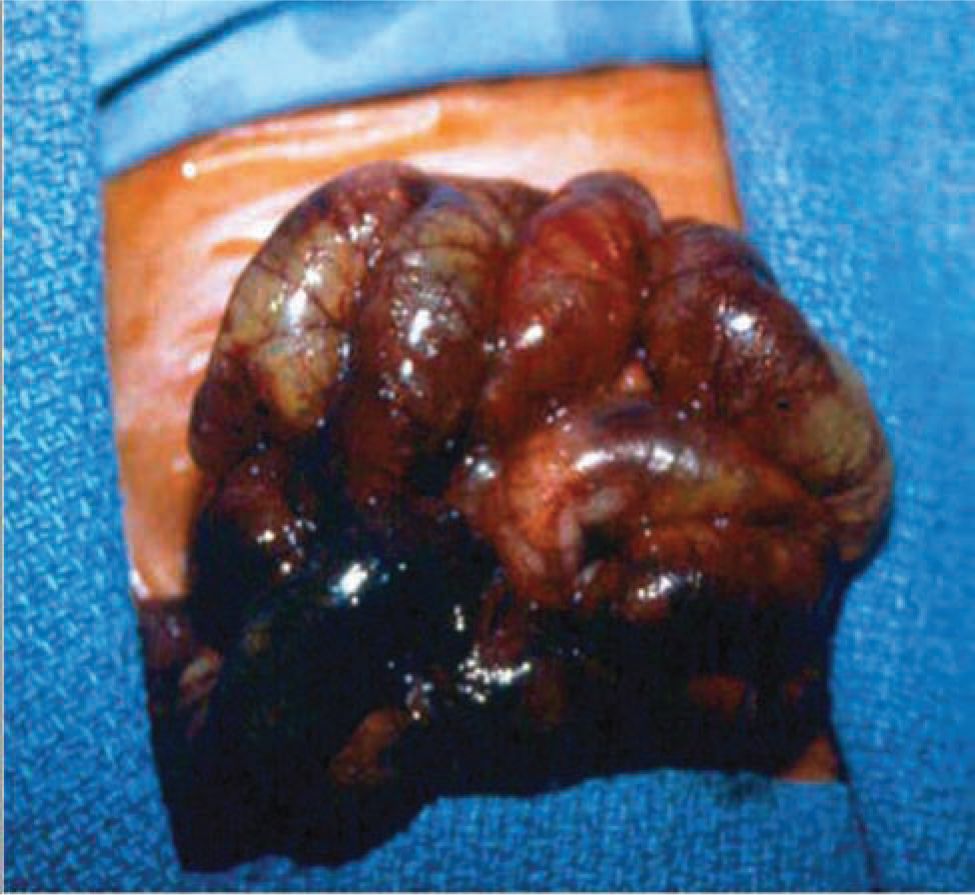
On gross examination, necrosis is noted in the diseased bowel segments, which are distended with thin walls. These affected areas display dark red to black or gray-green discoloration, and the mucosa is often hemorrhagic, ulcerated, and friable. Both the small and large intestines may be involved, and the diseased area(s) may be either continuous or consist of discontinuous segments (Figures 37-1 and 37-2). Although any segment of the intestine can be affected, the ileum and proximal colon are the most frequent sites. Pneumatosis intestinalis or gas within the wall of the intestine, when present, can be seen, and intestinal perforations may be noted.25
FIGURE 37-1 Intraoperative photograph showing segment of normal bowel (superiorly) and necrotized bowel (inferiorly).
FIGURE 37-2 Photograph showing completely necrotic bowel (necrotizing enterocolitis totalis).
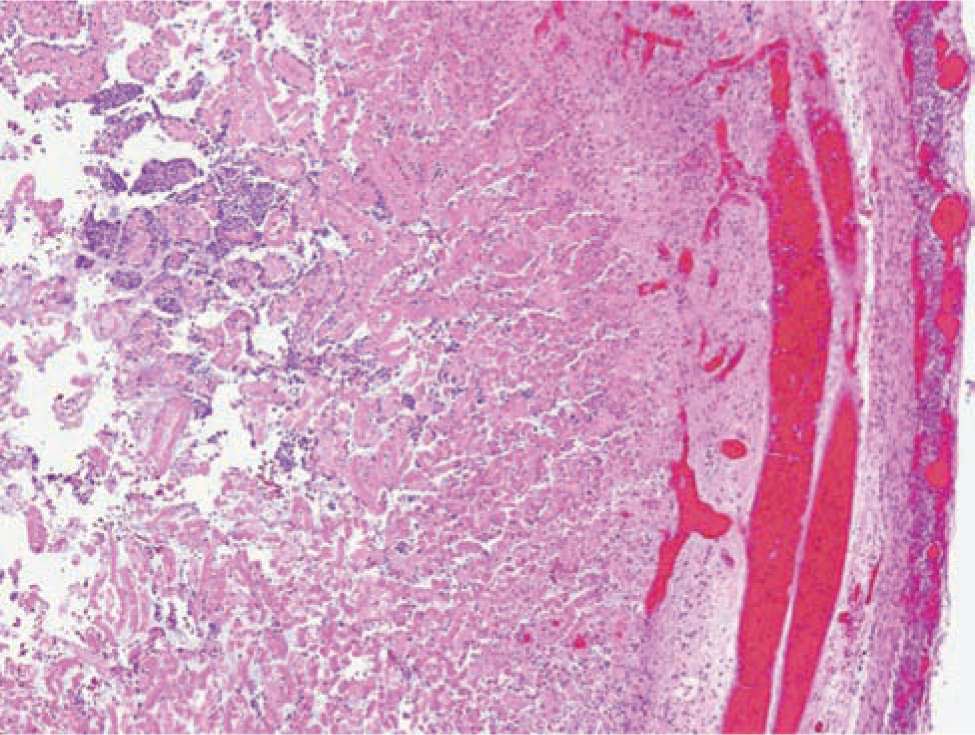
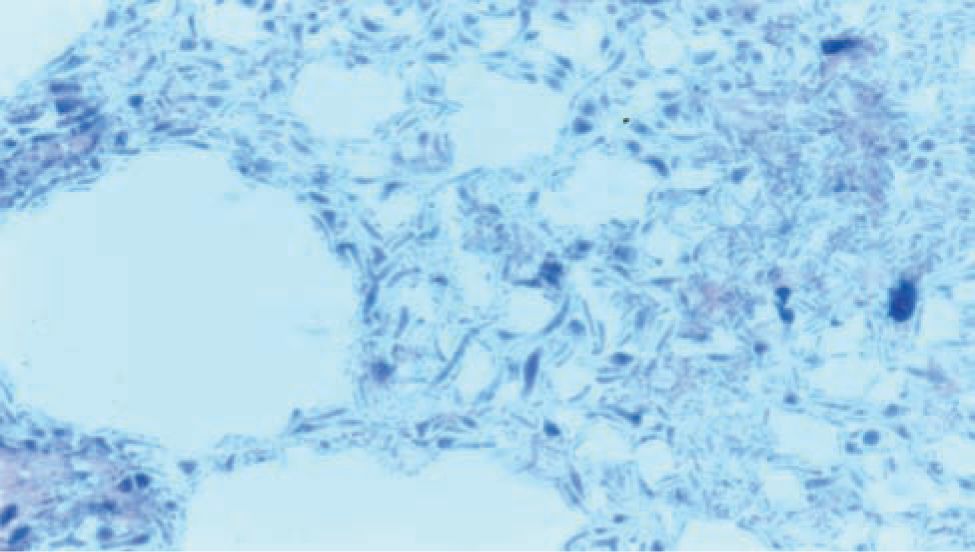
Histologically, ischemic (coagulation) necrosis and inflammatory changes are visible. These changes can be limited to the mucosa in mild disease but spread transmurally in severecases. Acute inflammatory changes are noted, and neutrophil infiltrate is the dominant finding. Reparative tissue changes, including granulation and fibrosis, and bacterial overgrowth are often noted (Figures 37-3 and 37-4).25
FIGURE 37-3 Histopathological bowel specimen showing necrotic changes.
FIGURE 37-4 Histopathological bowel specimen showing pneumatosis intestinalis.
Neonatal Gastrointestinal Immaturity
The preterm gastrointestinal tract is immature, which likely predisposes it to bacterial invasion and improper immune responses. Relative deficiencies of growth factors involved in intestinal maturation, including epidermal growth factor (EGF)26 and heparin-binding epidermal growth factor (HB-EGF)27 may play a role in this immaturity. The epithelial cells normally provide a physical barrier between the intestine and the rest of body. With decreasing gestational age, the protective mucous layer overlying the epithelium is less mature, and the tight junctions between individual epithelial cells are more permeable to pathogenic invasion.17 Motility is also decreased. In preterm infants, the gastrointestinal bacterial flora differ from that of healthy term infants, and their intestines are more likely to be colonized with pathogenic bacteria.28
Bacterial Role
Bacteria play an important role in NEC, and multiple bacterial species have been associated with the disease. The species of intestinal microflora and rate of colonization in extremely preterm infants differ from more mature infants.17 Some have theorized that a change in normal gastrointestinal tract colonization precedes the onset of NEC. Since no particular bacterial strain appears causative, it is presumed that NEC is not an infectious disease but instead is related to opportunistic infection occurring during the disease process. It is unknown whether an inflammatory reaction responsible for increased permeability allows bacterial invasion to occur in early NEC or whether bacterial invasion occurs first and triggers the hyperactive immune response.28
Inflammation
Inflammation is responsible for the cellular destruction and necrosis that is central to NEC. Although the specific inflammatory pathways in NEC are not completely understood, certain cytokines and nitric oxide (NO) are thought to play key roles.29,30
Inflammatory mediators associated with NEC include platelet-activating factor (PAF), tumor necrosis factor α (TNF-α), and interleukins (ILs), including IL-1, IL-6, IL-8, IL-10, IL-12, and IL-18. The pattern recognition receptor, toll-Like receptor 4 (TLR4), which recognizes gram-negative lipopolysaccharides (LPSs), has increased expression in preterm intestinal epithelium relative to adults. In preliminary animal studies, maternally fed preterm mice had less TLR4 expression than formula-fed mice. Thus, TLR4 is hypothesized to play a key role in the protective role of human breast milk.30
Increased amounts of NO are present in pathological intestinal specimens from preterm infants with NEC. NO synthase, the enzyme responsible for the formation of NO, exists in the gastrointestinal tract in both constitutive and inducible forms. At normal levels, NO likely plays an important physiological role by regulating vascular and mucosal integrity. However, it is produced in larger amounts in response to inflammatory cytokine release. At higher levels, NO creates oxidative compounds with cytopathic effects, such as peroxynitrite (ONOO–).31,32
Ischemic damage in preterm NEC patients likely develops secondary to a hyperactive immune response, instead of being the event that initiates NEC. Since NEC presents much earlier in term infants and frequently occurs following hypoxic and hypoperfused states such as septic shock, congenital heart disease, and hypoxic-ischemic events, ischemia may play more of an inciting role in term infants.30,32
Genetics
Only a small fraction of age-matched preterm infants with similar clinical courses develop NEC. Thus, it is likely that some infants have a genetic susceptibility, and identifying genetic risk factors for NEC has become a research priority. For example, a recent study found higher surgery and mortality risks in preterm infants with NEC who had reduced H-antigen secretion secondary to a polymorphism in the gene for fucosyltransferase (FUT-2), an enzymatic component of glycosylation in innate immunity.33
CLINICAL PRESENTATION AND DIFFERENTIAL DIAGNOSIS
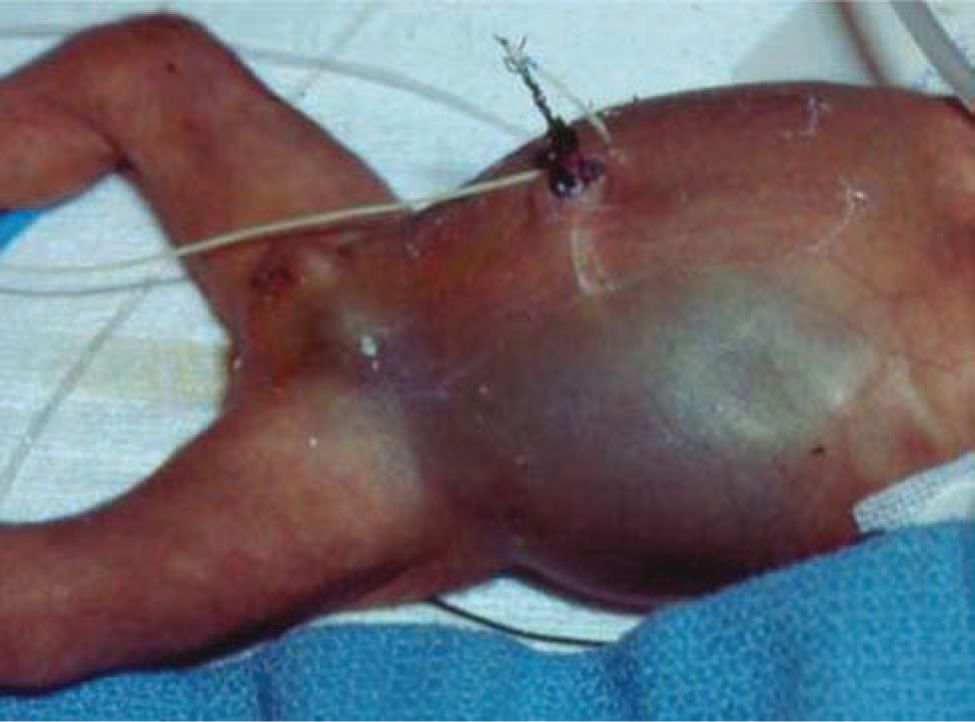
The physical examination remains a key component of evaluation for NEC. Abdominal distension is found in almost all patients with NEC. The abdomen may be initially soft to palpation but becomes firm and tender with disease progression. Palpable, distended bowel loops, decreased bowel sounds, abdominal wall erythema, or bluish discoloration may be noted. Abdominal wall erythema is highly predictive of NEC but is only present in 10% of patients (Figure 37-5).34 Lethargy is noted in severe illness. Vital sign changes include tachycardia, hypotension, and apnea and oxygen desaturation with resulting bradycardic episodes. Respiratory failure requiring intubation occurs in severe illness.
FIGURE 37-5 The abdomen of a child with bowel perforation shows distension and discoloration due to perforated bowel.
In its most mild or early from, necrotizing enterocolitis presents subtly with nonspecific gastrointestinal symptoms, including feeding intolerance or increased bilious or nonbilious gastric residuals, and signs that include abdominal distention and irritability. Besides NEC, the differential diagnosis for a stable infant in this setting includes decreased bowel motility secondary to prematurity or enteral feeding intolerance of nonspecific cause. Other causes of abdominal distension include anatomical obstruction, which is typically readily distinguished from NEC, and gastric distention from improper nasogastric tube placement. Hematochezia in the clinically well premature infant is most commonly caused by anal fissure, milk-protein allergy, or swallowed maternal blood.
Clinical instability ensues as NEC progresses. In severe cases of NEC, sudden instability may be the first presenting symptom. Sepsis is the major diagnosis that presents with findings similar to severe NEC. Ileus secondary to sepsis causes abdominal distension that may mimic NEC. In both severe NEC and sepsis, infants develop lethargy, apnea, poor peripheral perfusion, and abdominal tenderness. Fortunately, the same medical therapy regimen of holding feedings, starting antibiotics, and providing supportive care are appropriate for both conditions.
DIAGNOSTIC TESTS
Complete blood cell counts (CBC), electrolytes, and abdominal radiographs aid the clinician in the diagnosis of NEC. Serial physical examination and testing facilitate monitoring of worsening disease. Laboratory findings are nonspecific but can include thrombocytopenia; increased or decreased white blood cell counts with bandemia; elevated acute-phase reactants (C-reactive protein [CRP], CD64, or erythrocyte sedimentation rate [ESR]); hypo- or hyperglycemia; electrolyte instability, including hyperkalemia and hyponatremia; and metabolic acidosis. These laboratory abnormalities become more prominent as the severity of NEC increases.
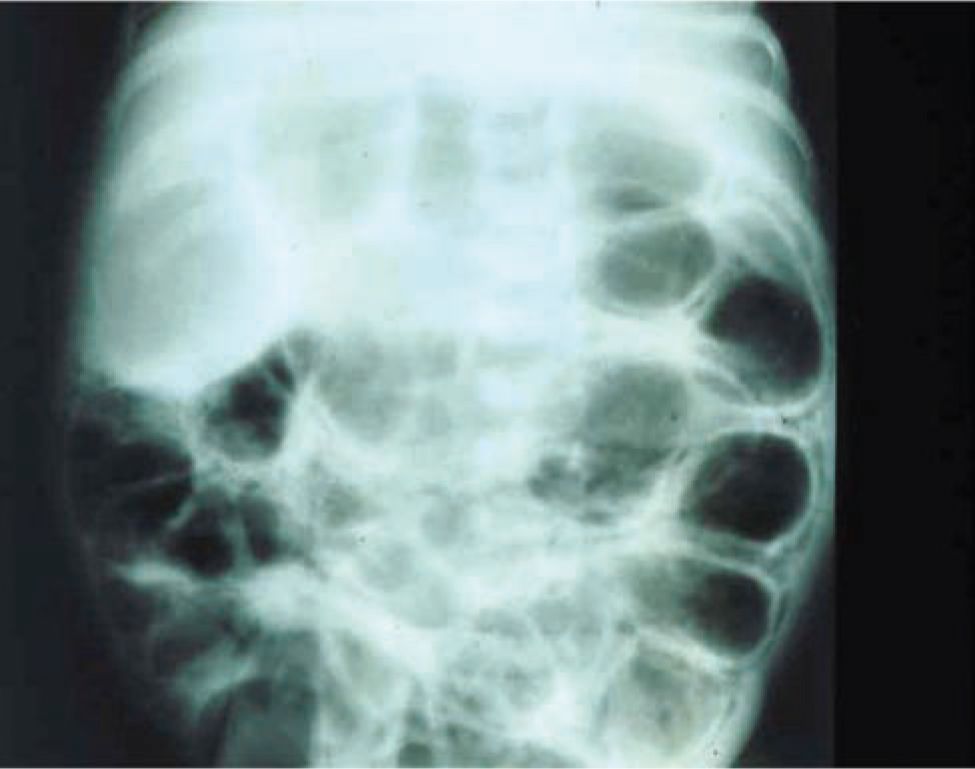
The plain abdominal radiograph remains the most important radiological modality for NEC diagnosis and severity assessment. The abdominal anteroposterior (AP) view and a second dependent view (left lateral decubitus or cross table) to aid in evaluation for pneumoperitoneum provide invaluable diagnostic information. Distended bowel loops, air-fluid levels, and thickened bowel walls are frequently found not only in early NEC but also in septic ileus and are thus nonspecific. Pneumatosis intestinalis is the most specific radiographic finding in NEC. Air bubbles are seen as lucencies within the bowel wall and are most often located in the right lower quadrant (Figures 37-6 and 37-7). Fixed bowel loops, portal venous air, and pneumoperitoneum are ominous signs. Pneumoperitoneum indicates perforation requiring surgical intervention (Figures 37-8 and 37-9). A gasless abdomen may be seen due to fluid-filled bowel loops but is nonspecific and can occur in sepsis or a patient receiving neuromuscular blockade.
FIGURE 37-6 Abdominal x-ray showing pneumatosis intestinalis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree