Hyperalimentation and Monitoring
INTRODUCTION
Nutrition support plays a crucial role in the management of premature neonates. Parenteral nutrition (PN) especially has continued to have an impact on the care of neonates who are unable to ingest sufficient enteral calories and fluids. The use of PN has evolved in the neonatal intensive care unit (NICU) to minimize the metabolic complications that interrupt normal growth. To deliver PN to neonates, those involved in the ordering and delivery of PN need to be educated and trained to ensure safety and standardization.1 This chapter provides a practical overview to provide optimal nutrition support to neonates in the NICU, keeping in mind the particular conditions and physiology of premature neonates.
INDICATIONS
Parenteral nutrition can be used as the sole source of nutrition and fluids for neonates. There are multiple conditions that can inhibit or limit the use of enteral nutrition. Patients can develop short-bowel syndrome from intestinal atresia, volvulus, or necrotizing enterocolitis. Motility issues can arise from Hirschsprung disease, meconium ileus, ileus as a result of generalized illness, or gastroschisis. Neonates with asphyxia or hypotension with use of vasopressors might not be clinically stable enough for the initiation of enteral nutrition. Preterm neonates on slowly advancing feedings will also require PN supplementation to obtain substantial calories and fluids until goal feedings have been reached. PN or enteral nutrition should be started within 24 hours after birth.2–5 More aggressive administration of both glucose and amino acids has led to the use of standard, starter, or “vanilla” total parenteral nutrition (TPN). Many of these solutions come premixed; many institutions still compound the mixture in the hospital pharmacy. These initial solutions usually contain 7.5% to 10% dextrose and 3% amino acids.6 The goal is to have a glucose infusion rate (GIR) of 6–8 mg/kg/min and amino acid administration of 2 to 3 g/kg/day.
ROUTE OF ADMINISTRATION
Peripheral venous access limits the osmolality of fluids that can be infused through it, which limits dextrose concentration to 12.5%. Increased osmolality that infuses through a peripheral catheter increases the risk of thrombophlebitis, although concomitant infusion of a fat emulsion can reduce venous irritation.7,8 Central venous access, either through an umbilical venous catheter (UVC) or a peripherally inserted central catheter (PICC) is generally needed. Positioning of these catheters must be confirmed with an x-ray showing the tip terminating at the junction of the superior vena cava and right atrium of the heart. Continuous infusion of heparin 0.5 IU/kg/h has been shown to reduce occlusions of PICC lines in neonates and can be added directly to the PN fluid.9,10
PARENTERAL NUTRITION COMPONENTS
Calories
There are many factors that can increase the metabolic demands of neonates, including increased heat loss, small for gestational age (SGA), fever, sepsis, respiratory distress, and the accelerated rate of growth. Neonates who receive a caloric intake as low as 70 kcal/kg/d have demonstrated a positive nitrogen balance. Weight gain can occur with parenteral intake of 80 to 130 kcal/kg/d if there is adequate protein intake. The caloric goal of premature neonates is 100 to 120 kcal/kg/d, and the goal of term neonates is 90–108 kcal/kg/d (Table 70-1).11 Calorie utilization is maximized if given in balanced allotments of macronutrients. Carbohydrates should be the main source of calories at 40% to 50%, followed by lipids at 30% to 40% and protein at 10%–30% of the caloric intake.12 Optimal calorie goals aim to sustain appropriate “catchup” growth. Excessive calorie intake must be avoided as this can result in excessive fat deposition in the subcutaneous tissue and liver as well as greater carbon dioxide production, which complicates any underlying respiratory conditions.13
Table 70-1 Recommendations for Parenteral Nutrition Macronutrients for Neonatesa
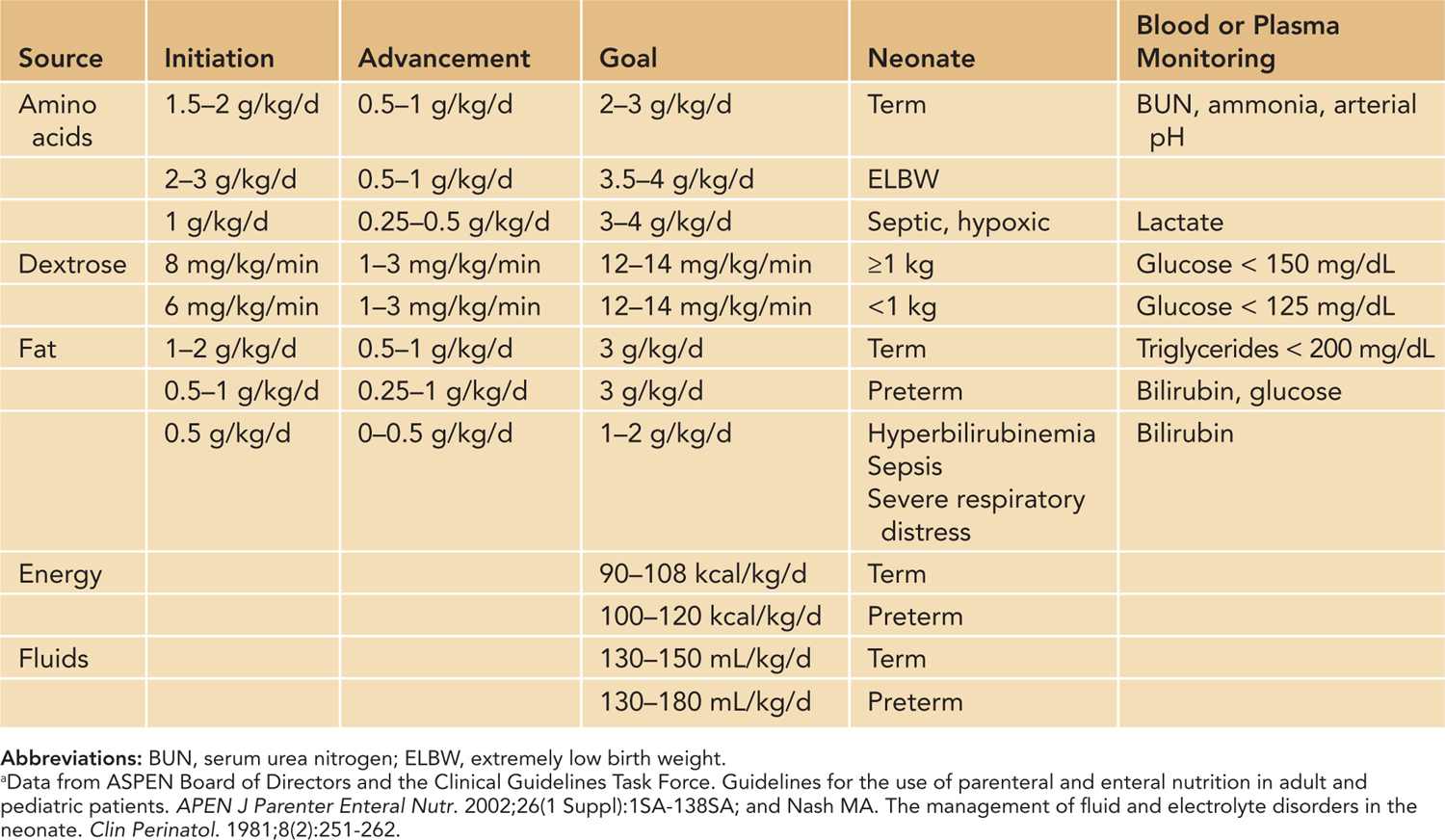
Fluids
The nutritional and caloric composition is a particular focus when discussing TPN, but the volume that is delivered is just as crucial (Table 70-1). Premature neonates have increased fluid losses because of radiant warmers, phototherapy, increased skin permeability, and respiratory distress. As a result of these factors, the premature neonate has higher volume needs than the term neonate. If there is fluid restriction, the concentration of nutrients needs to be increased, resulting in high osmolarity and issues with solubility. To help improve tolerance of these high-osmolar solutions, the amino acid–dextrose solution is typically run over 24 hours so the infusion rate can be as low as possible. Fat emulsions are also better tolerated if infused over 18 to 24 hours.
Carbohydrates
For neonates, carbohydrate delivery in PN should begin at approximately 6 to 8 mg/kg/min and be advanced, as tolerated, to a goal of 10 to 14 mg/kg/min.12 Dextrose is normally started at 10% concentration so that it will deliver a GIR around 7 mg/kg/min if infused at a rate of 100 mL/kg/d. As the GIR is increased, a trace amount of glucosuria is not uncommon, but the GIR may need to be decreased as serum glucose levels are also monitored (Table 70-1). Hyperglycemia in neonates can be associated with complications, including death, infections, visual problems, and intraventricular hemorrhage. The routine use of insulin to help prevent hyperglycemia in premature neonates has not been well established and can be associated with risk, such as lactic acidosis and death.11,14,15 Continuous insulin infusions at 0.01 to 0.1 units/kg/h may be necessary for managing hyperglycemia in neonates.12
Protein
Commercial pediatric amino acid solutions are formulated to result in plasma amino acid profiles that are comparable to breast-fed infants. These formulations contain taurine, tyrosine, histidine, aspartic acid, and glutamic acid and contain less glycine, methionine, and phenylalanine than adult amino acid formulations.11 Cysteine, a conditionally essential amino acid for neonates, is not added to pediatric amino acid formulations because of its short shelf life. Cysteine hydrochloride can be added to PN fluid at a dose of 40 mg/g of amino acids. Carnitine, required for optimal fatty acid metabolism, is also not added to pediatric amino acid formulations. There have not been any convincing studies to demonstrate that carnitine has any effect on lipid utilization and ketogenesis, although fatty acid oxidation is impaired if the tissue carnitine level falls below 10% of normal.16 Still, it has become practice to add L-carnitine 2 to 5 mg/kg/d to PN fluids, especially for neonates having difficulty with hypertriglyceridemia.12 This dose is the same quantity received from breast milk and infant formula.17 As stated previously, amino acids should be started within the first 24 hours of life and then advanced to the goal (Table 70-1). Newer retrospective data argue for starting amino acids within the first few hours of life.18
Fat
Fat constitutes a high-caloric nutrient source in any diet. Fat emulsions are currently available as 10% or 20% soy or soy-safflower oil. The use of 20% emulsion is preferred because it is more calorically dense yet has the same phospholipid concentration, has improved clearance of triglycerides and phospholipids, and has the same osmolarity as the 10% emulsion. These emulsions can prevent essential fatty acid deficiency (EFAD) at a minimum rate of 0.5 to 1 g/kg/d in the presence of adequate energy intake. If a neonate is in an energy-deficient state, then EFAD may not be prevented at this minimal rate because essential fatty acids will be oxidized to meet energy needs. Fat emulsions should be started within 3 days of life to prevent EFAD. Fat emulsions are safe to start within the first 24 hours of life and can be increased to a goal rate (Table 70-1). Fat emulsion delivery at a continuous rate over 24 hours optimizes lipid clearance. The maximum infusion rate should not exceed 0.15 g/kg/h, but this rate may need to be lower in very low birth weight infants or SGA infants. Heparin also stimulates the release of lipoprotein lipase, which may improve lipid clearance.
Jaundice and sepsis are not contraindications for using fat emulsions. There is a theoretic risk that fatty acids can displace bilirubin from albumin, resulting in increased serum unconjugated (free) bilirubin concentration, thus increasing the risk of kernicterus. Fat emulsion given at a rate of 1 to 4 g/kg/d did not show any effect on the bilirubin and albumin levels in neonates.19 Even with these findings, if the unconjugated bilirubin concentration approaches the level indicating an exchange transfusion, it is recommended that the fat emulsion rate should be decreased to 0.5 to 2 g/kg/d, and the rate should not be advanced until the bilirubin has decreased to 50% of the exchange transfusion level. Serum triglyceride levels can increase in the presence of bacterial sepsis, trauma, and stress and should be monitored. Although the risk of infection increases with the administration of fatty acids, this risk is not reason enough to avoid the use of fat emulsion.20
Electrolytes and Minerals
Close monitoring of electrolyte status is essential in neonates as daily adjustments are often necessary with the initiation of PN. The addition of electrolytes to PN may be deferred until the second day of life until regular urine output is ensured. Sodium is usually added once diuresis has begun, and potassium is added once good urine output and normal kidney function are established. Table 70-2 provides reference ranges of electrolyte requirements.
Table 70-2 Daily Electrolyte and Mineral Dosing Guidelinesa,b
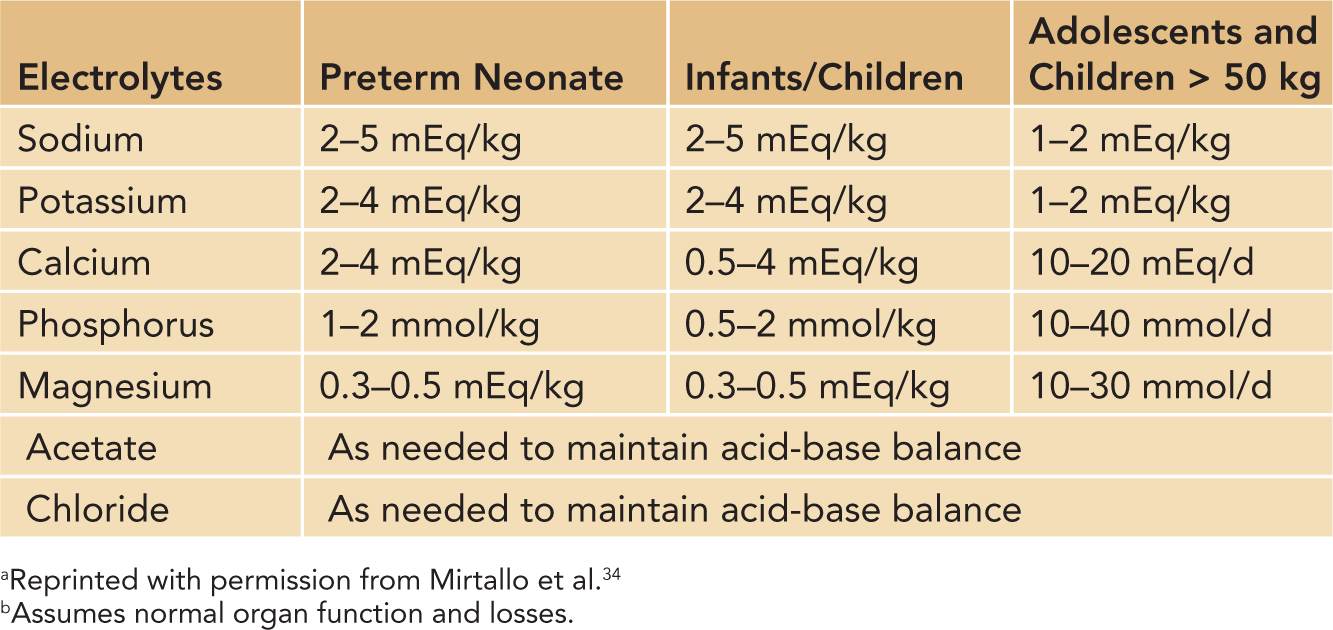
Calcium and phosphorus play a major role in neonatal PN because preterm neonates have not fully mineralized their bones. During the first 2 days of life, PN can contain less than maintenance phosphorus because of the concern of hyperphosphatemia. The maintenance dosing can be found in Table 70-2 but can also be listed as milligrams per liter, in which case it would be dosed at calcium 500 to 600 mg/L and phosphorus 350–500 mg/L of PN. The goal calcium-to-phosphorus ratio is 1.3 to 1.7:1. The difficulty with calcium and phosphorus administration is the concern of solubility. The solubility curves of calcium and phosphorus are multifactorial, but it has been clearly established that the pH of the solution plays a key role, so increasing the amount of amino acids, and possibly adding cysteine, as well as increasing dextrose can help increase the solubility.21
Trace Elements
There is no reason to avoid providing trace elements to neonates requiring short-term PN. The concentrations and composition of pediatric formulations are similar. Recommended dosing can be found in Table 70-3. Individual trace element products would need to be used to meet trace element needs because commercially available multitrace products would not meet these needs.
Table 70-3 Trace Element Requirementa,b
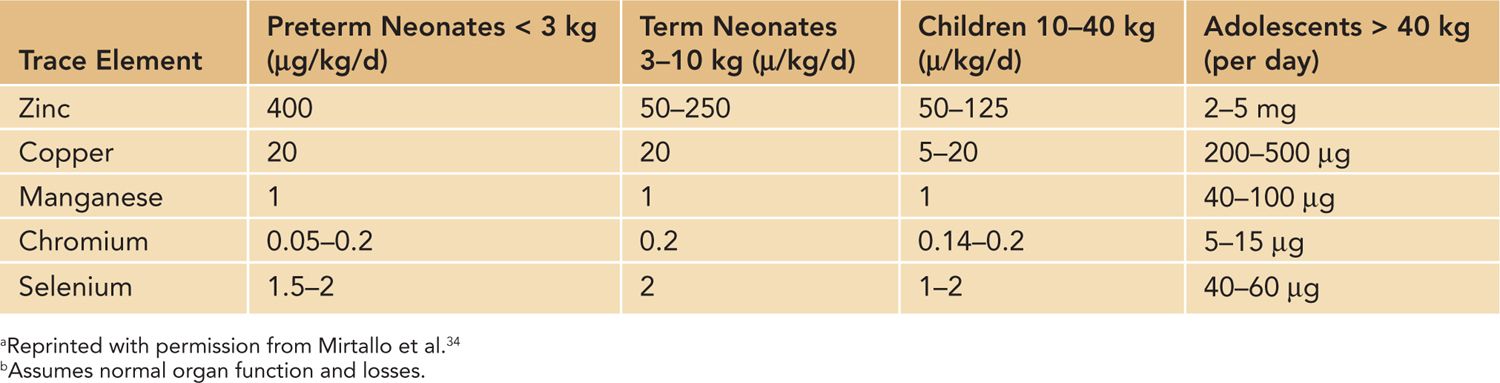
Zinc is important in cell growth and development. Additional zinc may be required in cases with elevated urinary and stool zinc excretion.
Copper is an essential component of several key enzymes. Additional copper may be required because of increased biliary losses secondary to a jejunostomy or biliary drain. Copper has been held from PN in patients with cholestasis, but cases of copper deficiency have been reported.22 If cholestasis develops, copper can be withheld from PN; then, monitoring copper and ceruloplasmin levels is crucial, and copper can be restarted individually if levels become deficient. Some recommend maintaining a dose of 20 µg/kg/d, but others have decreased the rate to only 10 µg/kg/day.22,23
Manganese is a component of several enzymes. Manganese deficiency has not been documented, although toxicity has been documented. Individually supplementing manganese should be done with caution, especially because it is already a contaminant of PN solutions. Manganese has been shown on magnetic resonance imaging (MRI) to deposit in the basal ganglia, leading to central nervous system symptoms such as Parkinsonism.
Chromium potentiates the action of insulin and has a key role in glucose, protein, and lipid metabolism. Chromium intake can be decreased in patients with renal impairment. Chromium is also a major contaminant of PN solutions and does not need to be routinely added to PN.
Selenium is a component of glutathione peroxidase. Supplementation is recommended in patients who continue on PN for longer than 30 days; levels should be monitored after that time. The dose should be decreased when renal dysfunction is present.
Multivitamins
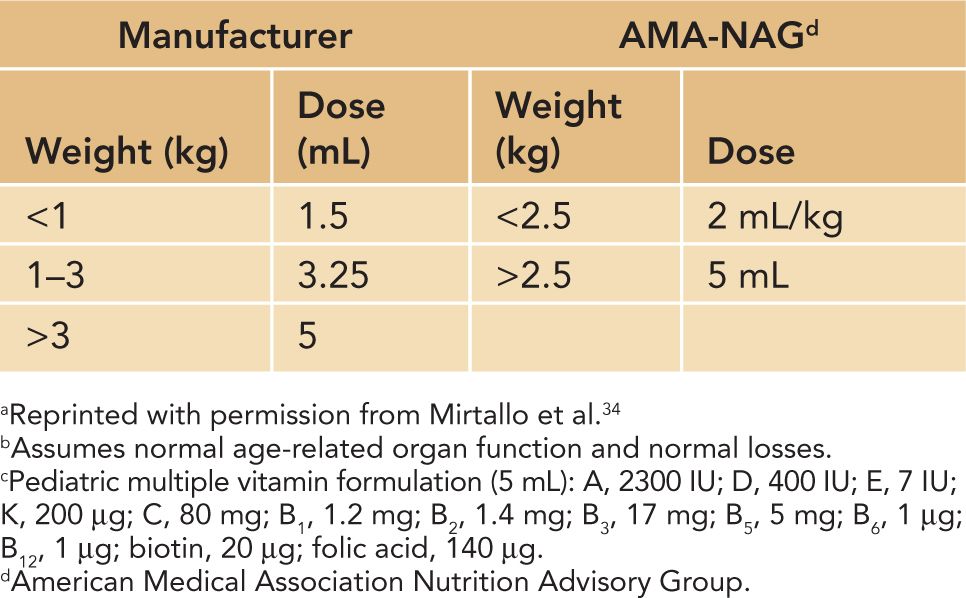
See Table 70-4 for recommended dosing of multivitamins.
Table 70-4 Daily Dose Recommendations for Pediatric Multiple Vitaminsa–c
MONITORING
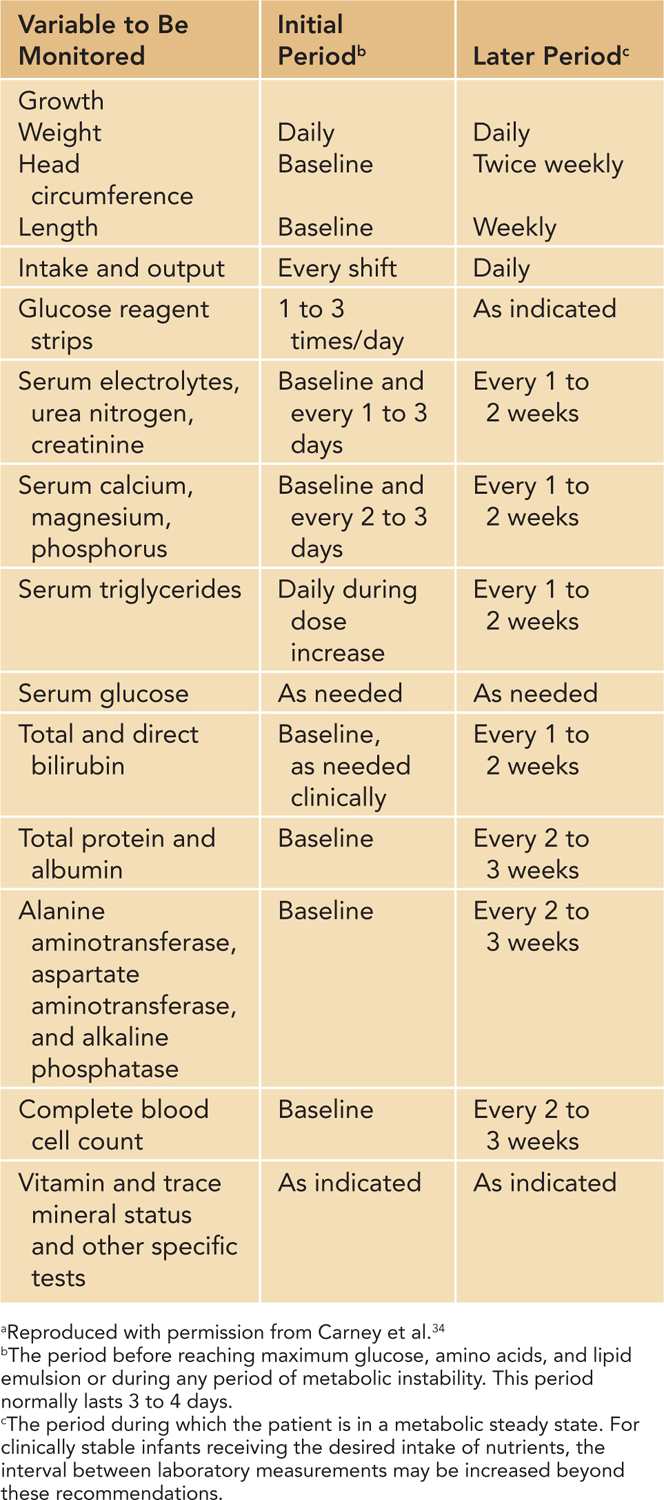
The guidelines for metabolic monitoring of neonates while on PN are shown in Table 70-5.
Table 70-5 Guidelines for Metabolic Monitoring During Parenteral Nutritiona
Catheter Occlusion
Central venous catheters used for the administration of PN can become occluded for several reasons. A thrombus or clot is the most common cause of a catheter occlusion. For this reason, it is recommended to add 0.25 to 1 U/L of heparin to the PN solution.24 If a thrombus is occluding the catheter, then 2 mg/2 mL alteplase can be instilled into the catheter at 110% of the catheter volume and left to dwell in the catheter for 30 minutes. If there is no response, leave in place for 2 hours; if no response, give a second dose, which can dwell up to an additional 2 hours. Lipid deposition can also occlude a catheter. In this case, 70% ethanol can be instilled in the catheter for 1 to 2 hours; the dose is 0.55 mL/kg (to a maximum of 3 mL). Calcium/phosphorus precipitate can be cleared from a catheter using 0.5 mL of 0.1 N hydrochloric acid. Acidic drug precipitate has been cleared with 0.2 to 1 mL of 0.1 N hydrochloric acid, and basic drug precipitate has been cleared from a catheter using 1 mL of 0.1 N sodium hydroxide or 1 mL of 1 mEq/L sodium bicarbonate.25
Cholestasis
Neonates are at high risk for developing cholestasis because prematurity, low birth weight, sepsis, and limited enteral intake are all risk factors. PN can increase this risk because manganese, copper, and aluminum can all have hepatotoxic effects. Recent studies suggested that the proinflammatory omega-6 polysaturated fatty acids in plant oil–based lipid emulsions (Intralipid, Liposyn III) and the presence of phytosterols contribute to the development of hepatotoxicity.26 Once a neonate on PN develops cholestasis, there are multiple strategies that can be implemented to help prevent and treat the cholestasis. The neonate must receive a pediatric amino acid formulation. Ensure that the neonate is not receiving excessive caloric intake. Decrease the fat emulsion to about 1 g/kg/d. Remove trace elements from the PN solution, while supplementing with zinc and selenium, then monitor copper and manganese serum levels and supplement individually if needed. Starting enteral feedings is the key to stimulate bile flow. Ursodeoxycholic acid 20 to 30 mg/kg/day given enterally is safe and effective and should be started as soon as possible once cholestasis is identified.27
Aluminum
Aluminum has long been known to be a contaminant of PN solutions. Toxicity from aluminum can manifest with neurologic impairment, decreased bone mineralization, microcytic anemia, and cholestasis. Calcium and phosphorus solutions have the most contamination, which puts premature neonates at highest risk because of their high demand for these minerals and poor renal clearance of aluminum by neonates. As a result, the Food and Drug Administration (FDA) mandated specific labeling requirements.28,29 Many organizations have also developed guidelines to help minimize the risk of aluminum toxicity.30,31 It has been shown that the FDA regulations cannot be met with the current PN solutions available.32 To minimize the risk, the PN solutions with the least contamination can be purchased and used by pharmacies.33
Weaning Parenteral Nutrition
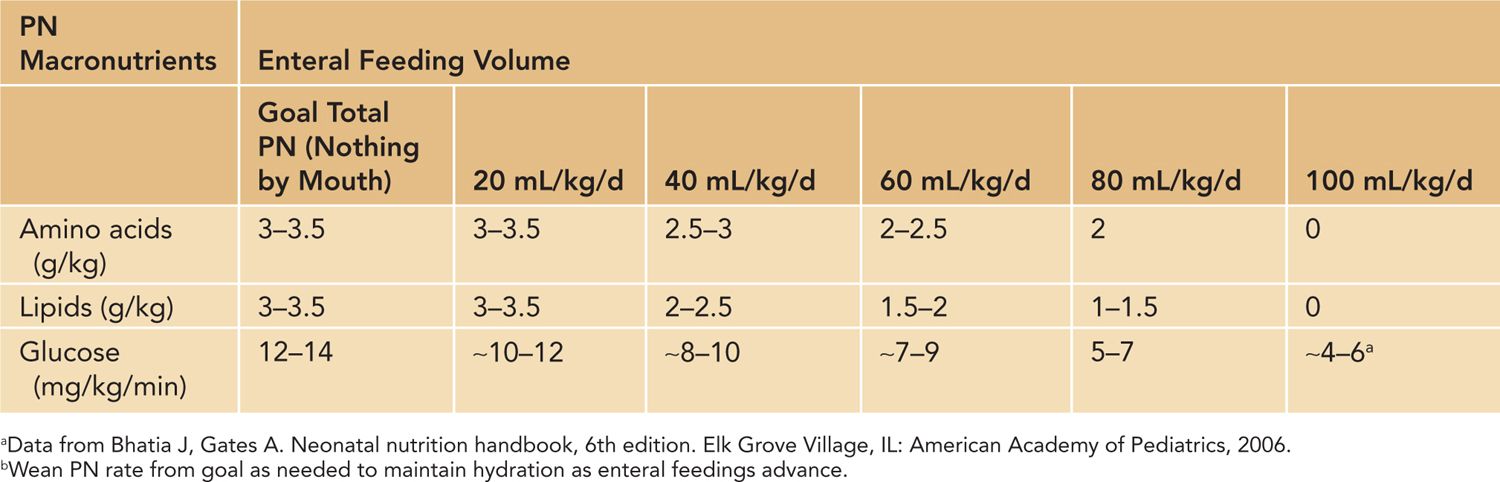
The ultimate goal of PN is to wean the infant from it and transition to enteral nutrition as quickly and safely as possible. Table 70-6 illustrates a tactic to wean PN as enteral nutrition is gradually increased, yet administer adequate nutrition.
Table 70-6 Weaning Parenteral Nutrition (PN)
Basic TPN Calculations
Guidelines for calculating GIR and for calculating macronutrient calories are shown in Table 70-7.



