Fig. 31.1
Modified flank positioning for laparoscopic procedures. (a) – Pediatric: In this picture, the child is positioned for right-sided surgery; note the padding and securing of the legs in a “figure 4” position with the bottom leg flexed and the top leg straight. Also, we test the bed by rotating it to the two extreme positions. (b) – Adolescent: In this picture the adolescent is positioned for left-sided surgery; note the padding and securing of the legs in a “figure 4” position with the bottom leg flexed and the top leg straight
Peritoneal access is obtained with a Veress or Hasson technique depending on surgeon preference; however, we caution against excessive force or manipulation during port insertion as to prevent tumor rupture, specifically in large renal tumors. For radical or partial nephrectomy, regardless of standard laparoscopic or robotic-assisted laparoscopic surgery, we prefer a periumbilical camera port and three working ports placed subxiphoid, in the midline infraumbilically, and in the ipsilateral lower quadrant (Fig. 31.2). When planning for adrenalectomy, we utilize a periumbilical camera port with working ports placed subxiphoid and in the ipsilateral midclavicular line at the level of the umbilicus. Additional ports may be placed infraumbilically for the 3rd robotic arm or in the contralateral upper quadrant for an assistant port (Fig. 31.3). Last, for RPLND, we use a periumbilical camera port and three working ports placed subxiphoid, in the midline infraumbilically, and in the ipsilateral midclavicular line. Additional assistant ports may be inserted in the contralateral midclavicular line to allow for retraction and suction (Fig. 31.4).
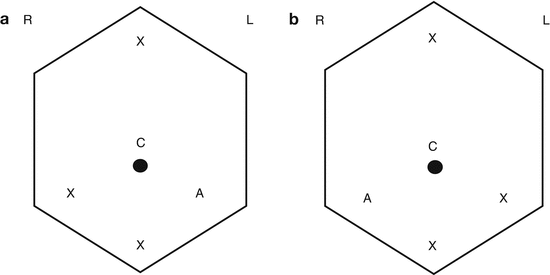
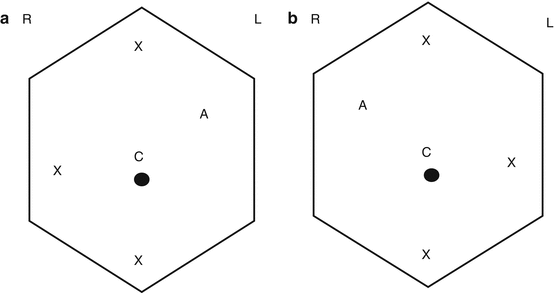
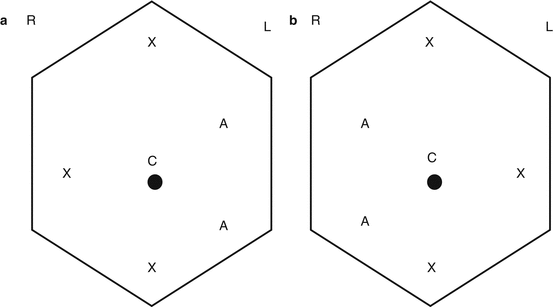

Fig. 31.2
Port placement for radical and partial nephrectomy. (a) – Right nephrectomy. (b) – Left nephrectomy. R Right, L left, C camera port, X working port, and A assistant port. Robotic working ports are 8 mm, standard laparoscopic working ports are 12 mm, and assistant ports are 12 mm. Of note, a robotic working port can be placed through a 12 mm standard laparoscopic port in case a standard laparoscopic instrument (i.e., vascular stapler) needs to be used via these ports

Fig. 31.3
Port placement for adrenalectomy. (a) – Right adrenalectomy. (b) – Left adrenalectomy. R Right, L left, C camera port, X working port, and A assistant port. Robotic working ports are 8 mm, standard laparoscopic working ports are 12 mm, and assistant ports are 12 mm. Of note, a robotic working port can be placed through a 12 mm standard laparoscopic port in case a standard laparoscopic instrument (i.e., vascular stapler) needs to be used via these ports

Fig. 31.4
Port placement for RPLND. (a) – Right RPLND. (b) – Left RPLND. R Right, L left, C camera port, X working port, and A assistant port. Robotic working ports are 8 mm, standard laparoscopic working ports are 12 mm, and assistant ports are 12 mm. Of note, a robotic working port can be placed through a 12 mm standard laparoscopic port in case a standard laparoscopic instrument (i.e., vascular stapler) needs to be used via these ports
Again, due to concern for tumor rupture, we encourage that all ports be placed under direct vision. All ports should be capable of accommodating a laparoscopic vascular stapler or should be convertible to accommodate a stapler to control the hilar vascular structures. In the setting of a robotic approach, we utilize all three of the working ports for robotic instruments, and so an additional assistant port can be placed to permit suction, retraction, etc. On a note about the port-site management, in many cases non-dilating trocars are utilized, and specifically the robotic working ports are non-dilating trocars. While there is literature to support either closing or not closing the fascia on laparoscopic port sites, it is our opinion that when using the relatively large-sized non-dilating robotic trocars and other trocars used to accommodate laparoscopic vascular staplers, closing the fascia of these incisions is beneficial to reduce the risk of port-site incisional hernia.
Standard postoperative care consists of intravenous (IV) fluid resuscitation, adequate pain control, resumption of a progressive diet on postoperative day 0, and 24 h of antibiotic coverage. As a note on the diet after RPLND, we commonly recommend a “no fat” diet for the 2 weeks after surgery to reduce the risk of a postoperative chyle leak. Our dieticians work with the patient and family on the appropriate education for this diet. The urinary bladder catheter is typically removed postoperative day 1 and activity encouraged. If a closed-suction surgical drain is left after partial nephrectomy, it can be pulled after the bladder catheter is removed, spontaneous voiding is resumed, and no increased drain output is observed. In cases of concern, the drain fluid may be sent for a creatinine level and compared to the serum level. Most reports describe a 2- to 3-day postoperative hospital stay after these laparoscopic or robotic-assisted laparoscopic surgeries.
Nephrectomy
The potential indications for minimally invasive radical nephrectomy (RN) in pediatric or adolescent patients include the most common primary renal tumors: WT, RCC, mesoblastic nephroma, and multilocular cystic nephroma. Additionally, there are reports of its use for both tumors of uncertain malignant potential, such as IMTs, and rare entities such as non-osseous Ewing’s sarcoma [30, 31]. As we lack level-one evidence to recommend minimally invasive renal surgery in this population, the risks and benefits must be discussed in this context and carefully described to the patient and family. Additionally, it is important that regardless of the indication, a full multidisciplinary discussion be undertaken preoperatively.
In keeping with the limited available data, we would recommend the following general guidelines for minimally invasive RN: (1) When WT is highly suspected based on age, medical history, or genetic factors, we recommend presurgical chemotherapy be strongly considered. (2) As with all MIS, in an attempt to replicate the open surgery, we recommend a transperitoneal approach which includes full exploration of the peritoneal cavity and a thorough regional LN sampling. (3) While the port-site incisions allow for a potentially improved cosmetic result, a generous Pfannenstiel incision is recommended for intact kidney/tumor extraction to prevent tumor rupture during manipulation. (4) Similarly, an appropriately sized endocatch bag should be used to prevent tumor spillage. (5) Given that tumor size is associated with tumor spill [32] and that tumor size can be reasonably appreciated on preoperative imaging, we would suggest that only smaller, low-stage tumors, with no signs of locally advanced disease (i.e., no LN enlargement or venous tumor thrombus) be approached in this manner. Specifically, after a review of the most extensive experience with minimally invasive RN in this population, Duarte et al. recommend this approach be reserved for masses with the largest tumor diameter of <10 % of the patient’s height [4]. (6) Last, the oncologic demands of the case take the highest priority and conversion to open surgery should be considered if any concerns arise.
We next proceed with a full exploratory laparoscopy to look for any signs of tumor dissemination. On right-sided cases we recommend liver retraction. Care must be taken if an external, fixed instrument is used as inadvertent movement can lead to liver injury. Alternatively, we have used a technique of passing a vessel loop or flexible guidewire through two percutaneously placed 14 gauge angiocatheters. The vessel loop or wire is positioned underneath the liver and secured outside the body on some tension with hemostats to provide liver retraction. Commonly, aggressive dissection of the lateral hepatic attachments is beneficial to reveal the upper retroperitoneum, especially for upper pole tumors. The colon is then mobilized medially and the retroperitoneum is exposed after taking down the root of the mesentery and “kocherizing” the duodenum to reveal the inferior vena cava (IVC). At this point the ureter should be identified over the psoas muscle at the level of the great vessel bifurcation. Using one instrument to gently support the ureter superiorly (taking care to avoid injury to the gonadal vessels), the dissection proceeds cranially working along the IVC until the gonadal vein insertion, which we recommend preserving if possible. Continuing in this manner, the renal vein is then encountered.
On the left side we recommend aggressive dissection of the lateral splenic attachments as with appropriate medial mobilization of the colon, the spleen will fall with gravity and provide excellent exposure of the left upper retroperitoneum. Similar to the right side, the dissection starts by identifying the ureter. On the left, the dissection proceeds along the lateral side of the aorta until the left renal vein is seen crossing the aorta. Additionally, the left gonadal vein can be traced to its insertion into the left renal vein.
Next, the dissection should proceed by “sweeping the knee” and elevating the kidney and ureter within Gerota’s fascia superiorly off of the psoas fascia. The robotic assistance of the 3rd arm or an assistant retracting or supporting the kidney and ureter is helpful to allow for two instruments to work at this objective. This visualization with superior elevation of the kidney will allow for identification of the renal vein anterior and the artery posterior. If identified at this point, it is typically a single artery, yet to branch; however, it is worth being always mindful for aberrant renal vessels. Prior to taking the vessels, we recommend completely dissecting them out to allow full visualization. This may require removing some lymphatic tissue surrounding the renal vessels. This should be kept for the final LN specimen. Once these vessels are prepared, we recommend taking the renal artery first, followed by the vein, both with a vascular stapler. Prior to taking the vein, the potential for tumor thrombus should be considered. If there is any concern, laparoscopic ultrasound can be of great assistance. Also, prior to firing the stapler the distal tip must be fully visualized as to not inadvertently entrap other structures. A laparoscopic suction device and laparoscopic sponge-tip instruments should be open on the field so that in case of stapler misfiring, vascular control can be achieved. After the hilar vascular structures are taken, the dissection continues cranially and may or may not include removal of the adrenal gland. After completing the superior dissection, taking great care to remain outside of Gerota’s fascia, the lateral attachments are taken. Next, the ureter should be dissected down to at least the level of the iliac vessels, and if there is concern about ureteral tumor extension, down to the bladder. The ureter can be taken with a clip or suture.
The specimen should immediately be placed in a laparoscopic endocatch bag which is then closed to prevent tumor spillage or soiling. Next, attention should be given to LN sampling. We recommend, at a minimum, removing all lymphatic tissue from the ipsilateral great vessel from the level of the bifurcation to above the renal hilum. The technique is similar to that described for RPLND where one instrument serves to elevate the LN packet as it is “split” over the anterior surface of the great vessel. The packet is then retracted as another instrument(s) dissects this free of the vessel. The robotic assistance of the 3rd arm or an assistant retracting the LN packet is helpful to allow for two instruments to work at this objective. These LNs should be placed in an endocatch bag and sent to pathology fresh as a separate specimen.
To remove the specimens, we recommend grasping the endocatch bag string through the ipsilateral lower quadrant port under laparoscopic visualization. This port can then be removed and its incision generously extended laterally and medially to accommodate removal of the kidney/tumor in the endocatch bag. This fascia may be closed and pneumoperitoneum reestablished to allow for a final inspection of the resection bed. We recommend irrigation with warmed sterile water, taking care to ensure hemostasis under conditions of reduced pneumoperitoneum. The patient bed can be rotated to allow the colon to fall back laterally and a final look with the laparoscope ensures there is no malrotation of the small bowel mesentery.
Partial Nephrectomy
(Please refer to accompanying Video 31.1)
The potential indications for minimally invasive nephron-sparing surgery (NSS) in pediatric or adolescent patients would mirror indications for open NSS. That is, the approach to NSS should not supersede the oncologic considerations. Thus, the currently reported indications include WT (bilaterally, in a solitary kidney, or in a patient with a WT predisposition syndrome) and RCC [7–9]. As with minimally invasive RN, given the current lack of level-one evidence to recommend minimally invasive renal surgery in this population, the risks and benefits must be carefully described in this context to the patient and family. In addition to our previous recommendations on minimally invasive renal surgery, if NSS is considered for WT it should be done utilizing the applicable COG or SIOP protocols following presurgical chemotherapy. Additionally, approaching a renal mass with NSS does not change the necessity of LN sampling. Lastly, we would recommend that minimally invasive NSS be reserved for ideally located masses (exophytic, noncentrally located) and for surgeons experienced with minimally invasive NSS.
The surgery begins with full exploratory laparoscopy followed by exposure of the retroperitoneum and great vessels with appropriate hepatic or splenic retraction as mentioned in the section on RN. The deviation from the RN approach is to start with the LN sampling prior to mobilizing the kidney. This is done by removing all of the lymphatic tissue from the ipsilateral great vessel from the level of the bifurcation to above the renal hilum. The technique is similar to that described for RPLND where one instrument serves to elevate the LN packet as it is “split” over the anterior surface of the great vessel. The packet is then retracted as another instrument(s) dissects this free of the vessel. The robotic assistance of the 3rd arm or an assistant retracting the LN packet is helpful to allow for two instruments to work at this objective. These LNs should be placed in an endocatch bag and sent to pathology fresh as a separate specimen. Complete LN dissection at this stage of the procedure is beneficial as it allows for clear visualization of all renal hilar structures and facilitates the vascular control for NSS.
We next completely mobilize the kidney as with RN. Then, using laparoscopic ultrasound guidance we delineate the location and extent of the mass. Gerota’s fascia is opened over the mass, and with a generous margin we dissect down to the renal parenchyma and slowly dissect the perirenal capsular tissue away from the normal kidney to expose the mass. Again, using laparoscopic ultrasound guidance, we note the extent of the mass and cauterize this outline on the capsule. We then turn our attention to renal vascular control and start by identifying the artery and vein and placing vessel loops around them with a clip and a short tail for rapid identification. Clamping the vessels for NSS is a matter of surgeon preference and experience. We prefer to clamp the renal artery with laparoscopically applied internal vascular clamps via the assistant port.
The fat overlying the mass is left in place to be sent with the specimen. Next, working with two instruments and suction from a bedside assistant, the mass is resected using cold scissors and direct visualization to reduce the potential for inadvertent tumor transection. A tip for this is to use two insufflators to allow for aggressive suctioning without losing pneumoperitoneum. Also, an additional port or the robotic 3rd arm should be utilized if it can be of assistance to hold the kidney or mass during the resection. The resection and tumor positioning should be planned out prior to vascular clamping so that the operative time under ischemic conditions is minimized. We then close any opened collecting system as needed and obtain hemostatic control with directed “figure of 8” sutures for exposed, transected vessels, applying thrombin gel, a surgical cellulose bolster, and separate renorrhaphy sutures through the renal capsule using the “sliding-clip” technique [33]. The vascular clamps are then removed. Of note, we typically use a dose of IV mannitol immediately before and after vascular clamping. The mass and nodes are placed in an extraction bag and removed via the lower quadrant port with adequate extension as described for RN. Specimens must be sent for frozen pathologic analysis to determine the need for additional resection and to ensure a negative margin. The resection site should be reinspected after reducing pneumoperitoneum and hemostasis ensured. A closed-suction drain can be left per surgeon preference.
Adrenalectomy
Neuroblastoma is the most common childhood adrenal malignancy and thus is the most common oncologic indication for MIS adrenalectomy. However, during the workup of an adrenal mass, less common entities such as adrenocortical carcinoma or pheochromocytoma may be encountered and could be reasonably approached by MIS. While initially, MIS was utilized as a diagnostic tool to biopsy pediatric adrenal tumors, there is increasing experience with therapeutic resection. For small, low-stage tumors with no evidence of invasion on preoperative imaging, minimally invasive resection may be considered if adhering to surgical oncologic principles. More specifically, size greater than 6 cm, enlarged veins, and involved adjacent organs or vessels are relative contraindications to MIS adrenalectomy for neuroblastoma [16].
As for the surgical approach, MIS adrenalectomy can be performed either transperitoneally or retroperitoneally, with multiple published reports describing both techniques. We prefer a transperitoneal approach as it serves to recapitulate the open surgery and the remaining report will focus on this technique. Additionally, given the varied venous drainage of the left and right adrenal, many surgeons consider left adrenalectomy to be less technically challenging. However, both left and right MIS adrenalectomy are feasible.
The surgery begins with exploratory laparoscopy. Subsequently, for right-sided cases, dissection of the lateral hepatic attachments and retraction of the liver is necessary. After identifying the hepatic flexure and upper pole of the kidney, the colon is reflected medially so as to expose the duodenum and the superior two-thirds of the kidney. The duodenum is “kocherized” medially to expose the IVC. We prefer to use a laparoscopic, expandable fanned retractor via an assistant port to rotate and retract medially on the IVC. At this point, dissection continues by creating a plane superior to the renal vein down to where the psoas muscle is visualized. Prior to starting this dissection, it is important to review the preoperative imaging and remain cognizant of the potential for upper pole renal vessels which should not be sacrificed. Next, using this plane to “sweep the knee” and elevate the upper pole of the kidney and the adrenal, small pillars of tissue are dissected along the lateral IVC and taken using a laparoscopic bipolar tissue-sealing device. This dissection is continued along the IVC as an assistant medially retracts and rotates the cava until the adrenal vein is encountered. This may be taken with a sealing device, hemostatic clips, or a vascular stapler per the surgeon’s preference. Next, we incise Gerota’s fascia along the upper pole of the kidney but leave posterior attachments intact to allow for inferior retraction of the kidney and subsequent movement of the adrenal. Additionally, the lateral attachments are kept to prevent it “falling down” into the working field. Once the adrenal is completely freed along the medial aspect, alongside the IVC, we use a bipolar sealing device to complete the superior and lateral dissection while inferiorly retracting the adrenal via its remaining renal attachments. The last step is taking the inferior, posterior renal attachments.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


