Fig. 10.1
Duplicated left collecting system; complete left ureteral duplication with obstructed superior moiety ureter (Courtesy of Matthew Timberlake)
Routine postnatal evaluation of duplication anomalies should include renal ultrasound (RUSD) and voiding cystourethrogram (VCUG) studies. RUSD can diagnose duplex kidneys, hydronephrosis, dilated ureters, and a ureterocele. Voiding cystourethrography can be used to detect vesicoureteral reflux and better characterize the ureterocele. In many renal duplication cases, the superior moiety is frequently dysplastic and is thus a small contributor to overall renal function. Nuclear renal scans are used to determine the differential function between the superior and inferior moieties. In the case of a poorly or nonfunctioning superior moiety, an extirpative procedure/partial nephrectomy with ureterectomy may be considered. However, where surgery is required but both the superior and inferior moieties contribute substantially to normal renal function, every effort should be made to preserve renal function to both moieties. For the purposes of this chapter, the author will focus on the scenario with a duplex system including complete duplication of the ureters and normal renal function to both the superior and inferior moieties but with a distal obstruction (ureterocele or ectopic insertion) associated with the superior moiety and a non-refluxing inferior moiety.
Indications
1.
Ureteral stricture disease (e.g., iatrogenic, polyps, congenital)
2.
Complete ureteral duplication
(a)
Obstructed superior (or inferior) moiety with non-obstructed and non-refluxing inferior (or superior) moiety
(b)
Inferior moiety with high-grade vesicoureteral reflux and non-obstructed and non-refluxing superior moiety
Contraindications
1.
Complete ureteral duplication with obstruction and/or reflux of both ureters
Preoperative Preparation: In addition to imaging and urine studies, all patients are given a diet of 24 h of clear or low-residue liquids depending on patient age to help reduce the bulk of stool in the colon. Patients are also given a suppository the night before.
Specific Instrumentation: The case description that follows is that of a robotic-assisted laparoscopic (RAL) U-U (see accompanying Video 10.1), which this author prefers; however, similar instrumentation and room setup can be used for a pure laparoscopic approach.
1.
Equipment
(a)
Infant or pediatric cystoscopy equipment
(i)
Appropriately sized ureteral catheters, double-J ureteral stents, and guide wires
(b)
da Vinci® robotic system
(c)
Robotic ports
(i)
8.5 mm or 12 mm camera cannula
(ii)
2 × 5 mm or 8 mm cannulae
(d)
Robotic instruments
(i)
5 mm
1.
Hook cautery
2.
Grasper (preferably Maryland type)
3.
Fine-tip needle driver
4.
Curved and round-tip scissors
(ii)
8 mm
1.
Same as above
2.
Curved “hot” scissors
(e)
Conventional laparoscopic instruments
(i)
Needle driver
(ii)
Grasper
(iii)
Irrigation/aspiration device
2.
Room and patient setup (Fig. 10.2)
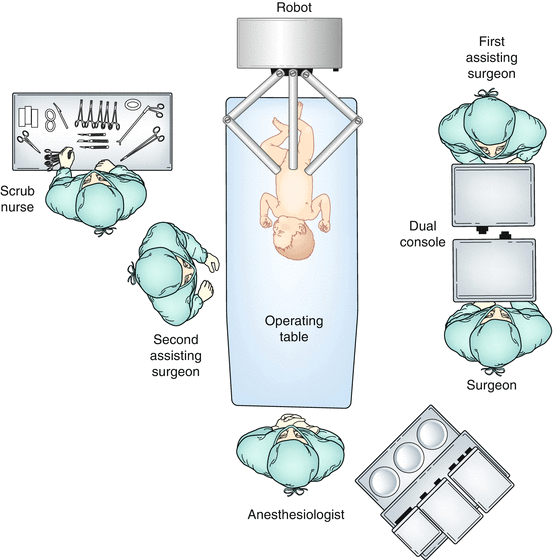

Fig. 10.2
Room setup for RAL ureteral surgery
(a)
Patient position – supine and secured to table to allow for table rotation and tilting prior to robot docking
(b)
Robot position – modified-side-, side-, or end-dock near the foot of the table depending on robot model and size of patient
Operative Technique
1.
Cystoscopy and retrograde imaging is performed with the patient in lithotomy position. A ureteral catheter is advanced into the recipient (ipsilateral non-obstructed) ureter; stent size can be determined at this time. Once this is completed, the ureteral catheter is then secured to a latex-free bladder catheter with a silk suture.
2.
Following catheter placement, the patient is repositioned supine and slightly frog-legged at foot of the table with all pressure points padded and then secured in position (Fig. 10.3). The patient is prepped and draped in a standard surgical fashion with the ureteral and bladder catheters included in the prep.
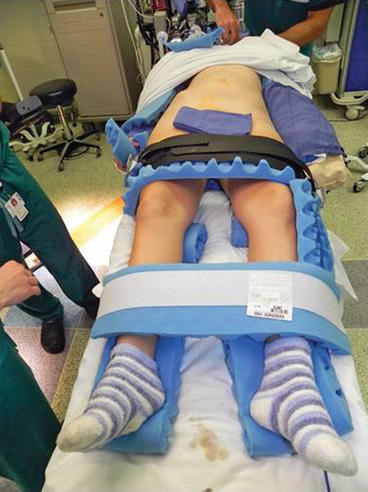

Fig. 10.3
Patient positioning and padding for RAL ureteral surgery (Courtesy of Patricio Gargollo)
3.
An 8.5 mm (or 12 mm, depending on size of the child) trocar is then placed through the umbilicus in a modified Hasson fashion. A 3–0 polydioxanone suture is placed through the fascia to help hold the trocar in place during surgery and to facilitate wound closure at the end of the procedure. The abdomen is then insufflated. Two 5 mm trocars are then placed under direct laparoscopic vision in the midclavicular line on both sides and 2–3 cm below the umbilicus. These two trocars can be moved more cephalad depending on the age of the infant/child to provide more room to operate intracorporeally (Fig. 10.4).
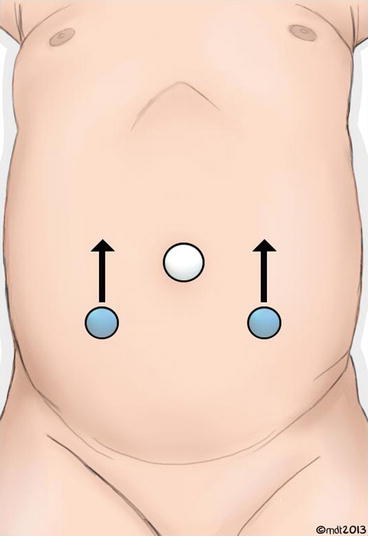

Fig. 10.4
Trocar positioning for RAL ureteral surgery. Lateral trocars can be adjusted cephalad depending on size of patient per arrows (Courtesy of Matthew Timberlake)
3–0 polydioxanone suture is again pre-placed through the fascia to facilitate wound closure at the end of the procedure. The table is then placed in 10–15° Trendelenburg position and rotated slightly so that the operative side is elevated (Fig. 10.5). The robot is then docked (Fig. 10.6).
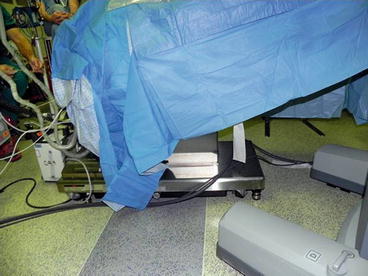
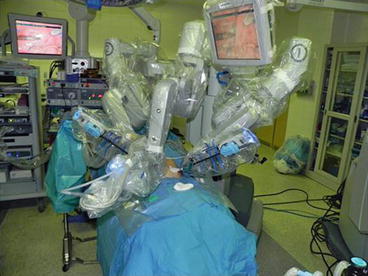

Fig. 10.5
Operative table rotated and placed in Trendelenburg position (Courtesy of Patricio Gargollo)

Fig. 10.6
da Vinci® modified side-dock position for RAL ureteral surgery (Courtesy of Patricio Gargollo)
4.




The ureters are approached in a transperitoneal fashion. A robotic grasper (Maryland preferred) and hook cautery are utilized through the 5 mm ports. The ureters are easily identified through the peritoneum and exposed by incising the peritoneum where the ureters cross the iliac vessels. The ureters are mobilized proximally and distally for approximately 2–3 cm in either direction using blunt dissection and electrocautery. Care is taken to avoid devascularization of either ureter. The ureteral catheter is visualized in the recipient ureter which is mobilized free from the donor (ipsilateral obstructed) ureter just caudal to the level of the iliac vessels. The hook cautery is then replaced with robotic curved scissors. The dilated ureter is then divided. If obstructed and refluxing, then the distal end of the divided donor ureter is ligated using polyglactin suture; otherwise, the distal end can be left alone.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


