Fig. 28.1
Callus patterns
Physical Stimulation
Mechanical Stimulation
Controlled mechanical stimulation is considered to be an important stimulus for the regenerate in distraction osteogenesis. There are preclinical and clinical evidences that physiologic weight bearing stimulates new bone formation and consolidation of new bone in distraction osteogenesis [27–30]. Controlled compressive but not shear force has been shown to affect the biologic nature of the healing response by early expression of BMP 2/4 and the messengers for collagen 1 and osteocalcin [31] and increase the size and strength of the regenerate [30, 32].
“Pumping” of the regenerate (also referred to as the accordion maneuver)—the procedure of alternative compression and distraction—is a well-known method to stimulate new bone formation in the clinical situation of poor regenerate [33, 34]. But, from the literature review, we found that it is still a conflicting issue. Mofid et al. [34], in an animal model of mandibular distraction, performed daily alternative compression and distraction at an amplitude of 1 mm/day for 3 weeks, and observed improved bone healing in terms of cortical thickness, cortical:cancellous bone ratio, and mineral apposition. In contrast, Greenwald et al. [33], in an animal model of mandibular distraction, distracted to a length of 2.5 mm (at a rate of 0.25 mm twice daily) for 5 days, then compressed 1.0 mm for a 2-day period, and redistracted to a length of 5 mm. They demonstrated no significant differences between experimental (“pumping”) and control group in radiological and histological parameter. The timing of oscillating motion is also a conflicting issue. Kassis et al. [35] showed that the effect of axial micromotion is effective only after the end of elongation rather than during active distraction. Further investigations are necessary to establish the effect and the optimal protocol for “pumping” including the timing, amplitude of sequential movement, and frequency.
Rhythm of distraction is another conflicting issue for enhancing bone regenerate. Ilizarov in an animal model demonstrated better callus in motorized distraction group (1/60 mm, 60 times/day) compared with the standard rhythm (1/4 mm, 4 times/day) [36, 37]. Mizuta proved that an increase in the distraction frequency (eight steps vs. four steps) in open-wedge osteotomies of the proximal tibia with hemicallotasis provides better bone formation, resulting in a shorter external fixation period [38]. Meanwhile, there have been reports that the rhythm of distraction does not significantly influence bone healing [39, 40]. Lengthening procedure is not confined to the bone and soft tissues are also important. Makarov et al. demonstrated in a goat model that a more fractionated rhythm of distraction was associated with enhanced preservation of muscle fibers and greater regenerative activity of the muscle [41]. Further investigation is necessary to clarify the benefit of the more fractionated distraction, and the optimal rhythm.
Low-Intensity Pulsed Ultrasound
Low-intensity pulsed ultrasound (LIPUS) is a form of mechanical stimulation that is delivered as high-frequency acoustic pressure waves. The physical process through which LIPUS stimulates bone healing remains unclear. It is speculated that ultrasound enhances fracture healing by inducing low-level mechanical forces at the fracture site, reproducing the effect of functional loading [42]. The mechanisms through which the mechanical signal is translated into a biochemical signal have not been fully understood but ultrasound seems to influence certain cellular reactions involved in each phase of the healing process such as inflammatory reaction, angiogenesis, chondrogenesis, intramembranous ossification, endochondral ossification, and bone remodeling [42, 43]. LIPUS is one of the best studied treatment modalities in distraction osteogenesis, but the results from preclinical experiments are still conflicting regarding its efficacy [44, 45]. There are well-designed clinical studies for the acute fracture healing, suggesting that LIPUS can be beneficial in the treatment of certain non-operatively treated fracture by shortening the time to radiological and clinical union [46–48]. Most successful reports with LIPUS are in the treatment of conservatively managed acute fractures. There are only scanty evidences for surgically treated fracture and most of them reported disappointing results [49–51]. Emami et al. concluded that low-intensity ultrasound treatment did not shorten healing time in acute tibial fractures treated with a reamed and statically locked intramedullary nail [49].
Pulsed Electromagnetic Field
Most of the available studies regarding pulsed electromagnetic field (PEMF) are related to its effect on the treatment of acute fractures [54–56] and suggest that PEMF may be beneficial in the treatment of certain non-operatively treated fractures. However, there are only a few reports about its effect on distraction osteogenesis [11, 56–58]. Though these studies are reporting supportive effect on enhancing callus healing, further high-level studies in human subjects are necessary before we can recommend PEMF as a valid treatment option.
Box 28.2 Biologic Stimulants
Local application
BMP
BMAC/PRP
Stem cells
Experimental—TP508, ED71, FGF-2
Systemic application
Parathyroid hormone
Growth hormone
Experimental—hyperbaric oxygen, androgens
Biologic Stimulation
Local Application-Bone Morphogenetic Protein
The BMPs are known to have a strong osteoinductive potential [59–61]. There are several preclinical studies on the effect of BMPs on distraction osteogenesis [5, 62–65]. Mizumoto et al. [65] used a single injection of rhBMP-7 into osteotomy gap on the day of surgery in a rat DO model and observed a larger amount of new bone throughout the distraction phase. Hamdy et al. showed that application of OP-1 early during the distraction phase in rabbit tibiae accelerated new bone formation by densitometry and micro CT analysis [62, 64]. Also, Song et al. [5] demonstrated enhanced bone formation by injection of BMP-2 at the end of distraction in rat femora. Several clinical studies have been reported using BMP-2 or BMP-7/OP-1 for open tibial fractures and spinal fusion [66, 67], but not for distraction osteogenesis so far. There are conflicting issues about the risk of cancer by using rhBMP-2 [68–73]. TGF-beta ligand is known to have the ability to alter cell signaling that can lead to tumor promotion, and certain BMPs are expressed during the growth of some types of cancer [68, 69, 71, 73–76]. Further investigations for optimal dose, optimal timing, and potential risk of various BMPs are required before they can be recommended for clinical use.
Bone Marrow Cells/Platelet-Rich Plasma
Osteogenic differentiation from bone marrow-derived mesenchymal stem cells (MSC) has several advantages when compared to the differentiation of other mesenchyme tissues [77]. First, bone marrow cells (BMCs) have abundant mononuclear cells. Second, the differentiation of osteoblasts from BMCs is well described and standardized. Third, due to the autogenous properties the complications such as infection, immunogenic reactions, and disease transmissions can be minimized. Fourth, the harvesting process of bone marrow cells using a Jamshidi vacuum aspiration is minimally invasive and has low donor-site morbidity. Lastly, we are not aware of any reports of malignant transformation of autologous BMCs in the literature [77]. Though a lot of animal studies using bone marrow transplant have shown that bone marrow has enriched osteoblast progenitor cells and it is a valuable bone graft in bone defect, there have been only limited investigations on its application in distraction osteogenesis [78, 79].
The PRP is abundant in various growth factors released from platelets, which have been known to increase vascular ingrowth and mitogenic effects on bone-forming cells [80–83]. Kawasumi et al. [82] observed that a high platelet concentration combined with osteoblastic cells in PRP accelerated new bone formation during distraction osteogenesis in a rat model. In contrast to these findings, Arpornmaeklong et al. [84] noted an inhibitory effect of PRP on osteogenic differentiation of marrow-derived pre-osteoblasts.
There have been few clinical studies using PRP in human subjects [85–87]. Latalski et al. [85] reported a shorter healing index in the PRP group in a retrospective human DO study with a small sample size. The role of PRP in bone regeneration is still a controversial issue and its mechanism is not yet fully understood [84, 85, 88–98]. BMCs and PRP have been used simultaneously showing favorable clinical results. Kitoh et al. [78, 99] studied transplantation of culture-expanded bone marrow cells combined with PRP that accelerated new bone formation during the distraction osteogenesis, especially in the femur.
Lee et al. [86] showed that the combined injection of bone marrow aspirate concentrate (BMAC) and PRP during surgery enhanced bone regeneration in human tibial distraction osteogenesis (Fig. 28.2). The difference in the abovementioned studies conducted by Kitoh et al. and Lee et al. lies in how to process BMC-culture expansion or concentration. Jäger et al. [77] described the strength of the concentration compared with culture-expanded method. An immediate autologous transplantation of bone marrow concentrate can prevent complications related to the reduced quality of the transplanted cells such as pre-aging due to telomere shortening, reduced viability, or dedifferentiation/reprogramming that is associated with in vitro cultivation. The risk of infection is reduced by decreasing the ex vivo time period; and injection can be performed by single-stage surgery. Although there is only limited clinical evidence, BMAC and/or PRP seems to be a promising treatment option. Though all these studies show a favorable prophylactic role of BMAC/PRP in enhancing the regenerate in DO, their role in established delayed/nonunion after DO is uncertain and warrant further studies.
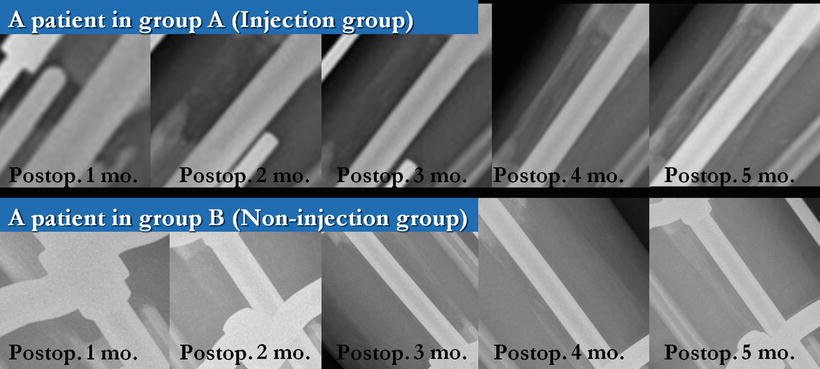

Fig. 28.2
Serial anteroposterior plain radiographs (postoperative 1–5 months, monthly) of the tibia in a patient undergoing lengthening over nail. More callus regeneration was seen in the injection group (group A). Images in group A were obtained from a patient in an injection group, showing postoperative 1- to 5-month states with an increment of 1 month. Images in group B (non-injection group) were from a patient in a control group with the same postoperative months above
Well-designed preclinical and clinical studies are necessary, especially to evaluate whether BMAC or PRP contributes more to bone formation—or if the effect is synergistic, the optimal timing of injections and the optimal concentrations and cell counts.
Stem Cells
There have been many debates about using biotechnology on fracture healing but there is little scientific evidence about the positive effect of tissue engineering on osteogenesis. Tissue engineering combines osteogenic bone marrow mesenchymal stem cells, synthetic scaffolds, and growth factors in order to form hybrid constructs. Stem cells have been used for other indications including the treatment of long bone defects [100], fracture healing [101], and avascular necrosis [102] apart from enhancing the regenerate in distraction osteogenesis.
Stem Cells
Stem cells can be of two types:
1.
Bone marrow-derived mesenchymal stem cells (MSC)
2.
Osteogenic differentiated progenitor cells
Bone marrow-derived mesenchymal stem cells (BMC) can be directed towards the osteogenic lineage if cultured with osteogenic supplements like dexamethasone, β-glycerophosphate, and ascorbic acid phosphate [103]. In a study done by Peters et al. [104] in rats using histomorphometric analysis, it has been shown that locally applied osteogenic differentiated progenitor cells are more effective than mesenchymal stem cells in enhancing bone healing.
Growth Factors
Growth factors may be added to improve the results of stem cell therapy. There are several osteoinductive growth factors including platelet-derived growth factor, insulin-like growth factor, and transforming growth factors. PRP contains all these growth factors [105]. PRP can also be a suitable carrier for cell transplantation because it coagulates immediately by an addition of thrombin and calcium. Combination of stem cells and PRP accelerates bone healing and bone remodeling process by stimulating angiogenesis [106].
In a study conducted by Kitoh et al. [99] in 46 patients of skeletal dysplasia and limb length discrepancy, the group that received transplantation of osteogenic differentiated stem cells (approx. 2.3 × 107 cells) and PRP around 3 weeks after the lengthening surgery had a shorter healing index of approx. 31 days/cm compared with the control group which had an average healing index of 55 days/cm and also had reduced associated complications.
Scaffolds
Stem cells may need a scaffold for osteoconduction. The 3-D structure of a scaffold determines the extent of cell migration and differentiation, bone ingrowth, vascularization, and mass transfer between the cells and the environment. All of these processes benefit from increased scaffold porosity, interconnected pore networks, large surface area-to-volume ratio and increased surface roughness [107]. Bioactive scaffolds aid in bone bonding. It is important for scaffolds to interact with surrounding tissues to induce specific cellular responses [108].
Box 28.3 Properties of an Ideal Scaffold
Increased and interconnected porosity
Large surface area-to-volume ratio
Same mechanical strength as bone
Bioactive
Biocompatible
Biodegradable
Optimal release profile
One method to achieve this goal is to construct the scaffold with biomaterial that can stimulate platelets to release growth factors. For instance, collagen is known to be as effective as thrombin in activating platelets [109]. The activated platelets would then release growth factors, which will enhance tissue regeneration, proliferation, and differentiation. Therefore, a scaffold made with collagen type 1 will likely be effective in bone formation and bonding.
The mechanical strength of a scaffold should approximate that of bone. Weaker scaffolds cannot support the skeleton, whereas stiffer scaffolds lead to stress shielding. The carrier should be biocompatible to minimize interference with bone induction. It must be biodegradable to minimize the effects on the biomechanical properties of the regenerate, and yet it must persist in vivo long enough to maintain bioactive elements at the site of implantation and optimize their release [110].
Scaffolds available for use with stem cells include PRP gel carrier, collagen, hydroxyapatite (HA) ceramic scaffold, resorbable polylactide membrane, and polycaprolactone (PCL). Collagen scaffold seems to be a good delivery vehicle for other graft materials [110]. Maracci et al. [100] have demonstrated that extensive long bone defects in human subjects treated with stem cell-seeded HA scaffolds have healed and integrated well with the host bone. Even after a 7-year follow-up the durability of the regenerated bone was good and there were no long-term complications noted.
Other Uses of Stem Cells
A case of avascular necrosis of femoral head being treated effectively by stem cells has been reported by Kim et al. [102]. Lee et al. [111] have reported successful reconstruction of 15-cm segmental defects by bone marrow stem cells and autogenous bone graft in central hemangioma of mandible. Trials are also being carried out by using stem cells in osteoporotic fractures [112]. “Osteogenic matrix coating,” a method to prevent loosening of joint prostheses by coating them with osteogenic cells or their precursors, has been proposed by Ohgushi and Caplan [113].
Osteogenic Progenitor Stem Cell Culture Technique
Our technique for procuring and processing the bone marrow aspirate and culture of osteogenic progenitor stem cells is explained below [101].
Approximately 3–5 ml of bone marrow is collected from the anterosuperior iliac spine and added to a container filled with 30 ml of 10 % FBS-α MEM and 350 units of heparin (Fig. 28.3). The mixture is then taken to the lab and centrifuged at 4 °C for 10 min, after which the supernatant was discarded and 20 ml of culture medium was added to the remaining pellets. The mixture is then filtered, 10 ml of the medium is added per T-75 culture flask, and the culture is initiated. The incubator is maintained at 37 °C with 5 % CO2. The next day, 50 μg l-ascorbic acid/10 ml and dexamethasone 10−7 M are added to facilitate cell differentiation into osteoblasts. The cell culture condition is evaluated using a light microscope, and the culture medium is changed on the fifth day of culture, after which the culture medium is changed every 3 days with the subsequent addition of l-ascorbic acid. On the 14th day of culture, nitro blue tetrazolium chloride 5-bromo-4-chloro-3-indolyl phosphate (NBTBCIP) staining was performed to confirm activation of the alkaline phosphatase. Twenty-four days after beginning the culture, Alizarin red staining is performed to detect newly produced calcium, and thus confirming that most of the cultured cells are osteoblasts. 1 vial (0.4 ml) contains autologous cultured osteoblasts over 12 million. Every patient may need 1–6 vials (12–72 million cells).

Fig. 28.3
Collection of bone marrow
Injection Technique (Fig. 28.4)
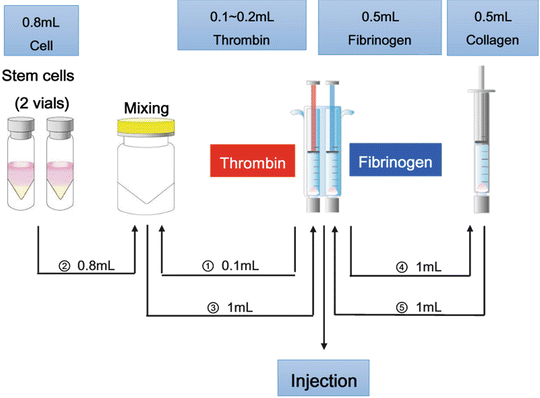
Fig. 28.4
Flowchart depicting the preparation of stem cell mixture
In the non-sterile area, transfer whole amount of the cell suspension in two vials into the mixing vial using an 18-G needle. In the sterile area, transfer only 0.1–0.2 ml of thrombin into the mixing vial and discard the rest of thrombin. Mix stem cell and thrombin in a mixing vial and transfer 1 ml into red (thrombin) syringe. Mix 1 ml of collagen and 1 ml of fibrinogen with two 3-ml syringes using a three-way connector (Fig. 28.5). Transfer 1 ml of mixed collagen and fibrinogen into blue (fibrinogen) syringe. Install the red syringe and blue syringe into Y-piece and implant stem cells with injector and spinal needle by checking the defect site through C-arm (Fig. 28.6). After injection, this becomes a gel within 3–5 min.
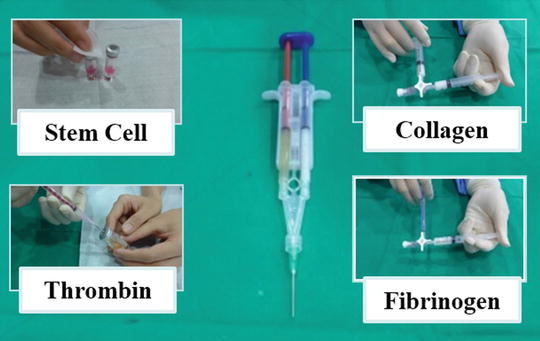
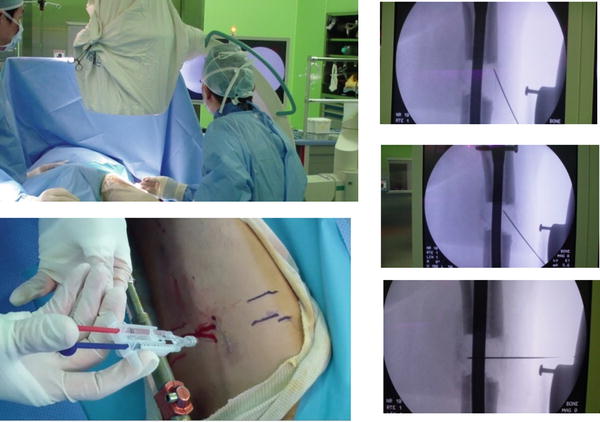

Fig. 28.5
Using a three-way connector, stem cell and thrombin are mixed in a red syringe. Similarly, collagen and fibrinogen are mixed in a blue syringe. Then both of these are attached to the Y-piece

Fig. 28.6
Injection of the stem cell preparation under C-arm control
Although the initial results are encouraging, further studies are necessary to assess the safety and efficacy of stem cells in distraction osteogenesis.
Other Experimental Local Stimuli
Osteogenic peptides are cheaper to produce as they are synthetic. They are less likely to lose their bioactivity during storage and delivery because of their short, linear structure.
TP508
One of these osteogenic peptides is TP508, which includes a 23-amino acid peptide that is the non-proteolytic receptor-binding domain of human thrombin. The effects of TP508 include changes in the inflammatory response, improvements in cell recruitment, and angiogenesis [114]. The doses of TP508 used in the experimental studies vary from 0.1 μg to 300 μg, and results differ depending on the dose. The animals given the higher dose had a larger area of newly formed bone [115–117].
ED-71
Yamamoto [118] reported that administration of 2-β-(3-hydroxypropoxy)-1α, 25-dihydroxyvitamin D3 (ED-71), an analog of synthetic vitamin D3, increased the bone mineral content (BMC) at the lengthened callus. According to Yamane et al. [119] ED-71 promoted both bone matrix formation and mineralization of the callus at 1 week after completion of lengthening, during which BMC increased markedly and may be effective for shortening the duration of therapy in callus distraction.
FGF-2
Fibroblast growth factor-2 (FGF-2 or basic FGF) is recognized as a potent mitogen for a variety of mesenchymal cells. In skeletal tissues, FGF-2 is produced by cells of osteoblastic lineage, accumulated in bone matrix, and acts as an autocrine/paracrine factor for bone cells [120]. FGF-2 shows variable regulations of proliferation and differentiation of cells of osteoblastic lineage and modulates bone formation. Aronson [121] has reported that the age-related deficits of endosteal bone formation were reversed by administering exogenous rhFGF-2 in an animal study. In another study Okazaki et al. [120] have reported that a single local injection of FGF-2 at the center of distracted callus facilitated the consolidation during bone lengthening in rabbit tibiae even under strenuous conditions of rapid and long distraction. In a study conducted by Jiang et al. [122], osteodistraction was applied in craniofacial bone of rabbits to observe the effects of MSCs with or without bFGF gene transfected on bone regeneration. They noted that excellent bone formation and highest BMD and BMC were achieved in the bFGF gene transfected group.
Optimal Timing of Biologic Stimulants
There is no clear evidence to guide us in terms of the timing of injection to achieve better bone regeneration. Angiogenesis has been shown to be of vital importance and intricately involved in the inflammatory response, soft callus formation, and transition from cartilaginous callus to bone [123, 124]. Proangiogenic factors such as vascular endothelial growth factor (VEGF) are especially important during early phase of distraction, when their expression is significantly higher than in later periods [125]. So, administration of growth factors during the early phases may stimulate neoangiogenesis and/or enhance the formation of regenerate bone. Several preclinical studies showed enhanced new bone formation by applying BMPs on the day of the surgery [65, 126, 127] and immunohistochemical analysis has shown high expression of growth factor receptors during the distraction phase [82, 125, 128–131]. In a randomized controlled trial, Lee et al. [86] clinically demonstrated that injection of BMAC and PRP on the day of surgery enhanced new bone formation (Fig. 28.7).
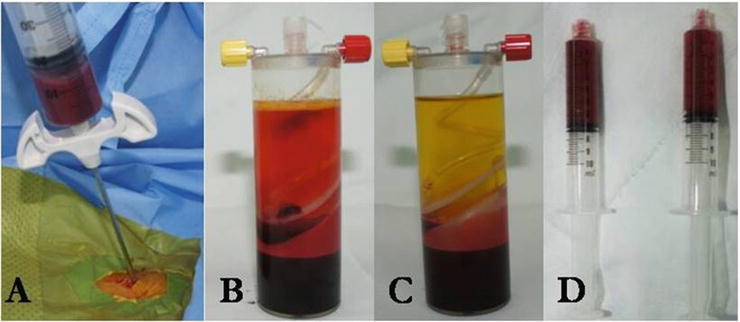

Fig. 28.7
BMAC and PRP are prepared in the operation room at the end of index surgery: 60 ml of bone marrow is aspirated at the iliac crest (a) and 60 ml of peripheral blood is obtained. Bone marrow and peripheral blood are centrifuged to make BMAC (b) and PRP (c). Six milliliters of the aspirate sampled from the mid-layer are prepared for the injection (d)
Hamdy and Rauch et al. [62, 132] observed high levels of expression of BMP-2, 4 and OP-1 during the distraction phase, with a rapid decline thereafter. They hypothesized that the best time for exogenous injection of BMPs would be at the end of the distraction phase, when the endogenous expression of BMPs is decreasing. But, they could not observe enhanced bone healing when OP-1 is injected at the end of distraction, but found twofold increase in bone volume when injected during early phase of distraction [62, 64]. There are also several reports of enhanced healing when applied at the end of distraction [85, 133, 134]. The optimal timing of the injection of the biological stimulants is an important but still unresolved issue. Hence further well-designed investigations are required.
Biological Stimulation: Systemic Application
Parathyroid Hormone
Parathyroid hormone (PTH) is one of the most promising therapeutic agents for osteoporosis. Human and animal studies have demonstrated that daily systemic injection of parathyroid hormone increases BMD [135–138]. PTH stimulates bone formation and resorption and can increase or decrease bone mass, depending on the mode of administration. Continuous infusions and daily subcutaneous injections of PTH stimulate bone formation similarly but have different effects on bone resorption and bone mass [139, 140]. It has been reported that the combination of systemic and local parathyroid hormone led to higher BMD, BMC, and bone area, a trend for greater radiographic-detected bone area and higher expression of osteocalcin in osteotomy sites when compared with the individual treatment or control groups [141]. Skripitz et al. [142] conjectured that since PTH seemed to have a greater effect on new bone formation than on normal bone remodeling, it might become useful for improving the incorporation of orthopedic implants and stimulating fracture repair. Neer et al. [143] reported that the clinical benefits of PTH reflect its ability to stimulate bone formation and thereby increase bone mass and strength. Given the enhancement of bone formation by the local stimulator such as BMP and the systemic application of PTH, combination therapies may be beneficial for bone tissue engineering.
The mechanism of this anabolic effect, however, has not been well established. It is widely believed that the anabolic effect of PTH may be due to increased osteoblast differentiation. Other report indicated that prevention of osteoblast apoptosis is a crucial mechanism for the anabolic effects of PTH on bone [144].
Growth Hormone
Systemic administration of recombinant homologous growth hormone (GH) has been reported to greatly accelerate ossification of bone regenerate in distraction osteogenesis [145]. GH administration increases bone turnover by enhancing both bone formation and resorption [146]. Correspondingly, an increase in the mechanical strength of the whole bone occurs, whereas the mechanical quality of the osseous tissue is equivalent in GH-injected animals and controls [7]. Various studies demonstrate that systemic administration of GH is able to enhance bone regeneration and mechanical strength of healing critical size bone defects in animal models [147–149].
Other Experimental Systemic Stimuli
Hyperbaric oxygen therapy has been used to treat a variety of diseases and has been described as helping patients who have delayed healing or bone defects [150]. This technology consists of intermittently administering 100 % oxygen at pressures greater than one atmosphere absolute entirely enclosed in a pressure chamber [150]. However, one systematic review [151] performed recently failed to locate any relevant clinical evidence to support or refute the effectiveness of hyperbaric oxygen therapy for the management of delayed union or established nonunion of bony fractures. Good-quality clinical trials are needed to define its role in DO.
Androgens such as testosterone are also known to have proliferative effects on osteoblasts and increase fracture healing by systemic and local stimulation of bone formation [152]. In clinical application, androgens may be a possibility to increase bone formation, especially in elderly patients. Furthermore, it may be possible to shorten postoperative rehabilitation because of the effects of androgens on muscles [153].
Anticatabolic Therapy: Systemic Application
Bisphosphonate
The potential anabolic effect of bisphosphonates has been reported [154, 155]. Recent advances in the pharmacological treatment of osteoporosis with bisphosphonates [156–158] have encouraged surgeons to explore their use in limb lengthening. In general, bisphosphonates have been considered antiresorptive agents with limited effect on osteoblasts [159]. The anabolic effect of bisphosphonates has been questioned as they are not internalized by osteoblasts, and therefore, they may not exert a direct effect on osteoblast activity. However, there is evidence to support that there is an effect of bisphosphonates on the osteoblast side not directly on mature osteoblasts but more likely on osteoblast precursors in the bone marrow. First, only nanomolar concentrations of bisphosphonates are needed to stimulate the production of osteoclast inhibitory factor, which is mostly secreted by osteoblast precursors [160]. Second, bisphosphonates have shown to stimulate fibroblastic colony formation by murine and human bone marrow both in in vitro and ex vivo cultures [161].
The use of external fixators or any form of fixation may be associated with stress shielding of the spanned bone interval in this case, the regenerate and the surrounding bone resulting in osteopenia. Eyres et al. [15] noted a mean decrease in BMD of 44 % in the tibia and 61 % in the femur distal to the regenerate in children undergoing limb lengthening. Maffulli et al. [162] noted that the BMC adjacent to the regenerate decreased to less than 40 % of its original value in 6 of 11 patients. There was a reversal of osteopenia, increased regenerate volume, and increased mechanical strength of the bone when bisphosphonates were administered. However, healing index remained very prolonged [163].
Im et al. [164] have shown that both risedronate and alendronate could increase osteoblast and osteoblast progenitor numbers in primary human trabecular cultures with enhanced expression of BMP-2, type I collagen, and osteocalcin. These effects are not limited to osteoblastogenesis but also through the promotion of osteoblast survival as per their efficacy as anti-apoptotic agents [165]. Furthermore, bisphosphonates were reported to enhance bone marrow mesenchymal stem cell osteogenic differentiation [166] and facilitate human adipose-derived stem cell osteogenesis for bone regeneration [167]. Not only bone formation but also bone resorption are highly activated in the regenerated bone, implying high bone turnover. Sufficient nitrogen-containing bisphosphonate (N-BP) causes a notable modulation in morphological properties of the regenerated bone through inhibition of highly activated bone resorption and eventually increased mechanical properties [168]. The main effects of N-BP are at the lumbar spine and proximal femur, where they stop bone loss, reduce fracture risk, and increase BMD [169]. In addition, N-BP are prescribed for the treatment of bone diseases such as osteoporosis, multiple myeloma, cancer metastases, and Paget’s disease.
However, the conventional systemic way of administration of bisphosphonates may have some undesirable side effects such as gastrointestinal ulceration and jaw osteonecrosis [169, 170]. Recent studies have indicated that local administration of a low dose of bisphosphonates was able to enhance bone regeneration in vivo [171–173].
Other Experimental Anticatabolic Therapies
The influence of calcitonin on various stages of bone formation has been investigated [174]. Weiss et al. [175] reported that when calcitonin is administered during the initial phases of bone formation, it increases bone formation due to a stimulation of proliferation of cartilage and bone precursor cells. In humans, serum calcitonin rises during pregnancy, growth, and lactation [176]. It is during these periods of calcium stress that a tonic antiresorptive hormone will best exert its effect to limit skeletal loss. This may be the primary role of calcitonin in skeletal conservation [177]. There is considerable support for the thesis that, in addition to its inhibitory effects on bone resorption, calcitonin enhances osteoblastic bone formation both in vitro and in vivo [178]. The putative anabolic osteoblastic effect of calcitonin may be of clinical importance in sustaining the bone formation rate despite inhibition of bone resorption.
Summary
Insufficient bone regeneration is an unpredictable and unsolved issue in distraction osteogenesis. Avoiding the detrimental factors is also important, but there are unavoidable factors like host-related causes including smoking, diabetes, and systemic illness. Biologic stimulation is more promising because it can be a concept of preemptive stimulation rather than the treatment after poor bone regeneration is observed. Efforts to improve regenerate bone formation are still in progress. The efficacy, safety, optimal dose, timing and route of administration, and cost-effectiveness of each of the agents that can enhance bone formation in distraction osteogenesis should be studied further by well-designed investigations.
References
1.
Jimi E, Hirata S, Osawa K, Terashita M, Kitamura C, Fukushima H. The current and future therapies of bone regeneration to repair bone defects. Int J Dent. 2012;2012:148261.PubMedCentralPubMed
2.
Iobst C. Limb lengthening combined with deformity correction in children with the Taylor Spatial Frame. J Pediatr Orthop B. 2010;19(6):529–34.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


