NCEP-ATPIII [75]
Modified WHO [9]
EGIR [76]
AACE [77]
IDF [1]
Simplified definition of metabolic syndrome [78]
Mandatory
…
Insulin resistancea >75th percentile glucose ≥110 mg/dL; 2 h glucose ≥140 mg/dL
Insulin resistancea (or fasting insulin) in >75th percentile
“High risk for insulin resistance” or BMI >25 kg/m2 or waist ≥102 cm (men) or ≥88 cm (women)b
Ethnic based waist: European: ≥94 cm (men) or ≥80 cm (women); Asian: ≥90 cm (men) or ≥80 cm (women)
Index of central obesityc (ICO) ≥.50
No. of factors
≥3
≥2
≥2
≥2
≥2
None
Glucose
≥110 mg/dL
≥110 mg/dL
≥110 mg/dL; 2 h glucose ≥140 ≥100 mg/dL
≥100 mg/dL; or previously diagnosed Type 2 DM
HDL cholesterol
<40 mg/dL (men)
<50 mg/dL (women) or on medications
<35 mg/dL (men)
<39 mg/dL (women)
<39 mg/dL
<40 mg/dL (1.03 mmol) (men)
<50 mg/dL (1.29 mmol) (women)
<40 mg/dL (1.03 mmol) (men)
<50 mg/dL (1.29 mmol) (women) or on medications
And/or
And/or
Triglycerides
≥150 mg/dL or on medications (1.7 mmol/l)
≥150 mg/dL
(1.7 mmol/l)
≥150 mg/dL
(1.7 mmol/l)
≥150 mg/dL
(1.7 mmol/l)
≥150 mg/dL or on medications
(1.7 mmol/l)
Obesity
Waist ≥ 102 cm (men) ≥88 cm (women)
Waist/hip ratio >0.9 (men); >0.85 (women); or BMI ≥30 kg/m2
Waist ≥94 cm (men) ≥80 cm (women)
Hypertension
≥130/85 mm Hg or on medications
≥140/90 mm Hg
≥140/90 mm Hg
≥130/85 mm Hg
135/85 mm Hg or on medications
Other:
Microalbuminuriad: albumin excretion > 20 mcg/min Or albumin/creatinine ratio ≥ 30 μgm/mg
Parameter | Score |
|---|---|
Age | |
<35 | 0 |
35–49 | 20 |
≥50 | 30 |
Abdominal obesity | |
Waist <80 cm (female), 90 cm (male) | 0 |
Waist 80–89 cm (female), 90–99 cm (male) | 10 |
Waist ≥90 cm (female), ≥100 cm (male) | 20 |
Physical activity | |
Exercise (regular) + strenuous work | 0 |
Exercise (regular) or strenuous work | 20 |
No exercise + sedentary | 30 |
Family history | |
No family history | 0 |
Either parent | 10 |
Both parents | 20 |
Insulin resistance syndrome (Syndrome X), described by Reaven, has insulin resistance as the main component and later the obesity part was added. The metabolic syndrome is given by ICD(10) code of E88.81 [6]. Prevalence of metabolic syndrome in nondiabetic population in European countries is 15 % (modified definition by WHO) [7] and in USA is 23 % of population (National Cholesterol Education Program/Adult Treatment Panel III criteria) [8].
The first definition of MetS was made by WHO in 1998 as the presence of insulin resistance and some of its components – impaired glucose tolerance (IGT) or diabetes mellitus type 2 – along with at least two of the following parameters: raised blood pressure, hypertriglyceridemia (and or reduced HDL cholesterol), central obesity, and microalbuminuria [9].
The basic defect in metabolic syndrome is now recognized as insulin resistance. The metabolic syndrome usually begins with central adiposity and subsequent increased B cell function leading to hyperglycemia [10, 11]. Hypertension develops as a result of the effect of insulin on sympathetic nervous system and on sodium metabolism, but the effect of other hormones like angiotensinogen, resistin, and leptin secreted from adipose tissue also contribute to hypertension. Insulin resistance causes significant changes in endothelial wall including abnormal nitric oxide (NO) metabolism and reduced PK/Akt signaling. Insulin resistance also causes structural and genetic damage to endothelial cell, so hypertension is caused by reduced vasodilatation and endothelial damage. Several studies have shown that apart from genetic predisposition, changes in lifestyle such as working shift duties, increased stress, sleep deprivation, and light exposure lead to metabolic syndrome [12].
In 2005, International Diabetes Foundation (IDF) detected abdominal obesity as a prerequisite for diagnosing MetS [13]. In 2008, the WCEP: ATP III included waist circumference, blood lipids, blood pressure, and fasting glucose in definition of MetS [14]. ATP III definition and IDF depend on waist circumferences while WHO definition depend on insulin resistance. Another issue in generalizing obesity in various populations is lack of uniform criteria in definition of obesity across various ethnic populations, which is addressed in IDF criteria.
Obesity is now an epidemic all across the world [15, 16]; when measured with NCEP–ATP III criteria, the prevalence of metabolic syndrome increased by 5 % during last 15 years. The increase is more evident in developing nations where food habits are changing from traditional to western culture [17].
Metabolic Syndrome and Cancer
Metabolic syndrome is associated with a number of cancers. The most commonly associated cancers are colorectal, endometrial, breast, and prostate [18]. Among gynecologic cancers, carcinoma endometrium has a strong association with MetS. It has been estimated that in the year 2012, cancers of the corpus uteri accounted for 319,605 new cases. This accounted for 4.8 % of the total cancers among women with an age standardized rate (ASR) of 8.3 per 100,000 women. There were an estimated 76,160 deaths due to endometrial cancers among women globally during 2012 [19]. The ASR for mortality due to endometrial cancer was 1.8 per 100,000 women accounting for 2.1 % of the total cancer deaths among women [1].
In a case control study done by Rosato et al. where 454 women with incident endometrial carcinoma and 798 controls were included, the multivariate odds ratio (OR) for development of endometrial carcinoma was 2.18 for type II diabetes, 1.77 for hypertension, 1.2 for hyperlipidemia, and 1.62–2.23 for various grades of central obesity. MetS is associated with odds ratio for endometrial cancer ranging between 1.67 and 2.77 when waist circumference was used and 8.40 when BMI was used to define MetS [20]. World Cancer Research fund defined obesity as a predisposing factor for endometrial carcinoma on the basis of evidence from large number of studies [21].
In a meta-analysis done in 2013, where the six studies were selected, metabolic syndrome was associated with increased risk of endometrial cancer (RR 1.89, 95 % CI 1.34–2.67, p < .001). When each factor contributing to metabolic syndrome was analyzed, the relative risk was 2.21 (P < .001) for higher body mass index or waist circumference, 1.81 (p = 0.044) for hyperglycemia, 1.81 (p = 0.014) for high blood pressure, and 1.17 (p < 0.001) for high triglyceride levels [22]. Another study done in Austria, Norway, and Sweden, which was a part of Metabolic Syndrome and Cancer project (Me-Can), showed the relative risk of endometrial cancer in metabolic syndrome to be 1.37 (95 % CI 1.28–1.46) [23].
Metabolic syndrome is associated with various other gynecological cancers as well. In Me-Can study the association between “MetS” and rare gynecologic cancer was explored [24]. The rare cancers included those of vulva, vagina, and other rare sites. The hazard ratio of developing these cancers were 2.08 (95, CI 1.29–3.37) when mean BMI was 29.7 kg/m2 when compared with women with mean BMI 20.8 kg/m2. This was statistically significant. For vaginal cancers HR was 2 (0.62–6.90) (p value = 0.027), but vulvar cancers and other cancers (cancer occurring in ligaments and otherwise not specified cancers) showed nonsignificant association.
Various components of metabolic syndrome and its relation to endometrial cancer will be discussed.
Obesity
From several studies it is evident that the key factor in endometrial carcinogenesis in MetS is obesity [20, 25]. Endometrial cancer risk in obesity is thought to be mediated through increased availability of serum estrogens and insulin resistance [26] (Fig. 35.1).
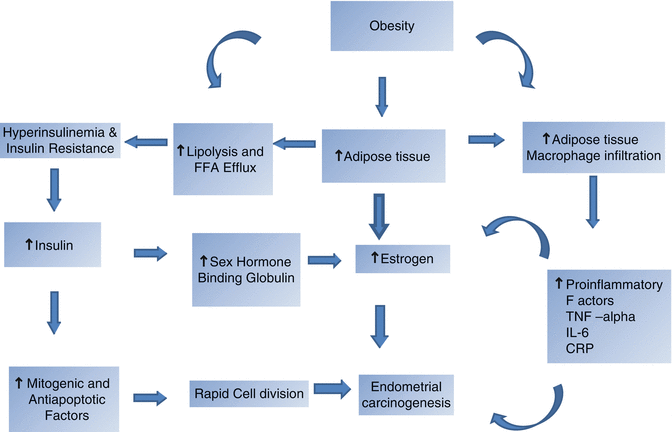

Fig. 35.1
Carcinogenesis in metabolic syndrome
In a Norwegian study involving 222 patients, there was a strong relationship between BMI and endometrial cancer (p < .0001). The adjusted relative risk (RA) was 0.53 (95 % confidence interval CI .19–1.47) for BMI <20 kg/m2, 4.28 (95 % CI 2.58–7.09) when BMI was 35–39 kg/m2and 6.36 (95 % CI 3.08–13.16) for BMI ≥40 kg/m2 [27].
Type I endometrial cancer, which is estrogen-dependent, is associated with obesity [28]. In a study involving one million Norwegian women, overweight and obese women had relative risk of 1.36 (95 % CI 1.29–1.44) and 2.51 (95 % CI 2.53–2.66), respectively, for endometrial carcinoma [29]. In a similar study done in Sweden among postmenopausal women aged 50. 74 years, overweight women (BMI 28–29.98) had 50 % increase in risk of endometrial carcinoma when compared to lean women (BMI <22.5 kg/m2). In the same study obese women (BMI 30–33.99) had three times risk, and markedly obese women (BMI ≥34) had six times increase risk of endometrial carcinoma [30].
According to a review study done in Europe, excess body mass accounts for 5 % of cancers in Europe, and endometrial cancers are caused by obesity in 39 % of women [31]. In a meta-analysis, overall risk ratio (RR) of endometrial cancer in a linear model increases 1.6 (95 % CI 1.52–1.68) for every 5 kg/m2 increase in BMI [32]. Weight gain in young adulthood increases the risk of endometrial cancer but weight gain in middle life does not increase the risk of endometrial cancer [33].
If the basic mechanism of endometrial carcinogenesis is considered, it is well understood that exposure to high level of estrogen plays a significant role [34]. Obesity exposes the women to higher levels of estrogen produced in adipose tissues and is now considered an etiologic factor for endometrial carcinoma [35]. Obesity also leads to insulin resistance and hyperinsulinemia [35]. Increased insulin causes carcinogenesis through mitogenic and antiapoptotic properties [36, 37]. Similarly insulin reduces serum sex hormone binding globulin and increases bioavailability of estradiol. Obesity also causes increased adipose tissue infiltration by macrophages, which secretes many proinflammatory mediators which include TNF-alpha, interleukin-6(IL-6), and C-reactive protein (CRP). These mediators cause increased invasion, progression, and metastasis [38, 39]. Increase in obesity, especially in developing nations like India, is definitely a cause for increase in incidence of endometrial carcinoma (Fig. 35.1).
Among the individual components of metabolic syndrome, obesity is the strongest risk factor for endometrial carcinoma.
Diabetes
The Norwegian study showed that women with diabetes mellitus had three fold higher risk of endometrial cancer (RR 3.13 95 % CI 1.92–5.11) when compared with women without diabetes mellitus [27]. In a study from Sweden, OR of type I diabetes mellitus was 13.3 (3.1–56.4) when compared to 1.5 (95 % CI 1–2.1) in type II diabetes [30]. The mechanism of carcinogenesis in a diabetic patient is insulin resistance, increased inflammatory mediators, and other hormonal factors [35, 40–42]. Another factor leading to carcinogenesis is increased blood glucose level, which makes glucose available for rapid cell division [35, 40–42]. Yet another factor is upregulation of glucose transport-protein, reactive free radicals, and increased products of glycosylation [43–46].
In a case control study done in Italy, there was no increased risk in juvenile diabetes (insulin dependant), while there was increased risk of endometrial cancer in insulin independent diabetes mellitus with (OR 3.1 (95 % CI 2.3–4.2) in ≥40 years. Similarly the OR in obese women (BMI >25–24) was 3.6 compared to 3 in nonobese women (BMI <25) [47].
Insulin resistance is caused by increased circulating fatty acids mostly derived from adipose tissue. Insulin resistance leads to hyperinsulinemia which also leads to increased level of circulating insulin-like growth factors. This causes endometrial hyperplasia [48–51]. The mechanism by which endometrial cancer risk is increased in diabetes is via two pathways. In the first one, insulin stimulates adrenal glands in postmenopausal women to produce more ovarian testosterone which is metabolized to estrogen in adipose tissue [52–54]. As described earlier, insulin reduces serum level of circulating sex hormone binding globulin and thus leads to hyperestrogenemia [54, 55]. The second mechanism is reduction in insulin-like growth factor-binding protein (IGFBP) which increases IGF-1 which stimulates endometrium and causes endometrial hyperplasia and carcinogenesis [48, 49, 51, 56].
Hypertension
It has been thought that hypertension promotes inhibition of apoptosis [57]. Many studies have shown that hypertension increases the risk of endometrial cancer [58]. In a case control study done at Italy, involving 3406 individuals, after adjusting all other variables, hypertension was associated with odds ratio of 1.6 (95 % CI 1.3 to 1.9) for endometrial cancer. In hypertension women with BMI ≥30 kg/m2 the risk of endometrial cancer (OR) was 4.9 when compared with nonobese women with BMI <25 kg/m2 [59]. The mechanism by which hypertension increases the endometrial cancer risk is poorly understood. It was postulated that hypertension may increase the risk of cancer by blocking apoptosis [57, 60]. Secondly, hypertension is usually associated with insulin resistance [20], hormonal imbalance, and obesity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


