Menopause
Marcelle I. Cedars
Michele Evans
The ovary is unique in that the age associated with decline in function (to frank failure) appears to have remained constant despite the increase in longevity experienced by women over the last century. Because the loss of ovarian function has a profound impact on the hormonal milieu in women and on the subsequent risk for the development of disease via the loss of estrogen production, improving our understanding of reproductive aging is critical to care for all women.
Human follicles begin their development during the fourth gestational month. Approximately 1,000 to 2,000 germ cells migrate to the gonadal ridge and multiply, reaching a total of 5 to 7 million around the fifth month of intrauterine life. At this point, replication stops and follicle loss begins, declining to approximately 1 million by birth. In the human male, the dividing germ cells become quiescent and maintain their stem cell identity. In the female, between 12 and 18 weeks gestation, the germ cells enter meiosis and differentiate. Thus, all germ stem cells have differentiated prior to birth. In the adult woman, the germ cells may remain quiescent, be recruited for further development and ovulation, or undergo apoptosis. Over time, the population of oocytes will be depleted, without regeneration, through recruitment and apoptosis until fewer than a thousand oocytes exist and menopause ensues. Approximately 90% of women experience menopause during the early 50s. The other 10% of women experience menopause prior to 46 years of age (often termed early menopause), with 1% of women experiencing menopause at an age younger than 40 years (premature menopause or premature ovarian failure [POF]).
Menopause occurs at a median age of 51.4 years, with the age range in normal women being 42 to 58 years. The age of menopause appears to be determined largely by genetics and is due to exhaustion of the oocyte pool. Menopause and the years preceding it are characterized by hormonal changes, decline in reproductive potential, and increased risk for physical and psychologic changes.
The average age of menopause has remained constant throughout recorded history. It does not appear to be related significantly to race, parity, height, weight, socioeconomic status, or age at menarche. Evidence suggests that genetic and environmental factors influence the age of menopause, although the specific nature of these relationships is characterized poorly. Given the strong association between age at menopause between mothers and daughters, this is likely a genetically determined trait. Environmental factors may not have a significant effect in themselves, but the interplay among environmental factors such as smoking (known to accelerate the age of menopause by 1.5 to 2.0 years), body mass index (BMI), alcohol use, and socioeconomic status and genetic risk may be important.
According to the 2000 census data, 35% of the population is age 45 or older and 21% are over 55 years of age. Currently, 7.3% are women 65 years or older (approximately 20 million women in the United States). Over 50 million women in the United States are in the menopausal transition or menopause.
Reproductive Stages
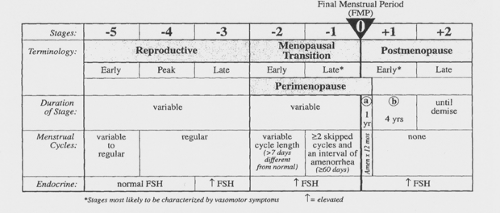
Reproductive aging is a continuum beginning in utero and ending with menopause. The stages along this continuum have been difficult to define. Numerous terms have been used clinically, to describe the end of this continuum, including perimenopause, menopausal transition, climacteric, menopause, and postmenopause. The Stages of Reproductive Aging Workshop (STRAW) was convened in July 2001 to address the lack of a pertinent reproductive staging system and to establish a nomenclature and guidelines as well as a consistent reproductive aging system for health practitioners, the medical research community, and the public.
The staging system takes into account menstrual cyclicity, endocrinology, and symptomatology, beginning with
menarche and ending with a woman’s demise. The foundation of the staging system is the final menstrual period (FMP). Five stages precede the FMP and two follow it, for a total of seven stages. Stages -5 to -3 are called the reproductive interval, stages –2 to –1 are termed the menopausal transition, and stages +1 and +2 are known as the postmenopause (Fig. 42.1).
menarche and ending with a woman’s demise. The foundation of the staging system is the final menstrual period (FMP). Five stages precede the FMP and two follow it, for a total of seven stages. Stages -5 to -3 are called the reproductive interval, stages –2 to –1 are termed the menopausal transition, and stages +1 and +2 are known as the postmenopause (Fig. 42.1).
The menopausal transition begins with variation in the menstrual cycle length (>7 days different from normal) in a woman with an elevated follicle-stimulating hormone (FSH) level. This stage ends with the FMP, which cannot be determined conclusively until after 12 months of amenorrhea. Early postmenopause is defined as the first 5 years following the FMP. Late postmenopause is variable in length, beginning 5 years after the FMP and ending with the woman’s death.
Although this staging system is said to include endocrinologic aspects of ovarian aging, it still depends largely on menstrual cyclicity as a key indicator of ovarian aging. It does include measurement of FSH; however, by the time FSH is elevated, even with cyclic menstrual cycles, oocyte depletion already has proceeded to such an extent that fertility (as a marker of reproductive aging) is diminished significantly. As noted previously, evidence suggests that genetic and environmental factors influence both the age of menopause and the decline in fertility, although the specific nature of these relationships is characterized poorly. Premature menopause can be due to a failure to attain adequate follicle numbers in utero or to an accelerated depletion thereafter. Potentially, either of these factors could be affected by genetic and environmental risk. The timing of menopause has a consistent impact on overall health with respect to osteoporosis, cardiovascular disease (CVD), and cancer risk. Over the next decade, it is estimated that more than 40 million women will enter menopause.
Oocyte Depletion
As discussed previously, the leading theory regarding the onset of menopause relates to a critical threshold in oocyte number. Approximately 1,000 to 2,000 germ cells migrate to the gonadal ridge and multiply, reaching a total of 5 to 7 million around the fifth month of intrauterine life. At this point, replication stops and follicle loss begins, with a reduction to approximately 1 million by birth and 500,000 to 600,000 by menarche. As the number of oocytes in the reserve pool continues to decline, menstrual irregularity, followed by cessation, will occur. The theory that menopause is triggered primarily by ovarian aging is supported by the coincident occurrence of follicular depletion, elevated gonadotropin levels, and subsequent menstrual irregularity with ultimate cessation of bleeding.
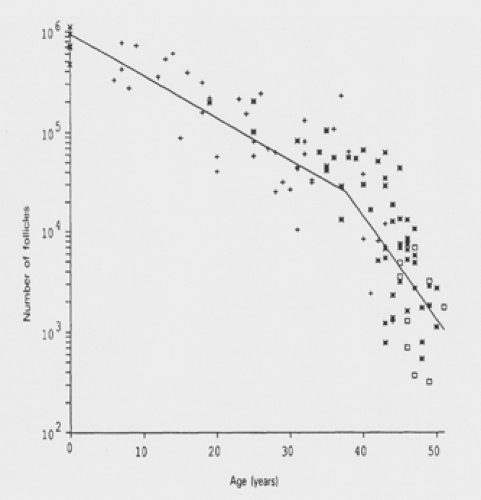
A mathematical model that predicts the rate of follicular decline has been developed (Fig. 42.2). It utilizes existing data, which ultimately shows a biexponential decline, with an acceleration in oocyte loss when the remaining oocyte number equals approximately 25,000. In this model, the decline occurs at 37.5 years of age. At this point, the rate of follicular atresia accelerates. In the absence of this acceleration, the model suggests that menopause would be delayed until age 71. The cause of this accelerated depletion is not well defined. It is also clear that if the factor influencing the rate of decline is follicle number and not age, other factors that might account for a diminished follicle number (genetic risk and possible toxic exposure) would lead to an earlier rate of accelerated decline and an earlier age of menopause.
Coincident with the decline in the number of follicles in the ovary, there appears to be an increase in random genetic damage within these structures. Evidence for this comes from an observed increase in aneuploidy in the offspring of older mothers and the observation that in women over
40 years old, oocytes harvested for in vitro fertilization are karyotypically abnormal approximately 40% of the time. Further examples in nature, such as Turner syndrome, shed some light on the process of oocyte aging. Individuals with this syndrome are, by and large, born with dysgenetic gonads that are devoid of follicles. Ninety-five percent of these individuals are aborted spontaneously prior to birth. If one examines the ovaries of a 20-week abortus, a full complement of oocytes is present. Two factors have been isolated from the ovary: oocyte maturation inhibiting factor (OMI) and luteinizing inhibitor, which may control the rate of maturation of follicles. It has been suggested that individuals with dysgenetic gonads may fail to produce adequate OMI, thus allowing all follicles to progress prematurely toward maturity. The control mechanisms are conceptual rather than factual at this juncture, and new information will have to accumulate before the factors governing human oocyte atresia are elucidated more clearly.
40 years old, oocytes harvested for in vitro fertilization are karyotypically abnormal approximately 40% of the time. Further examples in nature, such as Turner syndrome, shed some light on the process of oocyte aging. Individuals with this syndrome are, by and large, born with dysgenetic gonads that are devoid of follicles. Ninety-five percent of these individuals are aborted spontaneously prior to birth. If one examines the ovaries of a 20-week abortus, a full complement of oocytes is present. Two factors have been isolated from the ovary: oocyte maturation inhibiting factor (OMI) and luteinizing inhibitor, which may control the rate of maturation of follicles. It has been suggested that individuals with dysgenetic gonads may fail to produce adequate OMI, thus allowing all follicles to progress prematurely toward maturity. The control mechanisms are conceptual rather than factual at this juncture, and new information will have to accumulate before the factors governing human oocyte atresia are elucidated more clearly.
Endocrinology
The entire endocrine system in women changes with advancing age. The somatotrophic axis begins to decline during the fourth decade, prior to the loss of ovarian function. This decline in growth hormone is accelerated during ovarian failure and may, itself, accelerate the ovarian failure. However, pituitary concentrations of growth hormone, as well as adrenocorticotropic hormone and thyroid-stimulating hormone, remain constant into the ninth decade. Although the thyroid gland undergoes progressive fibrosis with age and concentrations of T3 decline by 25% to 40%, elderly women remain clinically euthyroid. β-cell function also undergoes degeneration with aging such that by age 65 years, 50% of subjects have an abnormal glucose tolerance test result. Frank diabetes is rare, however, occurring in only 7%. The female reproductive system, on the other hand, undergoes virtually complete failure at a relatively early age.
As noted previously, during the late fourth decade, FSH levels begin to rise even when cyclic menses continue. The most likely cause is a decrease in functional granulosa cells from the oocyte pool, with a decrease in inhibin B negative feedback allowing a monotropic rise in FSH. Early on, there also is a decline in luteal phase progesterone levels. As ovarian aging progresses, estradiol levels may be quite variable, with chaotic patterns and, occasionally, very high and very low levels. This dramatic variability may lead to an increase in symptomatology during the perimenopause (stages –2 to –1). As peripheral gonadotropin levels rise, luteinizing hormone (LH) pulsatile patterns become abnormal. There is an increase in pulse frequency with a decrease in opioid inhibition.
Estrogens
The main circulating estrogen during the reproductive years is 17-β-estradiol. These levels are controlled by the developing follicle and resultant corpus luteum. Oophorectomy will reduce peripheral estradiol levels from 120 to 18 pg/mL, which suggests that more than 95% of circulating estradiol is derived from the ovary. Other sources include the peripheral conversion of testosterone and estrone. Very small amounts are secreted by the adrenal gland. Because the two-cell theory requires aromatization of androgens produced by the theca in the granulosa cell, follicular exhaustion is associated with gradual declines in estradiol concentrations.
The predominant estrogen in the postmenopausal woman is estrone, which has a biologic potency of approximately one third that of estradiol. Estrone is derived largely from peripheral conversion of androstenedione. Extraglandular aromatase is found in liver, fat, and some hypothalamic nuclei. This activity increases with aging and with a higher fat content (also an age-related change). Estrone and estradiol production rates during the postmenopause are 40 and 6 mg/day, respectively. This compares with 80 to 500 mg/day for estradiol during the reproductive years. Essentially, all the estradiol in the postmenopausal woman is derived from conversion of estrone.
Androgens
Dehydroepiandrosterone (DHEA) and its sulfated conjugate, DHEAS, have been shown to decrease with aging, along with adrenal corticotropin responsiveness. DHEAS levels decrease in both men and women. The decline is greater in women and may be due to the relative estrogen
deprivation. Ovarian failure, at any age, accelerates this decline. Evidence suggests that physiologic levels of DHEA may protect against neoplasia, enhance insulin action, protect against osteoporosis, increase immune competency, and offer some cardioprotection. Changes in DHEA levels also have been associated with alterations in body composition that, in themselves, appear to impact cardiac and breast cancer risk. DHEAS levels also may have an impact on “sense of mental well-being.”
deprivation. Ovarian failure, at any age, accelerates this decline. Evidence suggests that physiologic levels of DHEA may protect against neoplasia, enhance insulin action, protect against osteoporosis, increase immune competency, and offer some cardioprotection. Changes in DHEA levels also have been associated with alterations in body composition that, in themselves, appear to impact cardiac and breast cancer risk. DHEAS levels also may have an impact on “sense of mental well-being.”
Androstenedione is the predominant androgen during the reproductive years, and production declines from 1,500 to 800 pg/mL in postmenopausal women. The postmenopausal ovary contributes only 20% to the circulating androstenedione. Testosterone levels also decline after menopause although not to the same extent as estradiol levels. Postmenopausal testosterone is derived from the ovary (25%), the adrenal gland (25%), and extraglandular conversion from androstenedione (50%). The postmenopausal ovary produces a larger percentage of testosterone (50%) than does the premenopausal ovary.
Systemic Effects of Declining Ovarian Function
The decline in ovarian function brings about profound changes in secondary sexual organs. The endometrium becomes atrophic, and the uterus decreases in size. Evidence is accumulating in animal models that the uterus may be partially responsible for the initial decline in reproductive capacity. On the other hand, data from human oocyte donor programs have shown that transfer of ova from younger donors to menopausal recipients produces normal gestations and offspring. It should be noted that these women are stimulated with an artificial sequential overlapping regimen of estrogen and progesterone. This produces an endometrium that is indistinguishable from that of the premenopausal state. The author’s data have shown high implantation rates in older women with a hormonally induced endometrium. Only those women who had received pelvic irradiation have responded poorly, suggesting an alteration of the uterine microvascular system.
The postmenopausal vagina, devoid of estrogen treatment, becomes smaller in both length and caliber. There is decreased elasticity of the vaginal wall, and the karyopyknotic index changes to show fewer superficial cells. The fallopian tubes contain both ciliated and secretory components. After age 60, cilia begin to disappear in the isthmic region, although they remain until a very old age in the ampulla and infundibulum. The mammary gland develops secretory potential at the time of puberty and maintains this function until menopause. Like other secondary sex organs, it is dependent on female sex steroids for its maintenance. With cessation of the production of estrogen and progesterone, glandular, ductal, and stromal involution occurs. The basement membrane thickens, and the luminal space becomes obliterated. Connective tissue of the lobule becomes indistinguishable from other types of connective tissue. There is an accumulation of adipose tissue in the breast, which occurs simultaneously with this involutional process.
Despite the involutional changes of the breast, 20% of patients with breast carcinoma are under the age of 50, with a median age of 55. There is evidence for a bimodal distribution of breast carcinoma, with the first peak occurring at 45 years of age and the second at 65 years. The portion of estrogen receptor–positive breast cancers increases until ages 60 to 74 years. Therefore, a dichotomy appears to exist that ducts and glands, which are rapidly undergoing involution as the result of failing steroid production, become susceptible to malignant transformation while retaining a receptor molecule (E2), which normally is self-induced.
Premature Ovarian Failure
Premature ovarian failure (POF) is a unique entity in which a woman undergoes changes consistent with menopause, such as amenorrhea, elevated FSH levels, and depletion of ovarian follicles, prior to the age of 40. POF occurs in 0.1% of women under 30 years of age and in 1% of women by age 40.
Genetic factors are thought to have a strong relationship with POF. There is a higher incidence of family history of early menopause and a suggestive increase in the family history of infertility as well as an increased incidence of familial cases of early menopause among patients with idiopathic POF. Twin studies have likewise noted a strong genetic component to the age of menopause. Although inheritance appears to be either X-linked or autosomal dominant sex limited, paternal transmission cannot be excluded. Women who have idiopathic early menopause (between the ages of 40 and 45) have a genetic pattern similar to those with POF. These observations support the hypothesis of common underlying genetic factors, which may lead to an early decrease in fertility, early menopause, and POF. Current recommendations advise testing of women with POF, or premature elevations of FSH, for the fragile X permutation (FMR-1). This knowledge may have impact on their own reproductive decisions and requires referral for genetic counseling given the multigenerational impact of this finding.
POF may not be the same as age-appropriate menopause, which results from the depletion of the primordial follicle pool, because POF may be reversible, with follicles in the ovary, estradiol production, and even pregnancies long after the diagnosis. When using ultrasonography to evaluate the follicles in women with POF compared with age-appropriate menopausal and young women on oral contraceptives (OCPs), the mean ovarian volumes were smaller in patients with POF compared with women on OCPs but not different from the women with
age-appropriate menopause. Approximately 40% of patients with POF had follicles in the ovary, albeit fewer than in the normal premenopausal women.
age-appropriate menopause. Approximately 40% of patients with POF had follicles in the ovary, albeit fewer than in the normal premenopausal women.
Menstrual Cycle
Prior to the menopausal transition, the average length of a menstrual cycle ranges from 21 to 35 days. The menopausal transition is defined partially by menstrual irregularity that occurs in response to changes within the ovary—specifically, a dramatic decline in follicle number (and granulosa cell content). As a result, inhibin B levels fall, decreasing negative feedback on FSH and causing a monotrophic rise in FSH. This early cycle rise in FSH may shorten the follicular phase due to accelerated folliculogenesis. Estradiol levels remain relatively constant with age until the menopausal transition, when they initially rise in response to increased FSH levels. As the ovary fails, progesterone levels decline, leading to a shortened (or inadequate) luteal phase. Thus, an early sign of waning ovarian function may be a decreased intermenstrual interval. Precycle spotting may also signal deficiencies in progesterone production. These clinical signs of reproductive aging indicate a poor prognosis for those women still interested in reproduction. As the FMP approaches and the oocyte complement declines to a critical level, estradiol levels fall, leading to hot flashes, vaginal atrophy, and accelerated bone mineral density (BMD) loss. Also, as the FMP approaches, there is a steady trend toward an increased mean cycle length. In a woman’s final 10 to 20 cycles, average cycle lengths characteristically are 40 to 42 days.
As menstrual irregularity increases during the menopausal transition, many women seek medical care. Treatment can be approached in several ways. After a complete history and physical examination, bleeding irregularities can be treated with different hormonal regimens, including OCPs, cyclic hormone replacement therapy (HRT), or progestin-only therapy. Many of these patients continue to ovulate, albeit irregularly, so the addition of cyclic progestin (without estrogen) may further increase cycle irregularity and does not offer contraceptive protection. Thus, treatment with OCPs or a combined, cyclic estrogen–progestin regimen is advisable.
There always is the risk of endometrial hyperplasia in this age group. Patients considered high risk (history of chronic anovulation or obesity or suspicious bleeding patterns such a watery, bloody discharge) should undergo endometrial sampling. Pelvic ultrasonography for measurement of endometrial stripe thickness is not a reliable predictor of risk in cycling women. For those who have a relatively new onset of bleeding irregularity (consistent with the menopausal transition in a previously ovulatory woman), initiation of hormonal treatment can be considered, with endometrial biopsy reserved only for those whose cycles fail to normalize after 3 months of therapy.
Postmenopausal bleeding always should be considered abnormal and must be evaluated accordingly. Bleeding can occur from the rectum, vagina, cervix, urethra, or uterus. A thorough history and physical examination is crucial. If the source of bleeding is uterine, a transvaginal ultrasonographic examination can be very helpful. If the endometrial stripe is thinner than 5 mm, the bleeding typically is the result of an atrophic endometrium. If the endometrium is 5 mm or thicker, it is imperative to perform a diagnostic test, either an endometrial biopsy or dilation and curettage, to sample the endometrium.
Menopausal Syndrome
Given the endocrinologic changes with aging, many symptoms associated with aging in women are due to estrogen deficiency, but the decline in adrenal androgens and growth hormone may contribute. Symptoms that definitely are a result of estrogen deprivation include vasomotor symptoms and urogenital atrophy. Osteoporosis is likely, largely due to estrogen deficiency, but this may be exacerbated by the relative growth hormone decline. The same may be said for the hormone-related changes of increasing atherosclerotic CVD and psychosocial symptoms including insomnia, fatigue, short-term memory loss, and depression. Both DHEAS and growth hormone may well have an impact on these age-related symptoms.
Vasomotor Symptoms
Vasomotor instability in the form of a hot flash (flush) is one of the most consistent and bothersome symptoms that women face as they enter the menopausal transition and subsequent menopause. Hot flashes result from estrogen deficiency and a resetting of the hypothalamic thermoregulatory set point. They occur in 65% to 76% of women who undergo spontaneous menopause or surgical oophorectomy. Symptoms may begin during the menopausal transition, when estrogen levels may fluctuate dramatically from cycle to cycle and even day to day.
A hot flash usually is characterized by intense warmth, described as “heat or burning” that usually begins in the head, neck, and thorax and can spread in waves over the entire body. It may be preceded by pressure in the head and may be accompanied by heart palpitations. The hot flash usually is followed by an outbreak of sweating, followed by chills as the body’s thermostat resets. The length of the episode varies from seconds to approximately 5 minutes, although episodes as long as 30 minutes have been described. The event frequency varies from a few per year to 30 per day.
For most women, the hot flashes commence prior to the FMP, although initially this may be perceived only as a sleep disturbance. In general, the episodes are noted more frequently at night, and the dysfunctional sleep pattern that follows may result in fatigue, irritability, loss of concentration, and depression, symptoms that often are elicited from
patients in the menopausal transition. More than 80% of women who experience hot flashes will experience them for longer than 1 year. Twenty-five percent of women complain of severe hot flashes. Without treatment, the symptoms usually subside slowly over 3 to 5 years. Investigators in a 25-year longitudinal study from Gothenburg, Sweden, with 1,462 participants found the prevalence of hot flashes to be at a maximum of 60% at 52 to 54 years of age. Interestingly, 9% of subjects still reported hot flashes at age 72.
patients in the menopausal transition. More than 80% of women who experience hot flashes will experience them for longer than 1 year. Twenty-five percent of women complain of severe hot flashes. Without treatment, the symptoms usually subside slowly over 3 to 5 years. Investigators in a 25-year longitudinal study from Gothenburg, Sweden, with 1,462 participants found the prevalence of hot flashes to be at a maximum of 60% at 52 to 54 years of age. Interestingly, 9% of subjects still reported hot flashes at age 72.
The etiology of hot flashes seems to be the withdrawal of estrogen rather than the state of hypoestrogenism. For example, women with Turner syndrome who have not been treated with exogenous estrogen do not experience hot flashes. Those who are treated with estrogen, which is later withdrawn, will experience symptoms of vasomotor instability. Obese women seem to be less symptomatic than matched controls with a lower BMI. The explanation may be that obese women are less hypoestrogenic secondary to peripheral conversion of adrenal androgens into estrone or that obesity lowers sex hormone–binding globulin levels, allowing a greater proportion of their estrogen to remain unbound and able to act on target tissues. Studies of hot flashes with external monitoring of skin temperature and resistance have shown a frequency of approximately 54 plus or minus 10 minutes. This frequency has been shown to interrupt random eye movement sleep and potentially contributes to some of the psychosocial complaints. Hot flashes are correlated temporally with pulses of LH; however, exogenous LH does not induce a flash, suggesting that there is some central mediator leading simultaneously to hot flashes and LH pulses.
Several biochemical alterations are associated with the hot flash. During the actual episode, there is evidence of a rise in plasma LH, epinephrine, corticotropin, cortisol, androstenedione, DHEA, β-lipotropin, β-endorphin, and growth hormone. Levels of estradiol, estrone, prolactin, thyroid-stimulating hormone, FSH, and norepinephrine are unchanged.
Treatment
Vasomotor symptoms are the most common indication for use of estrogen treatment in menopause and are also a Food and Drug Administration (FDA)-approved indication. Estrogen therapy, in either oral or transdermal form, has a greater than 95% efficacy for the treatment of hot flashes. Hot flash frequency is first notably reduced after 2 weeks of treatment, and the full effect of a certain dosage can be determined reliably after 4 weeks. Due to concerns over the risk–benefit analysis of HRT use, it is recommended that women be treated for vasomotor symptoms for short periods (1 to 4 years) and then gradually tapered because symptoms often recur when treatment is discontinued abruptly. For patients with contraindications to estrogen use, vasomotor symptoms can be treated, albeit less efficiently, with progestins, α2-adrenergic agonists (clonidine, methyldopa, lofexidine) and, possibly, antidepressants (selective serotonin reuptake inhibitors [SSRIs], venlafaxine hydrochloride [Effexor]). The SSRI/selective norepinephrine reuptake inhibitors (SSNIs) and gabapentin appear to show modest reduction in hot flashes when compared with placebo and should be utilized over herbal therapies that have not shown efficacy. Treatment side effects may, however, be a problem with these agents.
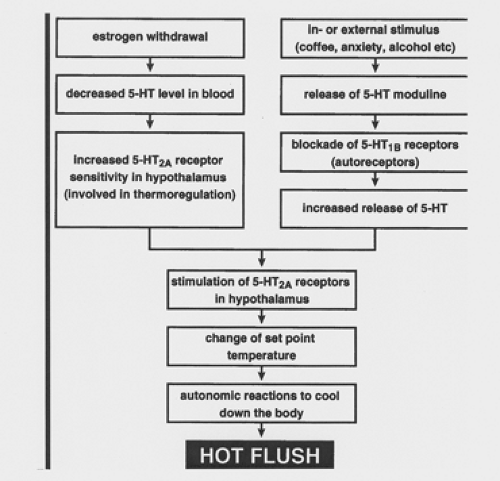 Figure 42.3 Possible mechanism by which a hot flush is induced. (5-HT, 5-hydroxytryptamine.) (From Berendsen HH. The role of serotonin in hot flushes. Maturitas 2000;36:155–164 , with permission.) |
The role of serotonin (5-hydroxytryptamine [5-HT]) in symptoms of menopause is being investigated increasingly. Serotonin levels fall with menopause, either naturally or surgically induced, and replacement estrogen is known to increase serotonergic tone. The 5-HT2A receptor subtype is thought to underlie changes in thermogenesis. Stimulation of this receptor may lead to changes in the set point temperature, leading to autonomic changes that cool the body. An increased skin temperature and sweating may result. Thus, an involvement for the 5-HT2A receptor in the etiology of hot flashes has been suggested. A theoretical model, illustrating a role for 5-HT in the mechanism of the hot flash, is shown in Figure 42.3.
Most studies using SSRIs for the treatment of vasomotor symptoms have been in patients with breast cancer. The differential between the antidepressant effects of these agents versus a direct effect on vasomotor symptoms may be more difficult to detect in this population.
Genitourinary Atrophy
Vagina
A decrease in circulating estrogen levels has deleterious effects on urogenital epithelium. Up to 50% of postmenopausal women experience symptoms of vaginal atrophy. The most common symptoms include dryness, irritation,
itching, burning, and dyspareunia. Atrophic vaginitis is associated with a rise in vaginal pH, which can lead to more frequent infections and worsening, irritative symptoms. A concurrent decrease in vaginal lubrication can lead to bleeding and decreased sexual comfort and pleasure.
itching, burning, and dyspareunia. Atrophic vaginitis is associated with a rise in vaginal pH, which can lead to more frequent infections and worsening, irritative symptoms. A concurrent decrease in vaginal lubrication can lead to bleeding and decreased sexual comfort and pleasure.
Estrogen replacement therapy (ERT) is an effective treatment for vaginal atrophy. The systemic dosage necessary for vaginal protection is somewhat higher than needed for bone protection (see below), and thus, topical therapy by means of creams or vaginal rings may be advisable to limit systemic absorption. Unless systemic HRT is required for vasomotor instability, local estrogen therapy can be used effectively to treat urogenital atrophy. Vaginal estrogen cream or tablets can be used daily for approximately 2 to 3 weeks and then twice weekly after initial symptoms have improved and vaginal vascularization (hence, hormone uptake) has increased. Treatment usually is long term, as symptoms tend to recur when estrogen is discontinued. The twice-weekly estrogen regimen can be used without supplemental progestin and without an increase in endometrial thickness. The dosage should be kept low, however, because the well-vascularized vagina is extremely efficient in the absorption of steroids. The new low-dose vaginal ring also may be used without progestin protection of the endometrium.
Vaginal estrogen frequently will improve symptoms of urinary frequency, dysuria, urgency, and postvoid dribbling. A direct effect to improve urinary incontinence is less clear. Alternatives to estrogen include vaginal moisturizers and lubricants. There is no evidence to support the use of Agrimony, black cohosh, chaste tree, dong quai, witch hazel, or phytoestrogens for the treatment of atrophic vaginitis.
Urinary Tract Infections
Urinary tract infections (UTIs) are common among women of all ages. Worldwide, an estimated 150 million UTIs occur annually. In the United States, UTIs account for more than $6 billion in health care costs. The incidence is highest in 18- to 24-year-old women at 17.5% and is 9% for women older than 50 years. In younger women, the major risk factors for recurrent UTI are sexual intercourse and spermicide exposure. In older, institutionalized women, the most important risk factors include urinary catheterization and functional status.
Healthy, postmenopausal women have different risk factors for recurrent UTI than those mentioned previously. Recurrent UTIs in healthy postmenopausal women are associated with urinary incontinence, cystocele, and increased postvoid residual volumes. Other significant risk factors include at least one episode of UTI prior to menopause, urogenital surgery, and reduced urinary flow. From the Heart and Estrogen/Progestin Replacement Study (HERS) of postmenopausal women with coronary heart disease (CHD), additional risk factors included diabetes, vaginal itching, and vaginal dryness.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree