Background
Introduction of human papillomavirus–based screening is ongoing in many countries, given its higher sensitivity and longer-lasting protection compared with cytology-based screening. However, optimal clinical management of human papillomavirus–positive but cytology-negative women is unclear, and additional studies with clinical follow-up are warranted.
Objective
The aim of the current study was to investigate the long-term outcomes of the clinical management used in a double-blind, randomized clinical trial of human papillomavirus screening conducted in the context of the routine, organized screening program in Sweden.
Study Design
Among 12,527 women aged 32–38 years enrolled in the trial, we followed up the 195 women who attended the colposcopy screening who were cytologically normal but persistently human papillomavirus positive (at least 12 months later; median, 19 months) in the human papillomavirus testing arm (n = 100) or were randomly selected from the control arm (n = 95). Women in the human papillomavirus testing arm were followed up with repeated human papillomavirus testing, cytologies, and colposcopies if persistently human papillomavirus–positive without cervical intraepithelial neoplasia grade 2 or worse. A similar number of random colposcopies and tests were carried out in the control arm. Women were followed up over 13 years for the main outcome measures: cumulative incidence of cervical intraepithelial neoplasia grade 2 or worse and cervical intraepithelial neoplasia grade 3 or worse.
Results
Among women who continued to attend and had continuous human papillomavirus persistence, all (40 of 40, 100% [95% confidence interval, 91–100%]) developed cervical intraepithelial neoplasia grade 2 or worse. There were no cases among women who cleared their human papillomavirus persistence (0 of 35, 0% (95% confidence interval, 0–10%) ( P < .001). Among women who had had human papillomavirus persistence but did not continue with repeated human papillomavirus tests (unknown persistence status), 56% (15 of 27 women) developed cervical intraepithelial neoplasia grade 2 or worse. Almost all cases occurred within 6 years. The intensive clinical management in the trial appeared to result in diagnoses of earlier cervical intraepithelial neoplasia grade 2 or worse but apparently did not prevent cervical intraepithelial neoplasia grade 2 or worse.
Conclusion
Women with human papillomavirus persistence will, in general, either become human papillomavirus negative or develop cervical intraepithelial neoplasia grade 2 or worse within 6 years, even with intensive clinical follow-up.
Related editorial, page 206
Cytological screening every 3–5 years reduces the cervical cancer risk by about 80% in countries with organized screening programs through early detection and treatment of precursor lesions (cervical intraepithelial neoplasia [CIN]). Clinical management of women includes referral to colposcopy, colposcopically directed biopsies, surgical removal of histologically verified high-grade lesions and posttreatment follow-up.
Human papillomavirus (HPV) is the major cause of cervical cancer. HPV-based screening is more sensitive than cytology in detecting CIN grade 2 or worse (CIN2+), providing a greater and more long-lasting protection against invasive disease. However, the optimal clinical management of women who screen HPV positive but cytology negative is unclear. Persistent infection with high-risk (HR) HPV is necessary for the development of cervical cancer. Most infections are transient, but women with a persistent infection have a high risk for development of CIN2+ and cervical cancer.
The HPV screening trial Swedescreen was nested in the population-based, organized cervical cancer screening program in Sweden. The clinical management of HPV-positive, cytology-negative women was a repeat HPV test at least 12 months later and, if type-specific HPV persistence was found, referral to colposcopy with colposcopically directed biopsies and endocervical cytology. In case of normal colposcopy 2 blind cervical biopsies were taken. At the first study colposcopy, 28 of 100 women with HPV persistence in the intervention arm and 2 of 95 women selected at random from the control arm had CIN2+. If women were not treated for CIN2+, they were invited for repeat yearly HPV testing and were, if persistently HPV positive, invited to an additional colposcopy.
The aim of the present study was to evaluate the long-term outcome of the clinical management of these women, using comprehensive nationwide linkages with the nationwide Swedish cervical screening registry.
Materials and Methods
Study population and data
The Swedescreen population-based randomized clinical trial (RCT) of primary HPV screening was started in May 1997. Women aged 32–38 years attending organized screening in 5 different regions of Sweden were invited to participate. In Sweden, women are invited for organized screening only if they have not had any recent opportunistic smears. No further exclusion criteria were used.
Since 1997, the trial participants have been followed up with registry linkages. Using unique personal identification numbers, linkages to the Swedish National Cervical Screening Registry (NKCx) have been performed up to December 2012 to identify all histological endpoints occurring among study participants, regardless of whether the histological endpoint was the result of study colposcopies. Until the primary endpoint of the study was reached (protection against CIN2+ in a second screening round), all histopathologies were re-reviewed by an expert pathologist.
For the long-term follow-up after this point, routine histopathological diagnoses were used and identified through linkage to the NKCx. The current analysis makes use of the re-reviewed histopathologies from the active study period and routine histopathological diagnoses that were obtained for lesions occurring outside the study colposcopies and during the long-term follow-up.
Randomization and masking
In total, 12,527 women were enrolled, following informed consent, and randomized 1:1 to HPV and cytology double testing (intervention arm, n = 6257) or cytology only (control arm, n = 6270). Women were randomized using computer-generated random numbers by an independent institute (the Cancer Registry of Stockholm). Further details on the study protocol have been published previously. Both the study participants and the colposcopists were blinded to the women’s HPV status. The trial was unblinded in August 2003, after the completion of the first study colposcopies and 3 years after recruitment concluded.
Intervention
HPV DNA testing
For all women, a Papanicolaou smear and a brush sample for HPV DNA testing were collected. All samples were analyzed in the intervention arm as well as a random sample of the control arm. HPV DNA testing was conducted using polymerase chain reaction with GP5+/6+ polymerase chain reaction primers and subsequent typing with reverse dot-blot hybridization. The assay correlated well to the HPV assay used in other clinical validation studies.
Colposcopy follow-up
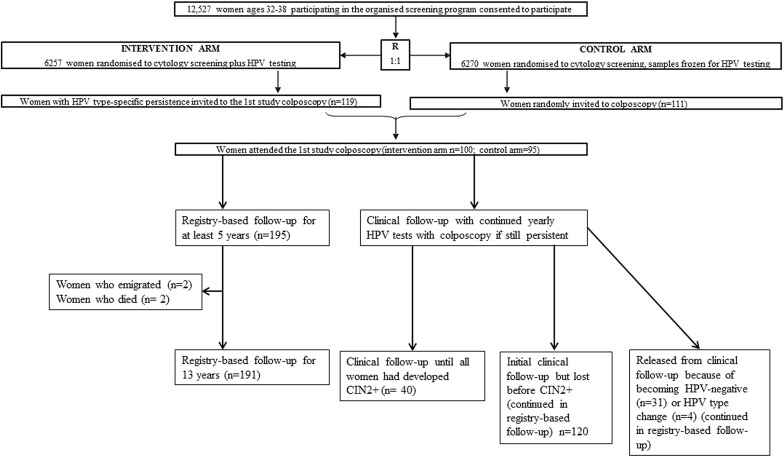
In the intervention arm, women who were HPV positive and cytology negative at enrollment were invited for a second HPV test at least 12 months later (median actual attendance, 19 months; interquartile range, 15–27 months). At baseline, 433 women were HPV positive, of which 341 were cytology negative and therefore invited to a second HPV test. A total of 270 women attended the second HPV test, of which 119 women (44%) had type-specific HPV persistence and were subsequently invited to colposcopy (100 women attended). In the control arm, 111 women were selected at random for mock HPV testing and subsequent colposcopies to control for ascertainment bias (95 women attended). The results of the first study colposcopies have been published.
Following the first study colposcopy, women who had not been treated for CIN2+ were invited for yearly repeat HPV tests. Throughout the study, all cytological abnormalities were managed according to the routine procedures used in the Swedish organized screening program. Women with continuous type-specific HPV persistence as well as a similar number of randomly chosen control women were invited for additional colposcopies ( Figure 1 ). Subsequent colposcopies were performed according to the same protocol and conducted by the same gynecologists as the first study colposcopies, except that HPV test results were unblinded.
The protocol included an examination of the transformation zone for visibility, maturation, and epithelial changes. Five percent acetic acid was applied to identify undifferentiated epithelia or inflammation as well as CIN. The dimension and border of abnormal areas were assessed by application of 5% potassium iodine to the ectocervix. All acetowhite areas, including metaplasia (undifferentiated epithelium), inflammation, and neoplasia were considered as abnormal colposcopies and biopsied. The endocervix was sampled with a cytobrush.
Blind biopsies were taken at 12 o’clock and 6 o’clock on the ectocervix close to the squamocolumnar junction if the colposcopy was considered to be normal, according to standard protocol at the time. Treatment and further management of the women based on the results of cytologies and histopathologies followed the routine procedures of the screening program.
Statistical analysis
The 195 women who attended the first study colposcopies were followed up using registry linkages. The start of the follow-up was the date of the first test in the RCT. The first instance of histologically confirmed CIN2+ or CIN3+ were identified through the study colposcopies and linkages to the NKCx. Women were censored at the last registered testing date, which is appropriate for screen-detected disease. There were no cases of invasive disease found during follow-up in either arm.
Linkages to the Swedish population registry found that 191 of 195 women were alive and resident in Sweden throughout the follow-up. Two women were deceased (both in the intervention arm) and 2 women had emigrated. All 195 women were alive and resident in Sweden for >5 years. Women were categorized as having a known persistence status (time frame determined as a positive HPV test result plus 1 year for the analysis), unknown persistence status (no HPV test performed 1 year after the last HPV test; in other words, more than 1 year since the last HPV test was taken), HPV type-specific clearance after persistence, and HPV negativity after persistence. Women who switched types were persistently positive for one type and then at a subsequent test were negative for the initial type but positive for a new type.
Absolute risks for CIN2+ and CIN3+ were calculated for the entire follow-up period (13 years). Cumulative incidence proportions of CIN2+ and CIN3+ were estimated using 1 minus the Kaplan-Meier curves.
All analyses were completed using STATA 11 (StataCorp, College Station, TX). The Swedescreen study was approved by the ethical review board in Stockholm, Sweden (DNR 1996/305). The long-term follow-up of the Swedescreen study had an additional approval from the ethical review board in Stockholm, Sweden (DNR 2012/780-32).
Results
In total, 195 women with a normal cytology attended the first study colposcopies (100 women in the intervention arm with persistent HR HPV infection and 95 randomly selected women from the control arm). The CIN2+ cases detected at the first study colposcopy have previously been described. During the long-term follow-up (up to 13 years), linkages were made to the national screening registry NKCx.
All 195 women were found to have had additional cytologies in the national registry (mean number of cytologies, 8; range, 4–17) and 192 of 195 of the biopsies from the per-protocol colposcopies were identified in the registry (mean number of cervical histopathologies, 2; range, 1–9). An additional 25 CIN2+ diagnoses occurred in the intervention arm, but no further CIN2+ occurred in the control arm.
Among the 102 women with initial HR HPV type-specific persistence (all 100 women in the intervention arm and 2 among the randomly selected women in the control arm), the absolute risk for CIN2+ over the long-term follow-up was 54% (55 of 102, 95% confidence interval [CI], 44–63%) and the risk for CIN3+ was 32% (33 of 102, 95% CI, 24–42%). By study arm, the risks for CIN2+ was 54% in the intervention arm and 2% in the control arm (log rank value of P < .001).
Persistence by type was as follows: 25 women with HPV 16, 11 with HPV18, 31 with HPV 31, with 6 HPV 33, 9 with HPV 45, and 20 with other HR HPV (not shown). Among the 25 women with a type 16 persistence, 68% (17 of 25, 95% CI, 48–83%) and 56% (14 of 25, 95% CI, 37–73%) developed CIN2+ and CIN3+, respectively (not shown). Persistence with HPV16 conferred the highest risk for CIN2+ followed by HPV 18 (7 of 11, 64% [95% CI, 35–85%]), HPV 31 (18 of 31, 58% [95% CI, 41–74%]), HPV 33 (3 of 6, 50% [95% CI, 44–100%]), HPV 45 (4 of 9, 44% [95% CI, 19–73%]), and other HR types (6 of 20, 30% [95% CI, 15–52%]) (not shown). None of the women in the control arm who were HR HPV negative at baseline developed CIN2+ during follow-up (not shown).
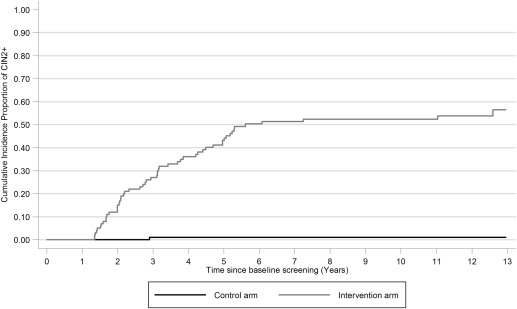
The cumulative incidence proportion of CIN2+ increased steadily in the intervention arm in the first 6 years of follow-up. However, after 6 years, only 3 additional cases of CIN2+ occurred ( Figure 2 ). For CIN3+, the pattern was similar, with only 1 additional case occuring after 6 years ( Figure 3 ). Among the 40 women who attended repeat testing and were known to be continuously HPV persistent, all 40 of 40 developed CIN2+ within 7 years of the baseline HPV-positive test result ( Figure 4 ); 63% (95% CI, 48–77%) and 98% (95% CI, 89–100%) had developed CIN2+ at CIN3+ after 6 years of follow-up, respectively.