Management of the Difficult Airway
Brent R. King
Carin A. Hagberg
Introduction
One of the most challenging tasks facing caregivers is maintaining the technical skills necessary for the management of the difficult airway. This is especially true for those providing care to pediatric patients. Data for anesthesiologists regarding closed malpractice claims show a higher frequency of adverse respiratory events in the pediatric population (1,2). The majority of these events were related to inadequate ventilation, airway obstruction, and complications of endotracheal intubation. Although corollary data from the emergency department (ED) have not been reported, it seems likely that if such adverse events are a significant problem in the controlled environment of the operating room, they are even more frequent in the ED.
Fortunately, the vast majority of children who require airway management for respiratory failure or airway protection in the ED are successfully managed using standard techniques such as bag-valve-mask ventilation (see Chapter 14) and endotracheal intubation (see Chapter 16). However, in some cases these methods fail, whether because of unique anatomic characteristics of the pediatric airway, acquired or congenital abnormalities, operator inexperience, or some combination of these factors. When failure occurs, an alternative technique must be quickly employed or the child may suffer irreversible hypoxemic injury. This chapter describes many of the alternatives to standard airway management, along with the rationale for their use. It is not intended to provide an exhaustive list of every available tool or device. Furthermore, certain techniques that might be used to manage a difficult airway are covered elsewhere within this text and therefore will not be described in this chapter, including blind nasotracheal intubation (see Chapter 16), percutaneous transtracheal ventilation (see Chapter 18), and surgical cricothyrotomy (see Chapter 26). Finally, the reader must understand that, like all types of medical technology, the tools and techniques used for managing the difficult airway are constantly evolving.
Definitions
Several important definitions are vital to a thorough understanding of the information that follows. Caregivers who manage the airway are often confronted with the responsibility of determining whether or not a patient will present increased difficulty for airway management.
Predicted Difficult Airway
Based on certain physical characteristics, some patients can be predicted to have a problematic airway. Such difficulty arises in regard to five different techniques of airway management: (a) bag-valve-mask (BVM) ventilation, (b) laryngoscopy, (c) orotracheal and/or nasotracheal intubation, (d) supraglottic airway placement, and (e) surgical airway. Since a given patient might require any one or more of these techniques, every patient should be evaluated for possible problems regarding all of them. Many patients will have more than one area of potential difficulty, and such considerations should dictate the type of airway management utilized (see below).
Known Difficult Airway
Patients who have had previous difficult or failed attempts at airway management are said to have a known difficult airway. Whenever possible, family members should be specifically questioned about any history the patient might have of prior problems with airway management. Likewise, those who have undergone a difficult or failed airway attempt should be advised to purchase and wear medical identification jewelry
indicating this (e.g., Medic-Alert bracelets), and if time permits, emergency providers should always look for such identifying jewelry when a patient requires airway management.
indicating this (e.g., Medic-Alert bracelets), and if time permits, emergency providers should always look for such identifying jewelry when a patient requires airway management.
Failed Airway
Although failed intubation—or the inability to place the endotracheal tube after multiple attempts—is a clear endpoint, there is no single accepted definition of a “failed airway.” The American Society for Anesthesiology (ASA) defines a failed airway as the inability to obtain a definitive airway after three attempts by an experienced laryngoscopist. Others describe a failed airway as any attempt in which the patient becomes hypoxemic. Critical to all definitions, however, is the concept that it is unwise for the same operator to continue using the same unsuccessful technique repeatedly while the condition of the patient deteriorates. Although simple, this can be a difficult concept to acknowledge in the heat of a resuscitation that is going poorly. Above all, each operator should approach every intubation with a planned primary technique and at least one alternative technique in mind.
Emergent Airway
When the patient requires immediate airway management, with little or no time for stabilization and assessment, a true emergency exists. In such cases, the operator’s options for management may be limited.
Urgent Airway
Many patients have impending respiratory failure or a waning level of consciousness and will require that their airway be secured. However, they are stable enough to allow the operator the opportunity to perform a somewhat more thorough assessment in order to determine the most appropriate course of action.
Elective Airway
Patients undergoing elective airway management are those who require endotracheal intubation, but not on an urgent or emergent basis. Airway management of such patients should be held to the same standards of care as patients electively intubated in the operating room.
Anatomy and Physiology
Detailed descriptions of the key anatomic features of the pediatric airway and important differences from the adult airway, as well as clinically relevant aspects of pediatric respiratory physiology, can be found in Chapters 12, 14, and 16. This section will therefore focus on anatomic and physiologic principles related to the difficult pediatric airway, with an emphasis on congenital and acquired abnormalities that pose the primary obstacles to successful airway management. Knowledge of syndromes that may adversely affect the operator’s ability to secure a definitive airway is crucial in caring for children with severe respiratory problems. Moreover, the presence of one anomaly mandates the search for others. Considerations regarding pediatric airway anatomy and respiratory physiology are described as they pertain to the five types of airway management.
Difficult Bag-Valve-Mask Ventilation
Inability to provide adequate BVM ventilation generally results from one or more of the following problems: inadequate mask seal, excessive gas leak, and excessive resistance to the ingress or egress of gas (3). While failure to intubate does not necessarily lead to hypoxia, since BVM ventilation can be often be continued temporarily as an effective means of respiratory support, failure to intubate and provide adequate BVM ventilation can quickly result in profound and life-threatening hypoxia. Interestingly, there is a paucity of literature describing the prediction of difficult BVM ventilation.
Problems with maintaining a mask seal during BVM ventilation can be anticipated in children with midfacial abnormalities. The most common congenital abnormality of the midface with the potential to affect the quality of the mask seal is cleft lip. Additionally, certain children have abnormal facial shapes (e.g., mandibular hypoplasia) that make it difficult or impossible to provide an adequate mask seal. The mask seal can also be adversely affected by severe trauma to the face. Of note, it may not be possible to properly apply a face mask with children who have dental appliances and other medical hardware that involve the oral cavity or the area between the nose and chin.
Obstruction can occur at any level from the oral cavity to the lower airway. For example, children with Beckwith-Wiedemann syndrome have an excessively large tongue, which can obstruct the airway. Angioedema can also cause a dramatically enlarged tongue. Congenital and acquired conditions involving the glottis and adjacent structures that produce airway obstruction (croup, bacterial tracheitis, foreign body, thermal injuries, etc.) can make BVM ventilation impossible. Finally, as described in Chapter 14, effective manual ventilation requires proper positioning of the head and neck to maximize airflow. Obstruction can occur even in a child with a relatively normal airway when the neck is immobilized in a cervical collar and cannot be adequately positioned. Neck mobility may also be limited in patients with certain congenital syndromes and obesity (4).
Difficult Laryngoscopy
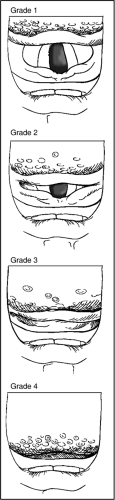
Difficult laryngoscopy is defined as the inability to visualize any portion of the vocal cords by conventional laryngoscopy. Many investigators include grades III (visualization of the epiglottis but not the vocal cords) and IV (visualization of only the tongue or the tongue and the soft palate) or grade IV
alone, according to the Cormack-Lehane original grading of the rigid laryngoscopic view (5) (Fig. 17.1). Difficulty in performing endotracheal intubation is the end result of difficult laryngoscopy and is dependent on the operator’s level of expertise, patient characteristics, and clinical circumstances. Thus it has been suggested that the definition of difficult intubation be based on a uniform understanding of the best attempt at performing laryngoscopy/intubation and should use the number of attempts and time as boundaries only (6). The best attempt should incorporate the effect of changing the patient’s position; the effect of changing the length or type of laryngoscope blade; and the effect of simple airway manipulations, such as conventional cricoid pressure, backwards, upwards, rightward pressure (BURP), and optimal external laryngeal manipulation (OELM) (see Chapter 16).
alone, according to the Cormack-Lehane original grading of the rigid laryngoscopic view (5) (Fig. 17.1). Difficulty in performing endotracheal intubation is the end result of difficult laryngoscopy and is dependent on the operator’s level of expertise, patient characteristics, and clinical circumstances. Thus it has been suggested that the definition of difficult intubation be based on a uniform understanding of the best attempt at performing laryngoscopy/intubation and should use the number of attempts and time as boundaries only (6). The best attempt should incorporate the effect of changing the patient’s position; the effect of changing the length or type of laryngoscope blade; and the effect of simple airway manipulations, such as conventional cricoid pressure, backwards, upwards, rightward pressure (BURP), and optimal external laryngeal manipulation (OELM) (see Chapter 16).
Difficult Endotracheal Intubation
Difficult endotracheal intubation is described as intubation that requires multiple attempts in the presence or absence of tracheal pathology. Endotracheal intubation primarily depends on an adequate laryngoscopic view of the airway. Problems that affect the operator’s ability to visualize the airway would be expected to negatively affect endotracheal intubation. Although rarely encountered in clinical practice, there are several of these potential problems.
Any patient with serious midface trauma or instability may prove difficult to intubate. Likewise, certain medical appliances or impaled objects involving the lower face may impede the passage of the laryngoscope blade. Facial abnormalities, such as an excessively long or short mandible or neck, will change the position of the airway as compared with normal patients and can make adequate visualization of the airway difficult or impossible. A classic example of such an abnormality is Pierre-Robin syndrome, a congenital disorder that includes severe micrognathia. Many patients have problems with the oral cavity itself, such as impaired mouth opening secondary to medical appliances, facial scars, or abnormalities of the temporomandibular joint; the large tongue of Beckwith-Wiedemann syndrome; and cleft palate. With cleft palate, the laryngoscope can slide into a cleft, impairing visualization and potentially causing trauma. Also, significant trauma to the structures within the oral cavity or to the extrathoracic airway can distort the normal anatomy and make laryngoscopic visualization impossible. Additionally, blood and other secretions may prevent the operator from obtaining an acceptable view of the airway.
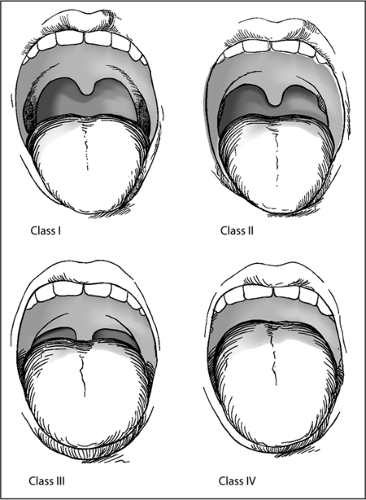
It may be possible to predict difficulty with endotracheal intubation based on the extent to which the palate, uvula, and tonsils can be visualized with the patient’s mouth open and tongue extended or depressed with a tongue depressor. Several investigators, most notably Cormack and Lehane (5) and Mallampati et al. (7), have demonstrated the relationship between this view of the airway and later difficulty with endotracheal intubation (Fig. 17.2). If the posterior palate is easily visualized, the patient is unlikely to have a difficult intubation,
whereas if only the tongue can be visualized, the likelihood of a difficult intubation is greatly increased. Unfortunately, most of these studies have involved patients presenting for elective surgery. For example, during the classic Mallampati evaluation, the patient sits upright on the edge of the bed with the neck extended, the mouth open, and the tongue protruding. Obviously, this type of assessment is not directly applicable to most emergency intubations. Yet in many cases, the emergency physician will be able to make at least a reasonable estimate of the patient’s Mallampati/Cormack-Lehane score. For the unconscious patient, it may be possible to briefly inspect the mouth by simply using a tongue depressor. Likewise, many patients with respiratory distress will nonetheless be able to cooperate with voluntary mouth opening. For crying infants and children, this inspection may in fact be relatively easy, although there is some evidence suggesting that these predictive scoring systems may be less accurate in younger patients.
whereas if only the tongue can be visualized, the likelihood of a difficult intubation is greatly increased. Unfortunately, most of these studies have involved patients presenting for elective surgery. For example, during the classic Mallampati evaluation, the patient sits upright on the edge of the bed with the neck extended, the mouth open, and the tongue protruding. Obviously, this type of assessment is not directly applicable to most emergency intubations. Yet in many cases, the emergency physician will be able to make at least a reasonable estimate of the patient’s Mallampati/Cormack-Lehane score. For the unconscious patient, it may be possible to briefly inspect the mouth by simply using a tongue depressor. Likewise, many patients with respiratory distress will nonetheless be able to cooperate with voluntary mouth opening. For crying infants and children, this inspection may in fact be relatively easy, although there is some evidence suggesting that these predictive scoring systems may be less accurate in younger patients.
Airway obstruction can also prevent successful endotracheal intubation, so a critical part of airway assessment is the identification of possible obstruction. The characteristic signs of this condition are familiar to most emergency physicians. Patients with stridulous breathing, dysphonia, aphonia, drooling, and/or so-called tripod positioning are likely to have obstruction of the extrathoracic airway. In some cases, the cause of the obstruction (e.g., a foreign body) can be identified and corrected promptly so that intubation can be performed without undue delay. However, many causes of severe airway obstruction are not amenable to immediate correction, and an alternative technique may be required. As with BVM ventilation, decreased mobility of the neck can be a
significant impediment, especially for the patient with airway obstruction, and it often greatly decreases the likelihood that intubation will be accomplished.
significant impediment, especially for the patient with airway obstruction, and it often greatly decreases the likelihood that intubation will be accomplished.
Difficult Supraglottic Airway Placement
With some notable exceptions, the devices used to “rescue” the airway when standard techniques have failed are placed in the supraglottic region with or without occlusion of the esophagus. Their effectiveness relies on lower airway resistance distal to the glottic opening. Consequently, in patients with significant airway obstruction or with obstructive lung disease, these devices may not provide effective ventilation, because the resistance to airflow is actually lower in the proximal portion of the upper airway (i.e., the oral cavity) than in the distal airway. Likewise, such devices may be difficult to insert when mouth opening is significantly restricted or there is distorted airway anatomy.
Difficult Surgical Airway
The term “surgical airway” is often used to describe several potentially life-saving methods of securing the airway by creating an external air passage into the trachea. However, all of these techniques share similar potential drawbacks of vital consideration for proper patient selection. For example, any method for securing a surgical airway requires the identification of external anatomic landmarks. If these structures cannot be readily identified (e.g., the patient is morbidly obese or has severe edema involving the neck), the likelihood of success on a timely basis decreases significantly. Furthermore, patients who have undergone prior airway surgery may have scar tissue or anatomic variation that adds another layer of complexity to an already difficult task. Finally, frank disruption of the airway may require direct surgical intervention, and therefore certain invasive airway techniques, such as retrograde intubation or cricothyrotomy, may not be effective.
Airway Management Algorithms
Airway Assessment
For anesthesiologists, preoperative evaluation is important in the identification of patients at risk for airway management problems, as any anatomical features and clinical factors associated with a difficult airway can be carefully evaluated (5,8,9,10,11,12,13). Unfortunately, emergency physicians rarely have the opportunity to perform such a detailed assessment.
It should be noted that many of the evaluations used for predicting a difficult airway in adults have not been extrapolated to the pediatric population. Patient cooperation cannot be assured, and therefore the airway exam is often limited. Even under the best of circumstances, some characteristics predisposing to a difficult airway will only be discovered after the patient has undergone a rapid sequence induction. Thus the emergency physician must rely on the most thorough assessment that can be performed given the time constraints imposed by the patient’s condition and should always be prepared with alternative techniques for the possibility of an unanticipated difficult airway. Clinicians should also be familiar with one or more of the published airway management algorithms (Figs. 17.3 and 17.4).
Anatomic features that can negatively affect airway management have been discussed previously (see “Anatomy and Physiology”). What follows is a brief description of a rapid, systematic assessment aimed at identifying patients at risk for the five types of difficult airway (Table 17.1). If, based on this evaluation, problems can be anticipated with intubation and/or the alternative techniques described below, then the operator should consider securing the airway without the use of paralytic agents.
The ED airway evaluation should begin with a brief external examination, the purpose of which is to identify features that might adversely affect airway management. These may include obvious characteristics like severe trauma, congenital facial abnormalities, and medical appliances, as well as more commonplace traits like obesity and the presence of facial hair. The operator should then (a) look for any problems the patient might have with opening the mouth; (b) estimate the patient’s Mallampati/Cormack-Lehane score; (c) assess the relative length of the mandible; and (d) determine the location of the hyoid bone, which serves as a landmark for the most caudal portion of the airway. Limited mouth opening, an excessively long or short mandible, and a more rostrally or caudally located hyoid bone all suggest that an optimal laryngoscopic view of the airway might be difficult to obtain. While guidelines for determining these proportions are well described for adult patients, the development of pediatric guidelines has lagged far behind. As mentioned, preliminary data indicate that the Mallampati classification may be an insensitive predictor of difficult intubation in the pediatric population (14). Also, Berry has suggested that the appropriate thyromental distance in infants should be one fingerbreadth (1.5 cm) (15).
During this evaluation, the operator should also be alert for clues suggesting airway obstruction and the degree to which the problems can be corrected by simple maneuvers designed to reposition the airway. Signs like snoring and noisy breathing, symptoms of upper respiratory tract infection, or a history of recurrent croup or problems with breathing while feeding suggests that the child has or is prone to extrathoracic airway obstruction. Such children may be difficult to ventilate with a BVM circuit or a supraglottic device.
Finally, the operator must determine whether there will be any significant impediment to moving the patient’s neck, as this can be an important factor in both obtaining an adequate laryngoscopic view of the airway and performing effective BVM ventilation. Patients with a suspected unstable cervical spine fracture whose head, neck, and torso are fully immobilized or those with congenital abnormalities that severely restrict neck mobility may be difficult to intubate and/or
manually ventilate, especially if another airway problem is present (large tongue, extreme rostral location of the glottis, etc.)
manually ventilate, especially if another airway problem is present (large tongue, extreme rostral location of the glottis, etc.)
When a patient is suspected of having a difficult airway, the operator should consider whether the procedure can proceed as planned or whether an alternative technique should be chosen. As mentioned previously, one of the most important questions is whether a paralytic agent should be used. If the airway exam suggests potential difficulty in more than one of the areas discussed (especially BVM ventilation), then intubation should be delayed whenever possible until equipment and personnel are available for an alternative technique, such as awake fiberoptic intubation with judicious use of sedation. Unfortunately, this is not always an option in the ED. The condition of the patient often mandates that a definitive airway be secured immediately. Consequently, many authorities recommend a so-called double setup approach to the patient with a potentially difficult airway. To perform a double setup, the operator or an assistant prepares the materials for orotracheal intubation (and/or alternative techniques) as well as those for a surgical airway. Prior to the administration of paralytic agents, the patient’s cricothyroid membrane is identified and the skin is cleaned with aseptic solution so that a surgical airway can be performed quickly should other methods fail.
TABLE 17.1 Rapid Airway Assessment | |
|---|---|
|
Unanticipated Versus Anticipated Difficult Airway
In the case of an unanticipated difficult airway, it is assumed in most algorithms that the patient who cannot be intubated has already received a paralytic agent as part of a rapid sequence induction regimen. In such cases, the first question is whether the patient can be ventilated with a BVM device. If so, then ventilation should proceed pending an alternative
technique or an intubation attempt by another clinician. If not, then the operator must act rapidly. This is the dreaded “cannot intubate–cannot ventilate” situation. In such situations, the operator should have an assistant prepare for one of the invasive airway techniques and, if possible, simultaneously attempt to place a rescue airway, unless the patient’s condition or anatomy suggests that there is little chance that such an airway would succeed. If the rescue airway fails, then an invasive airway must be established without delay. The simplest technique for most children is percutaneous transtracheal ventilation (see Chapter 18), which can initially be used to provide ventilation and then can be converted to a retrograde intubation or a wire-guided surgical cricothyrotomy.
technique or an intubation attempt by another clinician. If not, then the operator must act rapidly. This is the dreaded “cannot intubate–cannot ventilate” situation. In such situations, the operator should have an assistant prepare for one of the invasive airway techniques and, if possible, simultaneously attempt to place a rescue airway, unless the patient’s condition or anatomy suggests that there is little chance that such an airway would succeed. If the rescue airway fails, then an invasive airway must be established without delay. The simplest technique for most children is percutaneous transtracheal ventilation (see Chapter 18), which can initially be used to provide ventilation and then can be converted to a retrograde intubation or a wire-guided surgical cricothyrotomy.
Management of the true emergency airway should be performed in a similar fashion. In most cases, the child will arrive in the ED unconscious and apneic or will have high-grade upper airway obstruction. Once again, the central question is whether the child can be effectively ventilated with a BVM device. If so, then the operator can proceed with a brief assessment, followed by the appropriate intervention given the specific clinical circumstances. If BVM ventilation fails, immediate endotracheal intubation can be attempted, and if this is unsuccessful, a rescue airway procedure or surgical airway must be expeditiously performed.
Laryngeal Mask Airway
The laryngeal mask airway (LMA) is a supraglottic ventilatory device that consists of an oval inflatable silicon cuff in continuity with a wide-bore tube designed to fit the larynx of patients of various weights. The tube can be connected to a self-inflating bag or anesthesia circuit. LMAs in both adult and pediatric sizes (1 through 6) are available, and if used as conduits for intubation, these can accommodate 3.5- to 7.0-mm internal diameter (ID) endotracheal tubes (Fig. 17.5).
Select an appropriately sized LMA.
Remove the LMA from its packaging and inspect the cuff for leaks.
Completely deflate the cuff (a) using a commercial deflator or (b) by pressing the ventilating side against a flat surface and placing the fingers of the nondominant hand around the cuff while deflating the cuff with the dominant hand.
Lubricate the cuff using a water-soluble lubricant.
Open the patient’s mouth and, holding the LMA like a pen or pencil, guide the cuff along the palate and over the tongue. Continue with insertion until the LMA cannot be advanced further.
Inflate the cuff and connect the LMA to a bag and attempt to ventilate the patient.
If ventilation is not successful, check for an air leak or malpositioning of the cuff.
With the cuff fully deflated, the LMA resembles a small boat with a curved mast or smokestack.
Some operators prefer to insert the LMA with the cuff partially inflated. This may help to prevent complications associated with a malpositioned cuff.
It is important to follow the pharyngeal curve during insertion, keeping the cuff of the LMA against the palate as long as possible.
Most problems with the LMA occur when it is inserted improperly or when the patient is not adequately sedated. Improper depth of insertion, cricoid pressure, improper sizing, and neck mobility can all contribute to failure.
Special care should be taken when the LMA is used for the patient with a suspected cervical spine injury because placement of the device can cause the neck to move.
Indications
Although originally developed for airway management of routine cases with spontaneous ventilation, the LMA is now listed in the ASA difficult airway algorithm as an airway ventilatory device or a conduit for endotracheal intubation (3,16). It can be used in both pediatric and adult patients for whom BVM ventilation and intubation are difficult or impossible.
The LMA is unlikely to be effective in cases of upper airway obstruction or significant airway disruption. Additionally, the
LMA does not provide complete occlusion of the esophagus and therefore offers less protection against aspiration than other alternative devices or techniques. However, in many cases the benefits to be gained by effective ventilation outweigh such risks.
LMA does not provide complete occlusion of the esophagus and therefore offers less protection against aspiration than other alternative devices or techniques. However, in many cases the benefits to be gained by effective ventilation outweigh such risks.
 Figure 17.5 The LMA Classic. (Reproduced with permission from LMA North America, Inc., http://www.lmana.com/prod/components/products/lma_classic.html.) |
TABLE 17.2 Sizing Chart For The Laryngeal Mask Airway (Lma Classic) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Equipment
An array of pediatric and adult sizes are commercially available (LMA North America, Inc., San Diego, CA). An LMA of the appropriate size, a water-soluble lubricant, and a syringe to inflate the cuff are the only pieces of equipment required. The suggested weight for each size of LMA is listed on the side of the ventilating tube, along with the volume required to inflate the cuff (Table 17.2).
Procedure
After the LMA is removed from its package, the cuff should be inspected for damage. An essential component of LMA placement is the complete and proper deflation of the cuff. Although there is a special tool for this purpose, the cuff can be deflated without this tool, as follows. The anterior portion of the LMA cuff is placed against a firm, flat surface such as a table or countertop. The index and middle fingers of the operator’s nondominant hand are placed on either side of the cuff to compress it while a syringe in the operator’s dominant hand is used to remove all air until the cuff is completely empty. When properly deflated, the cuff has the appearance of a small boat with a long, curved mast or smokestack. After deflation, the LMA cuff should be lubricated with a water-soluble lubricant along its tip and posterior surface.
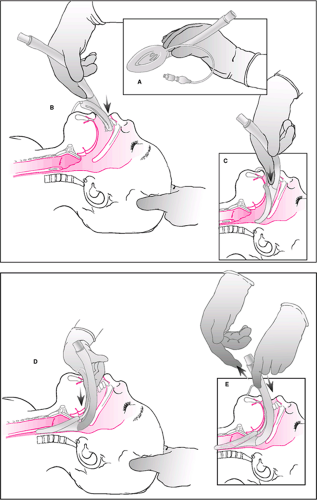
Two techniques for the use of an LMA are most commonly employed. To perform the first technique, the operator stands above the patient at the head of the bed in a position similar to that for a standard orotracheal intubation. The LMA is initially placed in the oropharynx with the ventilating portion facing the tongue. Holding the LMA like a pen or pencil (Fig. 17.6A), the operator uses the index finger of the dominant hand to guide the LMA along the patient’s palate until it cannot be advanced any further (Fig. 17.6B–E). At this point, the operator releases the shaft of the LMA, and the cuff is then inflated. Inflation of the cuff often helps the LMA to “seat” itself in the proper position. Once the LMA is in place, a resuscitation bag is attached, and the patient can be ventilated. The operator should then check for a leak. If a leak occurs at less than 15 cm H2O, the LMA may require repositioning or possibly removal and reinsertion. The second technique involves using the operator’s thumb to insert the device. With this method, the operator stands at the side of the bed facing the patient. Other methods have also been described in the literature, including insertion of the device with the ventilating portion facing the hard palate (17) and insertion with partial cuff inflation (18).
Complications
The most common complication associated with the placement of an LMA is lack of effective ventilation. This is usually dependent on the user’s skill and experience, the patient’s level of consciousness, and patient-specific anatomic or physiologic abnormalities (19). Placing the LMA correctly can be difficult in some patients. The mask may fold on itself either under or over its main axis. The incidence of failed placement is 1% to 5%, although the incidence tends to decrease with increasing operator experience (20). Failure to insert the LMA most often results from inadequate anesthesia, suboptimal head and neck position, incorrect mask deflation, failure to follow the palatopharyngeal curve during insertion, inadequate depth of insertion, cricoid pressure, and oral pathology such as large tonsils.
Laryngospasm and coughing may result from inadequate anesthesia, tip impaction against the glottis, or aspiration. Some operators prefer to insert the LMA with the cuff partially inflated in an attempt to avoid these problems. Complete epiglottis downfolding can increase the work of breathing and cause coughing and laryngospasm as well as complete airway obstruction (21).
Mask leaks or the inability to ventilate the lungs may also result from inadequate anesthesia, malpositioning of mask, inappropriate mask size, and high airway pressures. Hypoventilation due to coma or complete paralysis may lead to high end-tidal carbon dioxide concentration. Effects of pharyngolaryngeal reflexes like laryngospasm, coughing, gagging, bronchospasm, breath-holding, and retching may be associated with use of an LMA. Displacement of the LMA after insertion may be caused by inadequate anesthetic depth, a pulled or twisted tube, and an inadequately sized LMA. Patients who awaken with an LMA in place may have tube occlusion from biting.
Additionally, cuff deflation and tube removal at an inadequate depth of anesthesia can cause vomiting, severe coughing, and laryngospasm.
Several other complications of varying severity have been reported with use of an LMA. A known disadvantage of the device is the inability to protect against regurgitation
of gastric contents and pulmonary aspiration. Because the LMA does not isolate the trachea from the esophagus, the patient who has a full stomach or requires high airway pressures for adequate positive pressure ventilation is at greater risk for aspiration pneumonitis. However, when the indications and contraindications for the procedure are strictly observed, the likelihood of this complication is comparable to the likelihood with endotracheal intubation (22). In the ED, where every patient is assumed to have a full stomach, the potential risk of aspiration pneumonitis must be weighed against the advantages of the LMA in cases of difficult intubation and ventilation, when use of this device may be life-saving.
of gastric contents and pulmonary aspiration. Because the LMA does not isolate the trachea from the esophagus, the patient who has a full stomach or requires high airway pressures for adequate positive pressure ventilation is at greater risk for aspiration pneumonitis. However, when the indications and contraindications for the procedure are strictly observed, the likelihood of this complication is comparable to the likelihood with endotracheal intubation (22). In the ED, where every patient is assumed to have a full stomach, the potential risk of aspiration pneumonitis must be weighed against the advantages of the LMA in cases of difficult intubation and ventilation, when use of this device may be life-saving.
Intubating Laryngeal Mask Airway (Fastrach)
The Fastrach (LMA North America, Inc, San Diego, CA) is a special type of LMA that allows the patient to be ventilated in a manner similar to the standard LMA but also serves as a conduit for intubation. The Fastrach looks distinctly
different from the standard LMA and its other variations. It consists of a mask attached to a rigid stainless steel tube curved to align the barrel aperture to the glottic vestibule. The set includes an LMA with a stainless steel shaft covered with silicon (reusable version), a tracheal tube stabilizer (tube pusher), and a silicon-reinforced tracheal tube (Fig. 17.7). Whereas the standard LMA has a flexible tube resembling an endotracheal tube, the Fastrach has a rigid metal tube that is curved into a U shape. The Fastrach also has a large spatula-like handle near its distal end. Finally, within the cuff of the Fastrach is located a tiny lever that is designed to lift the epiglottis when the endotracheal tube passes through the metal tube.
different from the standard LMA and its other variations. It consists of a mask attached to a rigid stainless steel tube curved to align the barrel aperture to the glottic vestibule. The set includes an LMA with a stainless steel shaft covered with silicon (reusable version), a tracheal tube stabilizer (tube pusher), and a silicon-reinforced tracheal tube (Fig. 17.7). Whereas the standard LMA has a flexible tube resembling an endotracheal tube, the Fastrach has a rigid metal tube that is curved into a U shape. The Fastrach also has a large spatula-like handle near its distal end. Finally, within the cuff of the Fastrach is located a tiny lever that is designed to lift the epiglottis when the endotracheal tube passes through the metal tube.
Indications
The Fastrach is indicated for patients with existing or impending respiratory failure who require artificial ventilation and who do not have obstruction of the extrathoracic airway. Although designed for blind orotracheal intubation, it can be used in conjunction with lighted stylets, a fiberoptic bronchoscope, or the Flexible Airway Scope Tool (FAST, Clarus Medical, MN).
The Fastrach is contraindicated in patients with upper airway obstruction. Since the endotracheal tube is usually placed blindly, it is less likely to be successful when the patient’s anatomy is abnormal. The first-pass success rate is 93% to 97%, although the rate increases to 100% when the Fastrach is used in conjunction with a fiberoptic bronchoscope (23,24,25). Additionally, when used for ventilation, the Fastrach has the same disadvantage as a standard LMA in offering less protection of the airway than an endotracheal tube from aspiration of stomach contents into the lungs if reflux or regurgitation occurs.
Equipment
The Fastrach is available in three sizes (3 through 5) and can accommodate 6- to 8-mm ID endotracheal tubes. Although
the special silicon tubes are recommended for use with this device, standard polyvinyl chloride tubes may be used if warmed and softened or if used in conjunction with a fiberoptic bronchoscope.
the special silicon tubes are recommended for use with this device, standard polyvinyl chloride tubes may be used if warmed and softened or if used in conjunction with a fiberoptic bronchoscope.
Prepare the Fastrach in a manner similar to that used for a standard LMA.
Guide the Fastrach into position using the fingers of the nondominant hand, as described for a standard LMA; however, the rigid structure of the Fastrach dictates the path it will follow (the metal handle can facilitate correct placement).
When the Fastrach cannot be advanced further, inflate the cuff, attach a resuscitation bag to the tube, and attempt to ventilate the patient; if ventilation is successful, proceed to the next step; if not, replace or reposition the device.
Make sure that the patient is adequately oxygenated.
Lubricate the silicone endotracheal tube and inspect the cuff for leaks.
Pass the endotracheal tube into the Fastrach while simultaneously lifting the handle gently upward (an assistant should apply moderate pressure over the thyroid cartilage).
If resistance is felt, gently reposition the device; slightly more or less lifting of the handle or slightly more or less cricoid pressure may be effective.
Once the tube has been successfully passed, confirm its position with an end-tidal CO2 detector.
Inflate the cuff of the endotracheal tube, ventilate the patient a few times, then remove the adapter from the endotracheal tube and deflate the cuff of the Fastrach.
Begin removing the Fastrach; as the device approaches the top of the endotracheal tube, attach the rubber stabilizing bar to the endotracheal tube; continue to pass the Fastrach over the rubber stabilizing bar until it is completely out of the patient’s mouth; during this process, maintain control of the endotracheal tube.
After the Fastrach and the stabilizing bar have been removed, replace the adapter on the endotracheal tube and resume ventilation.
Secure the endotracheal tube using standard methods.
The Fastrach may not be perfectly aligned with the glottic opening. Just as in a standard intubation attempt, it may be necessary to manipulate external structures in order to pass the endotracheal tube.
The Fastrach can be used as a conduit through which a flexible fiberoptic scope or an airway guide is passed. The former method allows for direct visualization of the airway, while the latter provides tactile feedback confirming that the airway guide has passed into the trachea. Additionally, a lighted stylet can be passed into the Fastrach and used as a guide for proper placement of the endotracheal tube.
As the Fastrach is being passed over the endotracheal tube, special care should be taken to avoid dislodging the tube.
The endotracheal tube designed for use with the Fastrach is made of flexible silicone and reinforced with wire. It can be damaged very easily. When this tube is used, a bite block is recommended.
Procedure
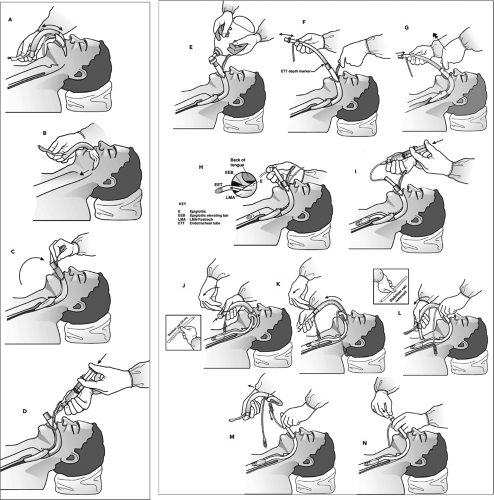
As with the standard LMA, proper use of the Fastrach requires that the cuff be inspected for damage and correctly deflated. The Fastrach is then placed into the oropharynx and guided along the palate into position (Fig. 17.8A,B). Unlike in the case of the standard LMA, the rigid nature of the Fastrach dictates the path that it follows; care should be taken to guide the device along the middle of the palate (Fig. 17.8C). The handle facilitates this process to some extent. Once resistance is met, no further advancement of the device is made, and the cuff is inflated (Fig. 17.8D). A standard resuscitation bag is attached, and ventilation is attempted (Fig. 17.8E). If ventilation is effective, then the operator can proceed with insertion of an endotracheal tube. The patient should first be adequately preoxygenated so that a brief period of apnea will be tolerated as the tube is inserted. The resuscitation bag is disconnected, and the special silicon endotracheal tube is inserted into the Fastrach and advanced (Fig. 17.8F). During advancement, the Fastrach should be lifted upward, and a slight amount of pressure should be applied over the thyroid cartilage (Fig. 17.8G,H). If resistance is met, the tube should be removed and reinserted.
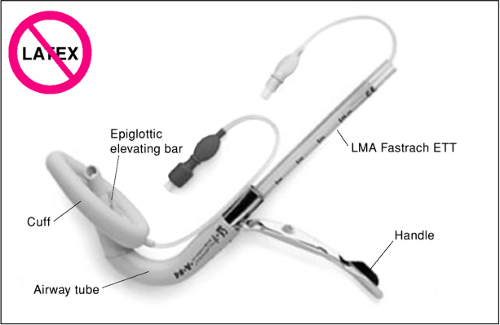 Figure 17.7 The intubating laryngeal mask airway (Fastrach). (Reproduced with permission from LMA North American, Inc., http://www.lmana.com/prod/components/products/lma_fastrach.html.) |
Once successfully placed, the endotracheal tube cuff is inflated (Fig. 17.8I). Proper placement should be confirmed by a colorimetric device or capnography (see Chapter 76). The cuff of the Fastrach can then be deflated (Fig. 17.8J). The tube connector is removed, and the stabilizing device is placed through the Fastrach and used to advance the endotracheal tube while the Fastrach is carefully passed over the flexible endotracheal tube and removed from the patient’s mouth (Fig. 17.8K,L). The stabilizing device is then removed so the pilot balloon can be passed through the shaft of the Fastrach (Fig. 17.8M). At this point, the tube connector is reattached to accommodate a resuscitation bag or ventilator (Fig. 17.8N), and the endotracheal tube is secured using standard methods. If a silicon endotracheal tube is used, insertion of a bite block is recommended, because these soft, wire-reinforced tubes are easily damaged and will not re-expand.
Complications
In addition to the previously described complications associated with the standard LMA, the Fastrach has its own potential complications, the most important of which is failure to pass the endotracheal tube into the trachea. In some cases, the Fastrach can be repositioned in order to facilitate passage; alternatively, the operator may attempt external manipulation of the airway as described above. Other solutions include the use of a fiberoptic bronchoscope, a lighted stylet, or an airway guide to direct the tube into the trachea. As with any form of endotracheal intubation, capnography should always be used to confirm that the tube is located within the trachea to avoid the potentially catastrophic complication of an unrecognized esophageal intubation.
Combitube
The Esophageal Tracheal Combitube (Tyco-Kendall, Mansfield, MA) is another supraglottic ventilatory device that can be used emergently as a rescue airway and is included in the ASA Difficult Airway Algorithm (3). The Combitube is a disposable double-lumen tube that combines features of a conventional endotracheal tube with an esophageal obturator airway. It has a large proximal latex oropharyngeal balloon, eight ventilatory holes, and a distal esophageal low-pressure cuff. It comes in two adult sizes based on height: a standard size (41 F) for adults taller than 5 feet and a small adult size (37 F) for those 4 to 5½ feet in height. Because the Combitube has a distal cuff which occludes the esophagus when inflated, it may offer a greater degree of airway protection than the LMA. The proximal balloon on the standard adult Combitube is inflated with up to 100 cc of air while that on the small adult Combitube is inflated with up to 85 cc of air. The smaller balloons require approximately 15 cc and 12 cc of air, respectively. Once placed, ventilation is possible with either tracheal or esophageal intubation. The operator must simply determine which lumen should be used for ventilation—the esophageal lumen (labeled “1”) for esophageal intubation versus the tracheal lumen (labeled “2”) for tracheal intubation.
Indications
The Combitube is indicated for patients without extrathoracic airway obstruction who require artificial ventilation and who cannot be intubated or ventilated using standard techniques.
It is especially useful when (a) direct visualization of the vocal cords is impossible, (b) access to the airway is limited, (c) there is ongoing massive airway bleeding, and (d) movement of the neck is contraindicated.
It is especially useful when (a) direct visualization of the vocal cords is impossible, (b) access to the airway is limited, (c) there is ongoing massive airway bleeding, and (d) movement of the neck is contraindicated.
Remove the Combitube from its packaging and inspect the balloons for leaks and/or damage.
Lubricate the entire distal portion of the Combitube with a water-soluble lubricant.
Insert the Combitube into the patient’s mouth; this can be done blindly or with the aid of a laryngoscope (in either case, the tube should be kept in the midline as it is advanced).
Advance the tube until the patient’s central incisors lie between the two black rings located on the proximal portion of the tube.
Inflated the balloons—proximal first, then distal.
Attach a resuscitation bag to the esophageal port (this port is the longer of the two, is blue in color, and is labeled “1”).
Attempt to ventilate; if the tube is located in the esophagus, ventilation will be successful.
If ventilation is unsuccessful, switch the ventilating bag to the “2” (tracheal) port and attempt to ventilate; if ventilation is successful, then the tube lies within the trachea.
If neither port provides effective ventilation, withdraw the tube until breath sounds are heard and attempt ventilation; the tube may have been advanced too far, causing the proximal balloon to obstruct the airway; if this attempt is unsuccessful, the patient likely has an occult airway obstruction, and another airway technique should be used.
Like the LMA, the Combitube is unlikely to be effective when the cause of respiratory distress is a high-grade upper airway obstruction. In addition, the Combitube was designed for patients who are at least 4 feet tall. This effectively eliminates use of this device for many children. Finally, the large oropharyngeal cuff is made of latex, and therefore the Combitube should not be used for patients who may have severe latex sensitivity unless there is no other means of ventilation.
Equipment
The Combitube comes assembled by the manufacturer in various packages: (a) a cardboard box containing the Combitube enclosed in a hard pack, two syringes, a plastic elbow connector, and a suction catheter; (b) a soft pack with all the same items; and (c) the Combitube by itself (Fig. 17.9).
It is important to select the correct size of Combitube for the patient to avoid complications such as esophageal perforation. The small adult size is intended for use in patients who are between 4 and 5½ feet in height. The standard Combitube is intended for use in persons at least 5 feet tall, although it may be more appropriate for those at least 6 feet tall.
The Combitube causes extensive stimulation of airway and gag reflexes. Consequently, it should normally be used in apneic patients who have a depressed level of consciousness.
The larger balloon is made of latex. Therefore, the Combitube might not be the best choice for latex-sensitive patients if an equally effective alternative airway technique is available. However, in a life-or-death situation, the benefits of the Combitube outweigh the potential risk of an allergic reaction.
Use of the Combitube can result in injury to the oral cavity, upper airway, and esophagus, and therefore the Combitube should always be well lubricated and inserted as gently as possible.
Procedure
After the Combitube is removed from the package, the balloons should be inspected for leaks or damage and then completely deflated. The entire distal portion of the Combitube should be lubricated with a water-soluble lubricant. The Combitube is inserted midline into the oral cavity either blindly or with the aid of a laryngoscope and then advanced until the patient’s central incisors are positioned between the two black rings on the tube (Fig. 17.10A). The proximal balloon is then inflated, followed by the distal balloon (Fig. 17.10B). A resuscitation bag is placed on the esophageal ventilating port, which is blue in color, longer in length, and labeled “1.” Ventilation is first attempted using this port (Fig. 17.10C). If the tube has entered the esophagus (as occurs in approximately 98% of cases), ventilation through this port should result in adequate chest excursion and effective gas exchange. However, if ventilation through the esophageal lumen is not effective, then it is possible the Combitube was placed in the trachea. Before attempting to reposition the tube itself, the resuscitation bag should be switched to the tracheal lumen (labeled “2”), and ventilation should again be attempted. If the Combitube is indeed located within the trachea, then ventilation
through the tracheal lumen will be effective (Fig. 17.10D). If ventilation cannot be accomplished through either lumen, the operator should consider occult airway obstruction or the possibility that the tube was advanced too deeply and the proximal balloon is obstructing the glottis.
through the tracheal lumen will be effective (Fig. 17.10D). If ventilation cannot be accomplished through either lumen, the operator should consider occult airway obstruction or the possibility that the tube was advanced too deeply and the proximal balloon is obstructing the glottis.
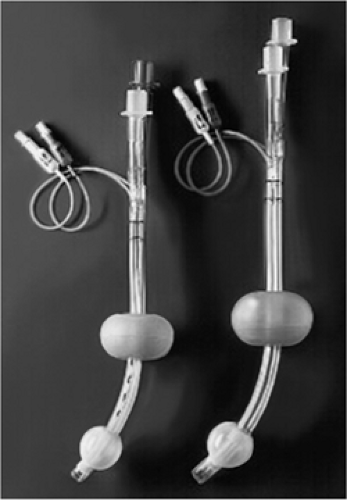 Figure 17.9 The Combitube. (Reproduced with permission from Nellcor, Pleasanton, CA, http://www.nellcor.com/prod/Product. aspx?id=259.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|