CHAPTER 7 In the last four decades, management of the patient with an abnormal smear has undergone a series of significant changes. The standard therapy in the late 1960s for any patient with a “positive” cervical smear was excision, utilizing either a cone biopsy of the cervix or, for the older patient who was no longer likely to bear children, some form of hysterectomy. Often these procedures resulted in considerable morbidity, such as the increased risk of miscarriage following cone biopsy, or even mortality following the more extensive hysterectomy techniques. When the colposcope was reintroduced to Europe and North America in the early 1970s, more appropriate, less aggressive, and more accurate techniques of therapy appeared. Initially these involved applications of the existing technology, refined by use, with the guidance of colposcopy following initial punch biopsy of any cervical lesion. This use of the colposcope as a biopsy director and treatment guide versus its use as a primary diagnostic and screening tool was the fundamental difference between Anglo–American–Australian and continental European practice. However, these differences appear to be diminishing and the colposcope is now largely used to confirm abnormalities detected by screening methods such as the Papanicolaou smear and human papillomavirus (HPV) testing. The precancerous nature of cervical intraepithelial neoplasia (CIN) has been discussed in Chapters 1 and 6. In recent years, there has developed a body of opinion that has questioned the treatment of all such CIN lesions and, as will be discussed in this chapter, evidence suggests that possibly all major-grade (CIN2, CIN3, high-grade squamous intraepithelial lesion (HSIL)) lesions should be treated, whereas minor-grade (CIN1, low-grade squamous intraepithelial lesion (LSIL)) lesions should be managed more conservatively, albeit with the aid of new methods of viral testing to determine the presence of high-risk HPV types, thereby identifying an increased risk of progression of minor-grade lesions as well as indicating the presence of high-grade lesions when minor-grade cytology exists. Over the last two decades the trend has been directed toward more conservative methods of managing CIN, with special emphasis being placed on techniques of local destruction. However, reservations have been expressed about the suitability of these modalities, especially with the development of invasive cancer in a number of women who had been treated conservatively, and also with reference to the accuracy of colposcopically directed biopsies. This coincided with the introduction of the loop diathermy excision technique (large loop excision of the transformation zone (LLETZ) or loop electrodiathermy excision procedure (LEEP)) and the straight wire and needle techniques. A large multicenter study covering over 13 000 treatments has recorded the continuing small risk of patients treated with conservative modalities to develop invasive cancer many years after their initial therapy. Notwithstanding these reservations, there is a great demand for conservative management in treating CIN lesions and, indeed, success rates for both conservative and excisional methods are similar. In this chapter, the topics of whom to treat, the newer methods available for treatment, the mechanisms for follow-up after treatment, and the reasons for failure will be considered. Initially, a detailed description will be given of the colposcopic and pathologic characteristics of CIN lesions that are a prerequisite to determining what, if any, treatment should be undertaken. Colposcopy plays a major role in the decision of what particular treatment should be given for any individual CIN lesion. As has been pointed out in Chapters 4, 6 and 7, the cervical transformation zone contains the CIN lesion within the colposcopically recognizable abnormal (atypical) epithelium. Before considering treatment, the colposcopist must examine three essential characteristics of the lesion. These are: (i) the limits and nature of the abnormal (atypical) epithelium; (ii) the risk and extent of glandular crypt involvement; and (iii) the extent of the CIN within the endocervix. After carrying out an initial visual examination, the colposcopist will be able to determine the outer limits of the lesion at the original squamocolumnar junction, and the internal limits at the new squamocolumnar junction. The lesion will usually be contained within the boundaries of these two junctions. If the new squamocolumnar junction cannot be adequately visualized because of its position within the endocervical canal, then the colposcopic examination must be deemed unsatisfactory. Once these limits have been determined, the nature of the lesion must be considered. As has been described in Chapters 4 and 6, it is now accepted that in colposcopic terms there are minor/low-grade and major/high-grade lesions. The minor-grade lesions are usually smaller than their major-grade counterparts, and are essentially ectocervical. As a SIL/CIN lesion becomes more severe, its size increases. Also, the larger the extent of the surface lesion, the more likely it is to involve the glandular crypts of the cervix. Extension of CIN into the endocervical crypts or glands occurs frequently and to a variable extent, as can be seen in Figure 7.1a. Colposcopically, the finding of gland cuffing (see Figure 6.106), the appearance of a rim of acetowhite epithelium around the gland or crypt entrance, suggests involvement of the crypt with CIN (Figure 7.1a). However, the depth of such gland extensions is impossible to determine colposcopically. A number of studies have shown the range of depth of glandular involvement in association with CIN3 lesions. Figure 7.1 (a) Diagrammatic representation of a cervical intraepithelial neoplasia (CIN) lesion involving the glands and crypts. Around the gland openings there is the colposcopic sign of gland cuffing (1); this indicates the presence of CIN involving the glandular elements. (b) Longitudinal section through a cone biopsy specimen. A full histologic assessment of the depth of glandular involvement requires an excision biopsy, that is, cone biopsy. However, the colposcopic punch biopsy taken by the colposcopist allows the extent and depth of crypt involvement to be determined, and provides an important prognostic sign. The importance of a pathology examination to assess the extent of crypt penetration by CIN can be seen in Figure 7.1b, which shows a longitudinal section through a cone biopsy specimen where the lesion is predominantly in the endocervical canal (1). The endocervical glands extend for a few millimeters into the stroma (2). Although the lesion has been completely removed at the ectocervical (3) and stromal (2) margins, the endocervical margin (4) cannot with confidence be said to be clear. The difficulties of determining the exact depth of glandular involvement by CIN has, to some extent, limited confidence in the conservative techniques, such as laser vaporization or cryotherapy. As “conservative” treatment methods have developed, it has become generally accepted that treatment, by either excision or tissue destruction, to a depth of approximately 6–7 mm perpendicular to the surface should be achieved to produce an optimal clearance rate. It is now felt that expansion of the gland and central degeneration or necrosis of the gland involved is an ominous sign of either impending or associated invasion. These changes should be noted particularly when the excision is reported as incomplete. The International Federation for Cervical Pathology and Colposcopy (IFCPC) approved a revision of the colposcopic classification and terminology in 2012 (see Table 6.1). A major recommendation was to define three types of transformation zone according to the size of the lesion, the position of the upper limit of the transformation zone, and the visibility of the upper limit of the transformation zone. Recognition of the transformation zone type allows the surgeon to individualize a therapeutic approach that would ensure the complete eradication of the lesion (Figure 7.2). Figure 7.2 Changing size of excisional biopsy according to the type of transformation zone. Adapted from Prendiville (2009), with thanks. Figure 7.3 shows how various treatment techniques can be tailored to deal with the wide and variable extent of a CIN lesion within the ectocervix, and especially within the endocervix. In Figure 7.3a, where the entire limits of the abnormal (atypical) epithelium (shaded) are readily visible to the colposcopist, a conservative (ablative) or a limited excisional technique can be used, destroying or excising tissue in a dome-shaped configuration to a depth of 6–10 mm. This represents a type 1 procedure. Where the lesion extends into the endocervical canal and the upper limits cannot be confidently seen (Figure 7.3b), an excisional technique must be used (i.e., cone biopsy, excision using a cold knife, laser, loop, or straight wire/needle), producing a cylindric defect; this being a type 3 procedure. Where there is a combination of a wide ectocervical and an extended endocervical lesion (Figure 7.3c), there is a combination of peripheral tissue destruction (1) along with a larger excision, which in this case involves removal of the central area (2). The removal of this central area represents a type 2 excision. It has already been mentioned that lesions extending into the endocervix prove difficult to remove completely. Various techniques, such as microcolpohysteroscopy and cervicoscopy, have been used to assess the endocervical involvement of CIN. Unfortunately none of them was found to be efficient. When CIN extends into the endocervical canal beyond the limits of detection with the colposcope, some excisional procedure that removes the entire length of the endocervical canal must be performed. In Figure 7.4, an endocervical lesion (shaded area) and the excisional defect produced by its removal are diagrammatically represented; this again representing a type 3 excisional procedure. Figure 7.5 shows a further, similar case; the abnormal (atypical) epithelium (high grade) is at (1) in the endocervix and the upper limits of its extent are arrowed. This epithelium is in a cervix that was treated some 2 years previously by local destruction of a CIN3 lesion. It is only with a “tailored” excisional cylinder-type biopsy that this area can be effectively removed without the need to remove large amounts of the ectocervix. Indeed, in Figure 7.4, the length of the excisional defect is 15–20 mm and the depth 6 mm; these are the dimensions that would be required to eradicate the residual CIN3 lesion seen in Figure 7.5. Complete removal can be achieved where prior endocervical assessment has been made before a tailored excisional procedure. As the role of conservative techniques of treatment developed, it was accepted as mandatory that the histologic nature of the cervical lesion would be known prior to treatment. An initial colposcopic assessment of the cervix is performed, and the most significant area of abnormality is biopsied. The skill in determining this area has to be acquired through a thorough understanding of all the morphologic manifestations of epithelial abnormality. The inexperienced colposcopist is advised to seek confirmation of any suspect epithelial type by carrying out a biopsy. Sometimes, when a variety of colposcopic appearances are present, multiple biopsies are appropriate. These must be carefully directed and documented. Even so, the diagnostic accuracy of colposcopy traditionally requires verification with histology, and the choice of the biopsy site relies on the colposcopy itself (Table 7.1). Several studies have examined the efficacy of colposcopically directed punch biopsies, and it was found that up to 10% of punch biopsies can underdiagnose premalignancy. It is obvious that the expertise of the colposcopist determines the accuracy of this type of biopsy. The authors feel that colposcopically directed biopsy can still be employed safely, but it is essential to perform multiple biopsies to reduce the risk of missing an early invasive lesion. However, the reservations expressed above about biopsy must be taken into consideration. Table 7.1 Indications for taking cervical biopsy Follow-up cytology ?HPV DNA See and treat Excision biopsy AGC, atypical glandular cell; ASC-?H, atypical squamous cells, cannot rule out a high-grade lesion; ASCUS, atypical cells of undetermined significance; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; MDT, multidisciplinary team. Women with persistent atypical cells of undetermined significance (ASCUS) or borderline nuclear abnormality (BNA) are at higher risk of developing high-grade CIN. If HPV is not used to triage ASCUS/BNA cytology then it is recommended by certain authorities (European and UK screening programs) to refer women with three consecutive ASCUS/BNA samples for colposcopic assessment. The interval between the tests should be 6 months to allow adequate time for the abnormality to resolve. A woman should be returned to routine recall only after a minimum of three negative cytologic tests each at least 6 months apart or colposcopic assessment indicating no abnormality. If only cytologic surveillance is used then it is recommended that there should be no more than three borderline samples over any 10 year period without a recommendation for colposcopy. Immediate referral for colposcopy is not a cost-effective option and may considered over management. It can be justified in selective cases such as ASCUS/BNA following treatment of CIN or when poor follow-up compliance is suspected. It is now accepted that the most effective and efficient method of management of these women is by the use of reflex high-risk HPV DNA testing. It is performed following a ASCUS/BNA cytology result and, in these cases, a positive HPV finding will dictate referral for colposcopy. If no abnormality is found during colposcopy or no CIN is detected on biopsy, a further HPV DNA test can be performed after 12 months. Similarly an ASCUS/BNA result with negative high-risk HPV DNA can be followed up with cytology in 12 months’ time. The recommendations of the American Society for Colposcopy and Cervical Pathology (ASCCP) for the management of ASCUS are shown in Figure 7.6. Figure 7.6 Reprinted from The Journal of Lower Genital Tract Disease, Volume 13, Number 5, with the permission of ASCCP © American Society for Colposcopy and Cervical Pathology 2013. The recommendations of the ASCCP for the management of atypical squamous cells: cannot exclude high grade (ASC-H) are shown in Figure 7.7. Figure 7.7 Reprinted from The Journal of Lower Genital Tract Disease, Volume 13, Number 5, with the permission of ASCCP © American Society for Colposcopy and Cervical Pathology 2013. Two management options can be proposed for this group: (i) repetition of the cytology and (ii) referral for colposcopy. Repetition of the Papanicolaou smear is an acceptable strategy, especially in young nulliparous women. In that case, the smear should be repeated at 6 month intervals at least until two subsequent negative smears have been obtained. It is prudent to refer the patient for colposcopy if one of the smears shows any grade of abnormal cytology or dyskaryosis. The Trial of Management of Borderline and other Low grade Abnormal smears (TOMBOLA) study, which was a UK screening program study examining women presenting with LSILs (mild dyskaryosis), found that more cases of CIN2+ were detected in women undergoing immediate colposcopy than in women who were monitored with follow-up cytology. In the immediate colposcopy group there was a lower rate of CIN2+ on exit from the study. Similar findings were confirmed in different meta-analyses and large trials. Considering the higher prevalence of high-grade CIN in cases of LSIL cytology compared with ASCUS, referral to colposcopy can be chosen as the preferred option. When colposcopy is satisfactory and shows no lesions, a repeat smear or high-risk HPV DNA testing in 12 months is recommended in European guidelines for quality assurance within the cervical cancer screening program. Women with a persistent LSIL cytology result or a single abnormal result with positive HPV test will need colposcopic assessment. It is better to avoid treatment in this group unless colposcopy-directed punch biopsy indicates high-grade CIN. To prevent possible overtreatment, patients should not be managed on a “see and treat” basis. However, it is also important to consider that progression to higher grade lesions increases with the length of follow-up, and the potential loss to follow-up. The recommendations of the ASCCP for the management of LSILs are shown in Figure 7.8. Figure 7.8 Reprinted from The Journal of Lower Genital Tract Disease, Volume 13, Number 5, with the permission of ASCCP © American Society for Colposcopy and Cervical Pathology 2013. Progression to invasive cancer is high in this group. Hence, triage using repeat cytology or HPV DNA detection is not indicated. Immediate referral (within 4 weeks in the UK) for colposcopy assessment is recommended by all professional bodies in the world. If colposcopy is satisfactory and the lesions appear to be high grade then treatment with excision of the transformation zone can be offered at the same time (see and treat); otherwise, colposcopy and biopsy to rule out the presence of high grade is recommended. Management should be decided according to the result of the punch biopsy, cytology, and colposcopic impression. The recommendations of the ASCCP for the management of HSILs are shown in Figure 7.9. Figure 7.9 Reprinted from The Journal of Lower Genital Tract Disease, Volume 13, Number 5, with the permission of ASCCP © American Society for Colposcopy and Cervical Pathology 2013. Detection of glandular intraepithelial or glandular invasive disease by cervical cytology is poor. However, the presence of atypical glandular cells in Papanicolaou smears is associated with a high frequency of underlying high-grade endocervical neoplasia or other types of cancers. For this reason, it is prudent to refer patients directly for colposcopic assessment. Endocervical and/or endometrial exploration is also indicated. If colposcopy cannot detect any abnormality then diagnostic conization should be carried out. When the glandular lesion is reported as endometrial in origin and if the woman is older than 35 years or if there is unexplained vaginal bleeding when the woman is younger than 35 years, a transvaginal scan, hysteroscopy, and endometrial sampling in addition to colposcopy is indicated to exclude endometrial carcinoma. Endocervical curetting is not a very sensitive method to detect or to exclude endocervical pathology and is not advised by the UK screening program or European Federation for Colposcopy. However, it is recommended by the ASCCP. The recommendations of the ASCCP for the management of women with atypical glandular cells are shown in Figure 7.10. Figure 7.10 Reprinted from The Journal of Lower Genital Tract Disease, Volume 13, Number 5, with the permission of ASCCP © American Society for Colposcopy and Cervical Pathology 2013. The introduction of the concept of low- and high-grade lesions in 1990 by Richart formalized the view, held by pathologists and clinicians for some time, that there seems to be a spectrum of epithelial change, from mild epithelial lesions at one end to undifferentiated invasive lesions at the other extreme. Many studies have shown that low-grade lesions (LSIL/CIN1) have a low rate of progression. However, the progression of LSILs increases by the length of follow-up. The cumulative rate of progression from LSIL to HSIL in 6 months can be 6% compared with 20% after 24 months. More recently, studies have emerged looking at the natural history of CIN1 in relation to infection with different HPV types. Women with CIN1 infected with non-high-risk HPV types can regress to normal cytology over 4–5 years. It is on the understanding that most low-grade lesions will undergo regression, compared with the potential for high-grade lesions to proceed to invasive cancer, that it is possible to make the following recommendations concerning which CIN lesions to treat. On the basis of the discussion in Chapters 6 and 7, it would seem wise to treat all lesions in this category of severity. It is unclear how urgent therapy is but, at present, no technique exists to tell the clinician when a lesion is likely to become invasive. While there is no doubt that CIN3 should be treated in view of the significant risk of progression to invasion, several studies have looked at the natural history of CIN2. Some studies show that CIN2 is an intermediate entity that lies between CIN1 and CIN3 and has some premalignant potential, although not as great as that of CIN3. Other studies show that it is much closer to CIN1 or benign disease, so it does not have real premalignant potential. Interobserver variation is poor for the diagnosis of CIN1 or CIN2. There is also poorer correlation between colposcopic and histologic diagnosis in CIN2, compared with that in CIN1 and CIN3. It appears that there is a significant regression rate of between 50% and 70%. The available evidence suggests that this group is not associated with a high risk of progression with time and significant regression may occur. Observation with serial monitoring by cytology and HPV test, with or without colposcopy, has been shown to be highly effective in detecting the development of major-grade lesions. Regression will reveal itself, and progression to a higher grade should be identifiable so that appropriate treatment can be instituted. The role of viral testing to determine the presence of high-risk DNA types is becoming an accepted part of the management protocol. There is a risk that a woman who has a diagnosis of CIN1 on biopsy may be harboring a higher grade lesion (CIN2/3 or adenocarcinoma in situ (AIS)) owing to the limitations outlined earlier regarding the accuracy of a punch biopsy. Consideration should therefore be given to referral cytology. If the Papanicolaou smear showed LSIL (mild dyskaryosis/BNA) or BNA–glandular, and the colposcopy examination is unsatisfactory, then excisional treatment is recommended. In the case of satisfactory colposcopy, it is acceptable to offer either treatment or follow-up with cytology and colposcopy in 6 months. In addition, a review of the cytology, histology, and colposcopic findings may be carried out. The incidence of cervical glandular intraepithelial neoplasia (CGIN) and adenocarcinoma of the cervix has been rising for the last few decades. However, the natural history of glandular precancerous lesions is not as well documented as its squamous counterpart (CIN). Glandular dysplasia is generally divided into low grade (glandular atypia/glandular dysplasia less severe than AIS) or high grade (AIS). Adenocarcinoma makes up approximately 15–20% of all cervical carcinomas whereas AIS constitutes only 2% of all intraepithelial lesions. This possibly indicates a shorter interval of progression of AIS to invasive cancer, or that many cases of AIS are missed. While screening with Papanicolaou smears has significantly decreased the incidence of squamous cervical cancer, it has not made any significant impact on the rates of adenocarcinoma. Although AIS has been well studied and many treatment protocols have been established, the management of AIS remains controversial, with treatments ranging from LEEP/laser cone biopsy and cold knife cone biopsy to hysterectomy. Because of the rarity of the disease, the published studies tend to have relatively few patients and are mostly retrospective in design. What is clear is that there is no place for the destructive methods of treatment, and that all AIS should be treated by excision. Historically, AIS has been treated by simple hysterectomy. However, because many of the women with AIS will not have completed child-bearing, conservative uterus-sparing approaches have been adopted. Having assessed the cervix, the colposcopist is now in a position to determine whether the lesion may be treated by one of two methods, namely excision or local destruction with preoperative biopsy. The choice as to which to use depends on certain criteria that must be considered by the colposcopist. These are as follows. Is the transformation zone fully visible? Is it a small or large lesion? Prendiville, in 2003, first proposed a geographical classification of the transformation zone and options of treatments according to the type of transformation zone (Table 7.2). Table 7.2 Treatment options according to type of transformation zone Completely ectocervical Visible LLETZ/LEEP Destructive treatments Completely ectocervical Visible LLETZ/LEEP (wider loop) Destructive treatments SWETZ/NETZ Partially endocervical Visible LLETZ/LEEP (wider loop) SWETZ/NETZ Partially endocervical Visible LLETZ/LEEP (wider loop) SWETZ/NETZ Laser cone Partially endocervical Not fully visible LLETZ/LEEP (deeper/longer loop) SWETZ/NETZ Laser cone Partially endocervical Not fully visible LLETZ/LEEP (deeper/longer loop) SWETZ/NETZ Laser cone Cold knife cone LEEP, loop electrodiathermy excision procedure; LLETZ, large loop excision of the transformation zone; NETZ, needle excision of the transformation zone; SWETZ, straight wire excision of the transformation zone. According to this proposal, any choice of techniques (excision or destructive) is likely to be successful for a type 1 transformation zone, whereas for a type 2 transformation zone an excisional technique is preferable. However, a larger size transformation zone would require a larger size loop. When dealing with a type 3 transformation zone, it is mandatory to use an excisional technique: type 3 also has a higher risk of incomplete excision. It is important for the colposcopist to be aware of the referral cytology, and also whether within that cytology report there is mention of any atypical glandular cells indicating the possibility of glandular intraepithelial neoplasia or adenocarcinoma. The issue of endocervical curettage and its effect on the choice of treatment has already been discussed. Once the prerequisites for treatment have been confirmed, a choice can be made between excision and local destruction. A meta-analysis of the performance of different treatment techniques (including LLETZ/LEEP, laser conization, cold knife cone biopsy, laser vaporization and cryotherapy) did not show any significant differences in terms of treatment failures, and the authors concluded that “there is no obvious superior surgical technique for the treatment of CIN in terms of treatment failures or operative morbidity.” The following conservative methods all depend on eradication of the localized lesion by its physical destruction. It is imperative that any such method destroys the CIN contained within the cervical glands or, more correctly, the crypts, as discussed above. Therefore, to be totally effective, these methods must destroy the tissue to a depth of at least 6–7 mm, especially if the CIN is situated in the crypts. There are four local destructive techniques: In recent years there has been a resurgence in the popularity of local destructive techniques because of the recognition that excisional techniques such as LLETZ/LEEP, knife cone biopsy, and laser cone biopsy are associated with adverse pregnancy outcomes. Local destructive techniques generally destroy less tissue, and hence cause less morphologic damage to the cervix. This, however, has to be balanced against the fact that histologic tissue is not available for confirmation of what has been treated, although a punch biopsy will have been taken beforehand. This means that there is no way to assess whether all the abnormality has been treated. Cryotherapy has been recognized as the most cost-effective, feasible approach to treating precancerous lesions in resource-poor settings. It is effective, has limited side-effects, does not require electricity, is inexpensive compared with other treatment options, and is technically simpler than other methods and therefore can be performed by non-specialized health-care providers. The principle of cryotherapy is that when a liquid vaporizes, or a gas expands, heat is drawn from the surrounding environment. Some of the earlier devices used for cryotherapy involved liquid nitrogen or Freon gas, but now practically all machines use either carbon dioxide, which is much more readily available, or liquid nitrogen. There does not appear to be significant differences between the performance of medical grade carbon dioxide and commercial grade carbon dioxide that is used in the manufacture of aerated beverages. The equipment (Figure 7.11) consists of a hand-held probe (1) that terminates in a hollow tip (2); this can be changed to suit the size and contour of the cervix. The gas is channeled through a narrow tube (3) into the tip, from which it is removed by a wider bore tube and exhausted to the outside air. The cryoprobe tip, which is placed against the area to be treated (Figure 7.12), is cooled by this gaseous expansion and the cervix is superficially frozen, resulting in cell death. Ideally the patient will already have had an initial colposcopic assessment, during which preliminary biopsies will have been made and reported on. Upon returning to the clinic, the patient is informed of the results and proposed treatment and should be allowed to ask questions and receive a full explanation of the procedure, its complications, and sequelae. In many resource-poor settings, however, colposcopy and pathology services may not be available. In these situations cryotherapy may be the only option available for treatment, and it forms part of the valuable “single-visit strategy,” where a rapid form of screening occurs (i.e., visual inspection with acetic acid (VIA)) followed by immediate treatment of any screen-positive women. Exceptions would be if an overtly malignant lesion is seen. This may well result in a certain degree of overtreatment, but, because the morbidity associated with cryotherapy is so low, the overall risk benefit ratio remains in favor of treatment. There are valid concerns regarding the successful treatment of large lesions with cryotherapy; therefore, most recommendations are that, for large lesions that occupy more than three quadrants of the cervix, or if the entire lesion cannot be covered by the cryoprobe, an alternative form of treatment, such as LLETZ/LEEP, should be performed. Another scenario where treatment with cryotherapy might be suboptimal would be if the lesion extends into the endocervical canal, in which case an excisional form of treatment is recommended. The patient is placed in the lithotomy position and a speculum is inserted into the vagina to expose the cervix. It is important to keep the cervix and probe tip clear of the vagina to avoid inadvertent damage. A suitable probe tip is then chosen and screwed into the cryoprobe, which is then inserted into the vagina under direct vision (Figure 7.13). The most effective, and now standard, technique is to use a freeze–thaw–freeze process: 3 minutes of freezing is followed by 5 minutes of thawing followed by another 3 minutes of freezing. The aim is to achieve a peripheral “ice ball” of approximately 4 mm diameter (Figure 7.14). This implies that the depth of freeze will also be approximately 4 mm, meeting to a large extent the depth-of-treatment recommendations mentioned above. At the end of the treatment cycle the probe is defrosted and gently removed from the cervix. The freeze crater (1) has completely covered the lesion with a small (3 mm) margin of normal tissue around (2). In Figure 7.15 an application (1) of antibiotic cream is being placed in the cervical crater; however, its use is controversial. Following treatment, the cervix will go through the usual repair process that occurs when tissue has been destroyed. The area will become demarcated and a slough will develop. In Figure 7.16, such a slough (1) can be seen; this will usually separate at around 10 days. At this time there may be some slight bleeding or increase in discharge, usually of a clear mucous consistency. Thereafter, new squamous epithelium will migrate inward from the periphery and cover the defect with a firm strong epithelium. Among patients, cryosurgery is the most acceptable of all the conservative techniques as it can be performed in an outpatient department and there is virtually no pain. At worst, a mild menstrual-type cramp will be felt. The most frequently reported sequela of cryosurgery is a profuse watery discharge that may persist for 2–3 weeks. Bleeding is relatively rare. If it does occur, it is usually slight, mixed with discharge, and most commonly occurs at the time of the separation of the slough. The cervix heals well, leaving a characteristic appearance of radial ridging. The squamocolumnar junction is often found to lie just within the endocervical canal. Some authorities feel that this is advantageous as it reduces the risk of new lesions occurring. Others consider it to be a disadvantage as it renders access and colposcopic monitoring rather more difficult. Infection of the sloughing area may occur but responds well to a short course of antibiotics and local cleansing. Although patient acceptability is very high, cryosurgery has a lower clearance rate than other conservative techniques. Some authors have claimed clearance rates of about 95%. However, the general experience appears to be that a rate of 85% is more frequently seen. Like other local destructive methods, the size of the lesion and not the biologic stage of the CIN influence the chances of success and of complete clearance. The Semm cold coagulator was introduced into gynecologic practice in 1966. It was primarily used for the local destruction of benign cervical lesions. Many authors have confirmed its wide applicability in the treatment of precancerous conditions of the cervix. The equipment (Figure 7.17) consists of a series of varying sized Teflon-coated thermoprobes that are rapidly heated electrically. The equipment is relatively cheap and has proved popular within Europe and the UK. Figure 7.17 The Semm cold coagulator and three thermoprobes. The patient is given a colposcopic examination in the standard manner, and the site and extent of the lesion are confirmed. A biopsy is taken and the patient returns to the clinic for definitive treatment if the conditions for safe usage of local destructive techniques have been satisfied. A “see and treat” policy involving immediate treatment following biopsy was tried in some clinical trials. This approach, however, relies on very high-quality colposcopy and is not generally recommended. They only use this technique when the entire limits of the lesion are fully visible and when there is no suggestion of an invasive lesion. Two to four biopsies are taken at this time. The thermoprobe is applied to the lesion, and two to five overlapping applications are required to adequately cover the abnormality (Figure 7.18). The thermoprobe heats the tissues to 120°C, thereby literally boiling the tissues. Cold coagulation at 120°C for 30 seconds can achieve a depth of destruction of at least 4 mm within the cervix. Hemostasis is automatic (Figure 7.19). Evidence from several studies showed initial clearance rates above 90%, and above 80% after 10 years. Authors do not recommend repeat cold coagulation for persistent and recurrent CIN3 lesions as this technique was found to be less successful. Figure 7.18 The thermoprobe is applied to the cervix and activated. Sloughing of the skin can be seen alongside the probe head. Figure 7.19 The treated cervix is dry with a central pale area of destroyed tissue. As with other conservative techniques, primary follow-up is with cytology (in some units cervicography is employed purely to monitor the appearance of the ectocervix). Colposcopy is used only when there is persistent or recurrent cytologic abnormality. The evidence shows that cold coagulation, like other conservative techniques, does not adversely affect pregnancy. The major advantage of cold coagulation is its relative affordability and wide applicability. The major disadvantages, as with other ablative techniques, are that the tissue destroyed is not available for analysis, the depth of treatment is difficult to determine, and its efficacy depends on high-quality colposcopy if used in a single-visit scenario. Low rates of complications such as preterm miscarriage or the need for operative delivery were reported in some studies. This simple and effective technique was extensively used worldwide in the treatment of CIN. The equipment is readily available in all operating theaters and is relatively inexpensive. This technique can be more effective than either cryosurgery or the CO2 laser for destroying larger areas of abnormality as reported in several studies. As an added safeguard, it is prudent to take further biopsies and/or endocervical curettage to make a better assessment of lesions that extend into the endocervical canal. The technique is, however, more limited in its application to lesions that extend onto the vagina. The greater accuracy and control over the depth of treatment achieved with laser surgery make this modality superior in these circumstances. The technique works by coagulating and destroying the epithelium and epithelium within glands. However, the latter may impair an adequate depth of destruction because it will stimulate the exudation of mucus, which, in turn, has a pronounced insulating effect; consequently, to achieve the required depth of destruction, application of heat must be continued until no more mucus is produced. The varied arrangement in number and topography of the clefts and glands within the cervix requires corresponding variation in the duration and depth of application of the heat source. Healing is usually complete within 4 weeks. Its major disadvantage is that its application causes pain, and usually a short general anesthetic is required, although some series have reported the successful use of local analgesia. All patients should be given a preliminary assessment, in which the cervix is examined with the colposcope and appropriate biopsies are taken. The results of these biopsies must be available before radical electrodiathermy treatment is begun. Either local or general analgesia can be used, and colposcopy is performed to confirm the initial findings. Though not essential, the cervix can be first dilated to counter the risk of postoperative stenosis. The cervix is then painted with Lugol’s iodine solution. The equipment consists of a standard diathermy delivery unit producing 40–45 W; the needle electrode is used to perform a series of radial cuts to 2–3 mm beyond the iodine-negative areas. This segmentation of the cervix is carried down to approximately 5–7 mm. The needle is used to slice radial cuts into the cervix to open any nabothian follicles and deal with any CIN that may extend into the gland crypts. The segments are then removed using a ball electrode (Figure 7.20); this leaves a conical crater that is at least 7 mm deep (Figure 7.21). Figure 7.20 A ball electrodiathermy device is being used to coagulate the abnormal (atypical) epithelium. The endocervix and part of the ectocervix have been obliterated to a depth of at least 10 mm beyond the lateral extent of the lesion. Pressure must be maintained on the diathermy probe because mucus exudation will insulate the tissue from the heat. Figure 7.21 A conical crater can be seen within the cervix. The clinician should take care not to touch the vagina with the diathermy unit during the procedure, as this would cause electrical burns. Postoperative cold-water douching of the area has been recommended, but has not been found by the authors to be necessary. A large study in Australia reported 97% eradication of CIN by a single treatment, This is similar to that of proponents of laser and cryosurgery, in that failures seem to be related more to the extent of the lesion or to a lack of firmness in the application of the heating current. Following radical electrodiathermy, the patient will experience the same problems as those found after laser treatment. Secondary hemorrhage may occur and is usually associated with infection. Scarring and stenosis are more common after electrodiathermy. Most cervices heal with an accessible squamocolumnar junction; there is often peripheral ridging and wrinkling, as found after cryosurgery (Figure 7.22). There has been reported an increased risk of premature membrane rupture and prematurity in women undergoing radical cervical diathermy in line with similar reported morbidity for excisional procedures which will be discussed below. Figure 7.22 The washed-leather appearance after electrodiathermy. The squamocolumnar junction is clearly visible just inside the endocervical canal. The use of the CO2 laser is now widely accepted as one of the most effective forms of treatment of CIN. Although at present the loop electrosurgical excision procedure has become more popular, there is no evidence to suggest that the results between CO2 laser vaporization and loop excision will vary significantly. The advantages and disadvantages of the two techniques will be described later. The term “laser” is an acronym for light amplification by stimulated emission of radiation. The laser converts energy, such as heat, light, or electricity, into a radiant form of energy that is produced at a specific wavelength, determined by the type of laser. CO2 lasers, for example, produce energy at a wavelength of 10.6 mm; this is in the infrared portion of the spectrum and invisible to the human eye. This energy can be focused on a specific spot, 1.5–2 mm in diameter, by a system of mirrors and lenses, and at its focal point the laser releases an enormous amount of energy. Any tissue at this focal point is vaporized at the speed of light. The laser itself is attached to the colposcope and at all times the area to be destroyed is under the direct vision of the person performing the laser surgery. Manipulation of the beam is extremely simple. Most lasers (Figure 7.23) are free-standing and have a cabinet that contains the helium and nitrogen gas mixtures and the tubes for developing the coherent light beam. The beam is transferred via a series of articulated arms, each with a surface-reflecting mirror at the junction. A column, to which the articulating arm is attached, is capable of movement both up and down and the control panel allows for a variety of power and time settings. The arm terminates either in a handpiece, which is used for free lasering in exposed areas such as the vulva, or, more commonly, in a micromanipulator that can be attached to a colposcope head. This latter arrangement allows the light to be reflected from the terminal mirror, which can be angled by the micromanipulator, which is coupled (1) to the front piece of the colposcope (Figure 7.24). A manipulator (2) at its upper part allows precision control over the emanating laser beam by free movement through a 360° arc. The lasers are arranged so that the light is “focused” at a focal length that maximizes the power density, and thus the cutting and vaporization potential of the device. Modern lasers have a variable spot-size facility (determined by adjusting the circular ring (3)), varying from 0.2 to 2 mm, to allow the laser to be used in various modes, such as cutting, vaporization, or coagulation. Figure 7.23 A free-standing CO2 laser machine with an articulated arm that connects to a micromanipulator which is attached to the colposcope. In this figure, a handpiece is at the terminal end of the articulated arm. The CIN lesion within the atypical transformation zone will have already been outlined using acetic acid. Its lateral borders can be easily defined by using Lugol’s iodine solution; this contrasts the normal area with the abnormal area and gives the operator an accurate indication as to where the most atypical lateral parts of the transformation zone exist. This procedure is adopted prior to all local destructive techniques. The lesion can also be outlined by the laser beam (Figure 7.29). Although the laser can be used without analgesia, and was used thus in the early days, it is now accepted that a combined analgesic and vasopressor agent will give a higher degree of patient compliance and acceptability. In Figure 7.25, a fine dental syringe and needle (1) is being used to inject at one of four sites (i.e., 12, 3, 6, and 9 o’clock positions) around the ectocervix immediately peripheral to the lesion site (2). Approximately 4 ml of solution is injected. In Figure 7.26, blanching is clearly seen when the fluid is injected to a depth of about 5 mm. Once the local analgesic has been injected, the appropriate setting is chosen on the laser machine (Figure 7.27). Many lasers have a super pulse mode that, because of the configuration of the beam geometry, produces less tissue damage. The aim of laser vaporization is to remove a block of tissue, the dimensions of which can be tailored to ensure the complete destruction of the lesion. This produces a dome-shaped defect that will incorporate any involved glands within the ecto- or endocervix (Figure 7.28). The procedure commences some 3–4 mm beyond the edge of the atypical transformation zone at the 6 o’clock position, and the laser beam is moved rapidly over the tissues. This rapid movement reduces thermal tissue conductivity and minimizes potential scarring. It also reduces the heat build-up within the tissues, thereby reducing uterine contractions, which may otherwise distress the patient. The operator should use the highest possible power density, because the longer the beam is in contact with the tissues, the more the surrounding normal tissues will be damaged and the slower will be the healing process. After the area has been outlined, the actual vaporization can be performed using one of two techniques. In the first, the procedure commences in the posterior area (1), the lesion (2) having already been visualized (Figure 7.29). The laser beam initially vaporizes a posterior area, thereby preventing blood from running down into the operative field. The beam is moved in multiple directions—diagonally, horizontally, or vertically—to prevent furrowing. The second technique, and the one used by the authors, involves a vertical movement whereby, commencing posteriorly, the beam is moved onto the endocervix and then down again, with each furrow of destruction overlapping the previous one, very similar to the action of mowing a lawn. One hand works the micromanipulator, and the other holds a small cotton bud or swab. As soon as bleeding occurs, the cotton bud is placed on that point to reduce blood contamination, because any blood will immediately take up a laser beam and so reduce the beam’s efficiency. Using this vertical approach, it is easy to recognize bleeding points as soon as they develop. Once the posterior lip has been destroyed, the anterior lip can be similarly dealt with. The vertical technique is methodic and also allows an even depth of destruction to be achieved. Penetration is usually to a depth of at least 6 mm and this should adequately deal with any CIN lesion within the cervical crypts. This technique is shown in Figures 7.30–7.33; punch biopsy had revealed the presence of papillomavirus infection and also CIN2–3. In Figure 7.30, the sharp lateral (outer) outline of the atypical transformation is seen; multiple biopsies in the peripheral area (1) showed the presence of subclinical viral infection, while biopsies taken at point (2), toward the endocervix, showed the presence of CIN2–3. The upper limit of the lesion can be clearly seen. Vaporization of the peripheral area to a depth of only 2–3 mm is shown in Figure 7.31, while in Figure 7.32 the central area has been ablated to a depth of about 8 mm. Some 6 months later the area appears as seen in Figure 7.33; the outer limit of the vaporization is at (1) and some metaplastic regenerating epithelium is at (2). The upper limit, the new squamocolumnar junction, can be clearly seen. The results for laser vaporization have been extremely satisfactory. The cure rate can be as high as 90–95%. There does not seem to be the increase in pregnancy morbidity that is found with excisional techniques such as premature membrane rupture or prematurity. The use of excision techniques was the mainstay of the management of cervical dysplasia and carcinoma in situ (previous nomenclature) for many years; these involved hysterectomy and then cone biopsy, the latter being the less radical technique. However, the use of cone biopsy in large numbers of young women caused much disquiet because of the attendant morbidity, and so it was not surprising that the locally destructive techniques, as described above, became popular. These methods are the treatment of choice for selected cases in which the entire abnormality is visible on the ectocervix, and in which there is no suggestion of invasion; this has been described under the prerequisites for treatment in section 7.3. The principal disadvantage of local destructive treatment, however, is that a histologic examination of the entire lesion is not possible, and early invasive cancer may remain undetected. Critics of the local destructive methods maintain that cytology, colposcopy, and biopsy together cannot exclude invasion with sufficient certainty to allow cone biopsy to be avoided. Several studies in the 1990s showed that excisional biopsy successfully detected microinvasive diseases that could have been missed had local destruction by laser vaporization been performed. These studies have led many authors to advocate the use of excision rather than local destruction techniques. At the moment, it must be left to the individual clinician to choose which technique gives the best results. The techniques used for excision are as follows: However, before discussing these individually, it is important to consider the special problem of CIN extension to within the endocervix. Patients are referred for colposcopy in the usual way, following the finding of a cytologic abnormality. Colposcopy is carried out in the usual manner, and any lesion is identified and its topography charted. Whereas with other conservative techniques the recommendation has been to make carefully representative biopsies and then bring the patient back to the clinic when a decision as to treatment has been made, with loop diathermy the possibility to combine the biopsy with treatment has led to the development of a “see and treat” policy for selected patients. It must be stressed that the patient must be effectively counseled and the procedure carefully explained. There is the risk of considerable psychologic trauma if this is not done. Clearly, the development of a rapid “see and treat” policy was advantageous in reducing unnecessary and traumatic clinic visits, but this enthusiasm had to be tempered with a desire not to overtreat patients with minor abnormalities. There has been a dramatic increase in the use of the large loop diathermy for removal of CIN lesions in the last two decades. The impetus in favor of this very simple device has meant that doubt has been cast on the efficacy of local destructive techniques, especially laser vaporization and cold coagulation. It is important to point out that, although excisional techniques using the loop have indeed discovered early invasive lesions in excised specimens, it does not necessarily mean that the local destructive techniques are redundant. It can be argued that many of the early microinvasive lesions now found by the use of excisional techniques would have been quite effectively destroyed by the use of local destructive techniques. Wire diathermy loops have been used for many years to remove large and small lesions from the cervix. Famous French colposcopist Rene Cartier popularized the use of small loops extensively to obtain biopsies from lesions on the cervix in the early 1980s in Paris. Latterly, Prendiville et al. have shown that by using large diathermy loops a useful biopsy can be obtained with simultaneous and complete treatment being achieved. This has all the advantages of other minimally invasive conservative therapies and the added bonus that a large block of material can be obtained for pathologic assessment. Loop diathermy is a very effective method for removing the entire transformation zone; it has high patient acceptability and a small morbidity rate. Many studies have already attested to the high success rate (95–96%) of this technique. The rate of recurrence and residual lesions can be 5.0% and 0.6% in the first and second years, respectively. The systems that are available consist of a combined cutting and coagulating facility (Figure 7.34); this can be blended to be used with a single device, the loop (Figures 7.35, 7.36). A wide variety of designs and sizes of loop are available. The device consists of an insulated barrel that can be inserted into a handpiece, very similar to that used in the standard operating theater diathermy systems. The barrel terminates in a wire loop that is insulated to a variable extent, depending on the individual manufacturer and the clinician’s desires. The wire is made in either a thin flexible form or a thicker more rigid form. The rigid form is rather easier to use but does produce more thermal damage to the specimen produced. The fine flexible wire (Figure 7.35) requires a delicate touch and an understanding of the three-dimensional nature of the block to be removed. Figure 7.34 A Valley Lab Force 2 electrodiathermy machine. These machines provide variable power for cutting and coagulation; the required power can be blended using a simple hand switch. The patient is “earthed” using an adhesive disposable jelly pad attached to the thigh. Use of the machine provides a high-quality cut resulting in minimal tissue damage through coagulation or thermal artifact. Hemostasis is easily and rapidly achieved. When the equipment is used in a modern colposcopy service it is important that there is no television interference. Figure 7.35 Four fine wire loops of various dimensions with two diathermy ball devices (Utah Medical Products, Inc.). (Courtesy of Professor W. Prendiville, Dublin, Ireland.) Figure 7.36 Two diathermy devices, fixed ball (upper) and moveable ball/roller ball (lower) (Utah Medical Products, Inc.). (Courtesy of Professor W. Prendiville, Dublin, Ireland.) Following removal of the large loop specimen, a defect remains (Figure 7.37); this must be carefully cauterized with the ball-headed cautery (Figure 7.36), thereby sealing the entire defect. Figure 7.37 The defect after large loop excision; the base may require careful cauterization to seal the surface. The lack of bleeding is entirely due to the injected vasopressor agent. The lack of thermal damage results from the use of the high-frequency electrosurgical unit. It is, however, essential to “seal” fully the surface using the ball electrode; otherwise, when the vasopressor agent wears off, profuse hemorrhage will occur. In this figure, the loop diathermy handle (1) with the loop attached has removed a small cone-shaped block of tissue (2) from the anterior lip of the cervix. The cone base is at (3). As with other conservative techniques, the patient is first told about the intended colposcopic assessment and possible treatment. With the patient in the lithotomy position, and a Cusco’s or Graves’ speculum with a smoke-extraction facility in the vagina, the cervix is exposed and the patient colposcoped in the standard manner; the limits of the lesion are identified as shown in Figure 7.38a–c, which depicts an area of abnormal (atypical) epithelium (high grade) (1) whose outer and inner (new squamocolumnar junction) limits can be seen. The application of Lugol’s iodine (Figure 7.38c) has the added advantage of delineating the limits of the lesion more clearly, especially after the injection of local anesthetic which sometimes causes bleeding and can obscure the acetowhite margins. Where the circumstances meet the protocol described above for the safe employment of a “see and treat” policy, the patient (as in the case in Figure 7.38) is informed of the treatment procedure to be followed. Figure 7.38 (a and b) A high-grade lesion with well-defined outer and inner limits (the new squamocolumnar junction is arrowed). An insulating diathermy pad is attached to the patient’s thigh, the smoke-extraction facility is attached to the speculum, and a local anesthetic is injected into the cervix (Figure 7.39). Using a fine needle on a dental syringe, the injection of local anesthetic with or without a vasopressor agent is delivered, taking care to inject peripherally to the lesion. Some authors recommend injecting at the four cardinal points—anterior, posterior, and lateral. However, the authors’ preference is to make a series of peripheral injections encompassing the transformation zone. In addition, if the desired excision is a deep one, then a deeper injection is administered into the anterior and posterior lips of the cervix. The injection should be made gently and superficially, at first causing blanching of the cervix; thereafter, the needle may be injected slightly more deeply, but not in the manner used for a paracervical block. It is important to avoid intravenous administration of the anesthetic, and therefore attention must be paid to the sensation of resistance when injecting. There should be some resistance felt as the desired location is within the stroma of the cervical tissue. The needle should be inserted no more than 5 mm deep. Figure 7.39 Injection of local anesthetic (Citanest 3% with octapressin) using a dental syringe. Depending on the model of the electrodiathermy machine used, the settings for cutting and coagulation are dialed in (refer to the manufacturer’s instructions). The best results are obtained with a blended setting of cutting and coagulation. An appropriately sized loop is chosen to encompass the entire lesion for removal in one block. In the authors’ experience, it is rarely necessary to use more than one “pass” of the loop. The “cut” button on the handpiece is pressed first before allowing the loop to enter the cervix (Figure 7.40). The wire is then followed, rather than pushed, through the cervix so that it exits beyond the lateral limit of the lesion (arrowed on Figure 7.40). It is important to allow the loop to pass easily through the cervical tissue and sometimes it may be necessary to wait a few seconds to allow the thin wire of the loop to do so. If excessive pressure is applied, the loop will start to bend, resulting in a shallower than expected excision. This movement can be made top-to-bottom or bottom-to-top, or even side-to-side, as the clinician prefers. Often the block sits on the cervix and can be lifted off with a small stick or forceps (Figure 7.41). Minimal bleeding should be experienced if the vasopressor agent has been injected correctly. Figure 7.41 The excised block of tissue in situ (arrowed) after a loop cone biopsy has been performed. Once the loop cone has been removed, the defect is carefully diathermied using the ball electrode; special attention should be paid to the skin edges where bleeding frequently occurs (Figure 7.42). The completed treatment leaves a shallow scoop defect in the cervix with a completely sealed surface (Figure 7.43). For additional hemostasis, Monsel’s solution (ferric subsulfate) may be applied to the base of the defect (Figure 7.44). The speculum should then be closed slightly to release the pressure from the speculum blades on the anterior and posterior vaginal fornices while maintaining colposcopic vision (Figure 7.45). This procedure often unmasks additional bleeding points, which may then be further diathermied. Healing following loop diathermy excision or cone biopsy is similar to that following other conservative procedures; it usually leaves a cervix with an accessible squamocolumnar junction (2) surrounded by a narrow rim of scar (1) (Figure 7.46). Figure 7.46 shows the cervix seen in Figures 7.40–7.45 some 3 months later, after healing. The large central area of columnar epithelium (3) is common following loop diathermy, as it is following laser surgery. Why there should be so much variability from patient to patient is not known. The large areas of ectopy commonly occur when a very wide lesion has been removed. The natural remodeling of the cervix does not appear to occur as frequently as it does following the removal of a smaller lesion. Figure 7.42 Cauterization of the base using a diathermy ball electrode. Figure 7.43 The completed treatment with the base of the treatment site having been sealed with ball diathermy. The endocervical tissue is arrowed. Figure 7.44 Application of Monsel’s solution (ferric subsulfate) to aid hemostasis. Figure 7.45 View of the cervix after partial release of the speculum. This maneuver shows the “real” view of the cervix with the excised edges (arrowed) tending to approximate. The CO2 laser is excellent not only as a vaporizing instrument but also, when the spot size is reduced to 1 mm or less, as a slow cutting device. The facility to reduce the spot size, and thus increase the power (power density) of the beam, gives the surgeon a very accurate instrument with which to remove lesions from the cervix, vulva, etc. In many centers, laser cone biopsy has effectively replaced the cold knife conization procedure because of its accuracy, relatively small blood loss, low complication rate, and the potential to remodel the cervix; this results in an easily assessed squamocolumnar junction in the majority of cases. The procedure can be performed either freehand or using the micromanipulator with the colposcope. The latter is the most frequently used. It is important to have a laser with a variable spot-size facility, and a suitable smoke-extraction device. It is essential that the speculum be light enough to allow maneuvering of the laser cone tissue and that the laser beam has access to the cervical surface. Fine Allis forceps or skin hooks can be used for grasping the cone, as shown in Figure 7.47. Figure 7.47 The multiple skin hook (Singer claw; manufactured by Rocket, of London). In many centers the procedure is performed under local anesthetic in an outpatient clinic, although obviously general anesthetic can be employed. The cervix is exposed using a Cusco’s or Graves’ speculum fitted with a smoke-extraction facility. The patient is colposcoped and the extent of the lesion identified. A vasopressor agent may be injected to reduce blood loss during the procedure. As with the standard laser ablation, the transformation zone and the lesion are first circumscribed, leaving a margin of 3 mm around any lesion. This cut is deepened to approximately 3 mm, and the edge of the cone block is grasped with the skin hooks and retracted downward and sideways (Figures 7.48–7.51). The laser is then used to deepen the incision circumferentially until the desired depth is reached, after which the incision can be directed centrally to dissect the upper limit of the block of tissue. This is achieved by grasping the tissue with a skin hook and pulling it to one side so that the laser beam begins to cut across the apex of the cone. Another technique which can be used to excise the apex is the use of right-angled scissors or a tonsillar snare. This has the advantage of leaving no thermal artefact. Figure 7.48 A cone biopsy being grasped by a skin hook and drawn down. As this happens, tension is applied to the tissues, which can be easily divided by playing the laser beam over the surface. By moving the hooks around the periphery of the cone and steadily deepening the vertical cut, a cylindrical block of tissue is developed to an appropriate depth, usually 20 mm. If necessary the base of the cone defect can be biopsied to determine whether there is any residual lesion within the endocervical canal after the cone has been removed (Figure 7.52). At the end of the procedure, the cylindrical defect remains; this can be easily measured using any simple measuring device (Figure 7.53). Sometimes it may be necessary to extend treatment at the base. Others have recommended cutting a small dimple in the base, by undercutting with the laser the area just distal to the endocervical cut edge. This results in eversion of the endocervical epithelium, and it is believed that this simple procedure minimizes the risk of cervical stenosis. After the specimen has been excised, hemostasis is achieved by increasing the spot size of the beam, thus reducing its power and moving the beam across the bleeding points. A diathermy ball for hemostasis can also be an alternative effective technique, particularly if there is heavier bleeding. NETZ is a modification of loop diathermy. A straight wire electrode made of tungsten is used like a knife. A diathermy loop is an inflexible instrument with a fixed diameter of 1.2–2.5 cm. The amount and shape of excised tissue is determined by the size and shape of the loop used. It is not possible to see the deep cutting edges of the loop, which can lead to residual diseases within the canal. Larger lesions are usually removed in multiple pieces or removed by laser cone biopsy or cold knife cone biopsy. Both of these procedures are expensive and much more risky procedure than the NETZ procedure. The amount of tissue removed by needle diathermy is fashioned in the same way as with a cold knife or laser. However, hemostasis can be achieved more effectively and efficiently. It has been reported that NETZ procedures produced a better rate of clear margins of resected cone at both the endocervical and ectocervical margins. Preparations of the patient are similar to those for a LEEP procedure. However, needle diathermy needs lower power settings to complete the procedures. These authors use a 15 W cutting and 15 W coagulation setting with blend level 1. Some authors suggested using only the coagulation setting at a higher power level. The power setting may need to be adjusted according to the type of needle used. With the diathermy unit on blend 1 (using both cutting and coagulation), the active part of needle is inserted into the cervical tissue outside the transformation zone at the 12 or 3 o’clock position. The needle is moved slowly and gently in a clockwise direction to complete the circle. The cervix may need to be pushed centrally or to the other side by means of the hook to complete the initial circle. The straight wire can be bent at the junction of the proximal one-third and distal two-thirds to create a 30° angle. The needle is reinserted at the 12 o’clock position and slowly turned counterclockwise to the 6 o’clock position to create the cone and complete the excision. Effective hemostasis is achieved by coagulation with the same needle. The excised cone specimen should be immersed intact in neutral buffered formalin for up to 24 hours’ fixation depending on its size. After processing, longitudinal sections are examined and both the endocervical and ectocervical margins are assessed. Radial blocks are sometimes taken if the os is circular or open, but this technique has disadvantages in that the blocks end up being wedge-shaped, thereby causing problems with embedding and sectioning. The edges of the cone may prove difficult to interpret. It is not unusual to find two zones of thermal injury. The zone at the margin of resection, which is approximately 50 μm thick, is characterized by extensive carbonization and charring. The other zone is much more variable in thickness and is characterized by tissue coagulation but lacks charring. The coagulated zone can extend from 130 to 750 μm thick. Figure 7.54 shows an example of these zones of injury at the edge of a cone biopsy (1). This thermal damage can be avoided at the apex of the specimen by the use of scissors or a tonsillar snare as described above. The specimens produced by loop diathermy procedures are of high quality. Figures 7.55–7.58 show how minimal is the thermal artifact on the cut edges of the specimens, and how easy it is to interpret the histologic changes on the ectocervical and endocervical ends of the specimen. There is no significant difference in the biocharacteristics or extent of thermal damage in histologic specimens following CO2 laser conization and after excision biopsy using the loop electrosurgical excision procedure. In Figure 7.55 a long loop cone biopsy is presented with minimal thermal damage (1); the excellent quality of the material over the lesion (2) with progression into the endocervical canal (3) can be seen. A high-power view of this in Figure 7.56 shows the thermal damage (1), gland infilling (2), and the surface lesion at (3). In Figure 7.57 another excellent specimen (stained with Van Gieson’s stain) shows a CIN3 lesion (1), whose ectocervical limits are seen between the arrows, with “clear” glands (2) and margins both ecto- and endocervical. In Figure 7.58 there has been incomplete excision at (1) and the excellent quality of the material facilitated the making of this important diagnosis. A histologic report of incomplete excision of CIN by LLETZ does not equate with residual disease. The high treatment success rate of LLETZ means that a report of incomplete excision should stimulate close colposcopic and cytologic follow-up to identify the small number of women with residual CIN after therapy. For years, the standard therapy for treating CIN and conserving the cervix was cold knife cone biopsy. This valuable technique was somewhat superseded by the laser (CO2) cone and then by the loop diathermy cone biopsy (LLETZ/LEEP). Prior to the widespread availability of colposcopy, many cone biopsies were performed “blind” using this cold knife technique. Consequently, there was tremendous variation in the amount of cervical tissue removed, and subsequent trauma to the cervix was significant. Large cone biopsies tended to be effective in dealing with the CIN at a “price,” but there was a higher risk of pregnancy complications; smaller cone biopsies were less traumatic but tended not to remove the fundamental CIN problem. Colposcopy allows an accurate assessment to be made of the size of the lesion and, consequently, the clinician can more accurately determine and tailor the size of cone (excision) biopsy required. No patient should receive treatment for an abnormal smear without first being given a colposcopic examination. The aim of a cone biopsy is to achieve a combination of diagnostic and curative management. The tissue to be removed is the entire transformation zone with an extension into the endocervical canal to encompass the entire lesion. How deep this extension should be remains difficult to assess and the various methods employed to overcome this problem are discussed above. There are a number of indications for cone biopsy. The indications for cone (excision) biopsy (using either cold knife, CO2 laser, or loop diathermy) are as follows: It must be recognized that in many parts of the world, where the newer excisional techniques, namely CO2 laser and loop diathermy, are not available, there remains an important place for cold knife cone biopsy. With the introduction of many reversible short-acting analgesics, such as midazolam, it has been possible to perform extensive surgical procedures with the patient semiconscious and free from pain. The patient should be placed in the lithotomy position. A Simm’s or Auvard’s speculum is placed in the vagina, exposing the cervix. The patient is recolposcoped and the site of the lesion confirmed. It is usual to utilize a technique for reducing blood loss during the procedure. The most frequently used hemostatic techniques are either to insert lateral sutures to encompass the descending branches of the cervical arteries or to use a vasopressor agent that is injected directly into the stroma of the cervix. The most popular agents are dilute epinephrine in local anesthetic, felypressin in local anesthetic, or bupivacaine and octapressin. Injections of these agents should surround the periphery of the ectocervical margin of the lesion and not be injected directly into the lesion because of the theoretic risk of implantation of CIN deep into the cervix. Some clinicians recommend the use of Lugol’s iodine solution to assist in locating the ectocervical margins of the lesion; it is of little help in outlining the new squamocolumnar junction. With a tissue forceps the cervix is grasped peripheral to the lesion and drawn down; the ectocervical incision is made with a pointed knife blade (a wedge), completely circumscribing the transformation zone and the lesion (Figure 7.59). It is often easier to begin the incision posteriorly and then carry it on anteriorly, so that any bleeding runs away from the incision. Manipulating the cone with a tissue forceps, a vertical incision is then made into the cervix, angled toward the upper part of the endocervical canal (Figure 7.60) so as to remove a conical block of tissue (1) (Figure 7.61). The necessary depth of this cut is the great imponderable. It would appear that a depth of between 15 and 20 mm will clear most endocervical extensions. If the lesion extends for a large distance across the ectocervix and up into the endocervical canal, the upper limit of which can be clearly seen, then it will be necessary to perform a shallower cone excision. However, if there is little to be seen on the ectocervix and the lesion disappears out of sight into the endocervical canal, as in Figure 7.62, then a proportionately longer cone will be needed. Fortunately, the latter situation rarely occurs in younger women, so that the risk of future pregnancy complications, resulting from damage to the cervical sphincteric mechanism, does not apply. Figure 7.61(b) Gross photograph of a knife cone biopsy specimen, “os up,” prior to fixation. There is a suture at 12 o’clock for orientation. Figure 7.63 (a and b) Scheme showing the technique for developing cervical skin flaps and inserting Sturmdorf sutures. The purpose of the Sturmdorf suture is to cover the posterior aspect of the bare cervical stump with a loose skin flap. The basic procedure is to draw the midpoint of the edge of the skin flap into the cervical canal on a double thread. The needles mounted on these two threads secure the flaps to the stump. It is initially anchored at the posterior cut end of the canal by transfixing the posterior wall of the stump with the two ends of the thread; one on the left side, in the 5 o’clock position, and one on the right side, in the 7 o’clock position. (a) The route taken by each end of the suture after it has been picked up at the midpoint of the edge of the posterior skin flap. The stitch commences by traversing the skin edge at 1A, and some surgeons prefer to use a double hitch or knot at this point. In any case, this is the midpoint of the suture length and each half will be inserted separately on each side with one end taken and inserted in the progression marked on the diagram from 1 to 4; the other free end is then likewise inserted. (b) The effect when the stitch has been pulled tight and tied. Since the stitch embraces a major part of the cervical stump between its two halves or ends, it has value as a hemostatic agent and there is no need to worry about vessels bleeding from the ends of the stump. Traditionally, considerable efforts have been made to repair the cut surface of the cervix following cone biopsy. Figure 7.63 shows the use of one such previously popular technique (Sturmdorf) in which sutures were inserted to draw over the raw surface flaps of cervical epithelium that had been previously developed. The result at the time of surgery is commendably neat (Figure 7.64). However, the scarring and disfigurement that can occur during the healing process plus the risk of covering over any residual precancerous lesion, thereby rendering satisfactory follow-up difficult or impossible to achieve, must first be considered. So long as local hemostasis can be achieved, it is better to simply freeze or cauterize the base of the cone cavity and allow spontaneous healing to occur. This results in the new squamocolumnar junction being accessible to cytologic and colposcopic follow-up. Alternatively, simple tamponade with a roll pack or large tampon can be used. Figure 7.64 The cervix has been fully reconstituted with the Sturmdorf stitches tied both anteriorly at the 12 o’clock position (1) and posteriorly at the 6 o’clock position (1). The cervix is covered by skin, except for a narrow band on each side; these disappeared when the anterior elevating stitch was cut. Figure 7.65 A diagrammatic representation of the wide extension of abnormal (atypical) epithelium onto the ectocervix; such a combination requires both vaporization and excision. This epithelium extends high into the endocervical canal (shaded) (1) and an excisional procedure will be needed for its complete removal. The abnormal (atypical) epithelium on the ectocervix (2) had already been biopsied and shown to be cervical intraepithelial neoplasia; this will require vaporization. The arrows point to the outer limits of the vaporization procedure. Uncommonly, the area of the atypical transformation zone is extensive, reaching to the periphery of the cervix and onto the vagina. It may extend well within the endocervix (Figures 7.65–7.67), giving rise to an unsatisfactory colposcopy. Previously, such an extensive lesion would have been treated by surgery, with removal of a large part of the cervix and vaginal fornices. A combination of wide peripheral laser vaporization and a deeper central vaporization or laser/loop diathermy excision allows the clinician nowadays to deal effectively with the entire area without removing large volumes of the cervix and causing serious surgical damage (Figure 7.68a). It is important to recognize that in a small proportion of cases a congenital transformation zone may be present with the typical pale acetowhitening extending beyond the cervix onto the vaginal fornices, usually more commonly seen anteriorly and posteriorly. There is often a fine mosaic pattern present. This has little propensity to malignant change and does not require treatment. Carefully documented biopsies should be taken prior to embarking on any treatment to this area. Figure 7.66 (a) A large area of abnormal (atypical) epithelium (dense staining acetowhite) extends high into the endocervix (1) and onto the ectocervix (2) requiring excision, for the central area (1), and vaporization, of the peripheral area (2). (b) Shows the difficulty in finding the upper limit of the lesion. Figure 7.67 An extensive area of abnormal (atypical) epithelium with extension high into the endocervix (1), onto the ectocervix at (2), and the vaginal fornix (3). Punch biopsies are performed at areas (2) and (3) to exclude the presence of invasion, after which these parts of the cervix were treated with vaporization. Because of the unsatisfactory nature of the central area (1) an excision procedure was performed. Figure 7.69 A normal cervix after laser cone biopsy; this shows the result some 6 months after treatment. The edge of the treated area is at (1), the regenerating epithelium at (2), and the new squamocolumnar junction at (3). The procedure may be performed under local or general anesthetic. Biopsies will have been taken of the peripheral area of the transformation zone. In Figure 7.67, for example, a colposcopically unsatisfactory central area (1) exists with an obvious minor- or “intermediate”-grade tissue present at (2) and (3). Biopsies taken at positions (2) and (3) showed CIN3. The central area (1) was removed by excision biopsy and revealed very early microinvasive carcinoma; the peripheral areas that showed CIN3 were subjected to laser vaporization. When lesions extend onto the vaginal fornices (Figures 7.66, 7.67), it is often prudent to perform the procedure under general anesthetic on a day-case basis, because the vaginal skin is extremely sensitive and it may not be easy to achieve complete analgesia using local anesthetic agents. With the patient in the lithotomy position, a colposcopic examination maps out the lesion. It is useful to circumscribe the outer limits of the atypical transformation zone by laser. Cone biopsy is performed first using either laser or loop diathermy. Once the central area has been removed, the periphery can then be vaporized to a depth of 5 mm (Figure 7.68). The fibers of the cervical stroma can be seen at the base of the treated area with minimal attendant bleeding. In Figure 7.68a, the CIN lesion extended to the vaginal fornix (arrowed); this contrasts with the lesion seen in Figure 7.67, where it extended to the vaginal mucosa (3). In the cases pictured in Figures 7.66–7.68a, the peripheral area had already been sampled by punch biopsy, performed in the colposcopy clinic; this had revealed CIN3. If there is any colposcopic or histologic suggestion of invasion, then undercutting, using the laser in the superpulse mode, of the abnormal (atypical) epithelium in the vaginal fornix or vagina will be necessary. This technique is described in Chapter 8. The end results of the laser “top hat” procedures, involving excision of the endocervical extension of CIN, are also employed when using laser vaporization, loop diathermy excision, and loop diathermy cone biopsy. Figure 7.68a is a good example. Routine hysterectomy in the primary management of CIN is now rarely indicated. There are many thousands of cases reported in the literature, and the incidence of recurrence of clinical invasive cancer of the vaginal vault is surprisingly low. In most of these recurrences, there was a suspicion that there had been a failure at the initial examination to recognize the extension of the CIN onto the vagina, as shown in Figures 7.66 and 7.67. The indications for hysterectomy have now been clearly defined and are as follows: For the patient requiring hysterectomy, there is little doubt that the optimal therapy is to use either a vaginal hysterectomy or a minimal access technique, such as a laparoscopically assisted vaginal hysterectomy (LAVH) or laparoscopic total hysterectomy (LTH). Such a technique allows accurate identification and delineation of the site of the cervical abnormal areas and reduces the risk of leaving a residue of precancer behind. This is of particular value for the patient who has a lesion which extends from the cervix onto the fornices of the vagina (2.4%). The treatment of patients with persistent cytologic abnormalities using standard abdominal procedures is marred by a significant risk of leaving behind slivers of the cervix and vagina which harbor areas of CIN and may go on to develop invasive lesions at a later date. A significant advantage of the LAVH or LTH is the freedom given to the surgeon to remove the ovaries in those patients where it is appropriate to do so. If hysterectomy is to be performed after conization, then it would seem that the procedure should be carried out sooner rather than later. In the follow-up of such patients, it is the authors’ practice to visualize the vault with a colposcope at 4 and 12 months after hysterectomy, and then to undertake periodic vault cytology annually for 5 years and then every 2–3 years after that. The immediate postoperative problems seen after laser cone biopsy are essentially the same as those found after other excisional or local destructive procedures have been performed on the cervix. While the procedure is being carried out, hemorrhage may occur as a result of incomplete division of the vessels, or it may be associated with secondary infection of the defect site. Treatment is usually conservative, although occasionally surgical management may be required and, more rarely, supportive blood transfusion may be necessary. Discharge is a common accompaniment to the healing process, and often lasts for a few weeks. So long as significant infection does not occur, no special treatment is needed. Pain is rarely felt following the procedure, even in the immediate postoperative period. Longer term problems may arise as a result of variability in healing of the cervix. Most patients will be left with a cervix that has an accessible squamocolumnar junction with a small area of columnar epithelium visible (Figures 7.69, 7.70). However, it is not uncommon for the cervix to appear as shown in Figure 7.71, where there is a large area of exposed columnar epithelium. Such situations may result in complaints of postcoital and intermenstrual bleeding or discharge. It may be necessary to stimulate metaplasia of this area by applying cryosurgery, cautery, or even laser vaporization to the columnar epithelium. For most patients, however, active treatment is not necessary. Rarely, the cervix may scar, producing a partial (Figure 7.72) or complete stenosis of the os. This may be identified as an incidental finding at the time of cytologic review. Occasionally, the patient notices an alteration to the menstrual pattern with prolonged or reduced flow, and dysmenorrhea may develop. Mechanical dilatation of the cervix or cutting back the scar with a laser usually deals effectively with the problem. Figure 7.70 A frequent finding after both laser conization and laser ablation is the appearance of a prominent “button” of columnar epithelium (1). This tends to appear after the removal of large volumes of tissue, leaving a wide defect. The edge of the regenerating area is at (2). Figure 7.71 An area of “ectopy” (columnar epithelium exposed on the ectocervix) developed 6 months after laser vaporization of a cervical intraepithelial neoplasia lesion. Figure 7.72 A partially stenosed os after laser cone biopsy; the squamocolumnar junction is not accessible. The use of brush cytology or endocervical curettage is mandatory for proper follow-up assessment in such a case. Figure 7.73 and 7.74 A hysterectomy specimen showing the presence of complete cervical stenosis following a cone biopsy 4 years earlier. In Figure 7.73, a pyometra had developed in this postmenopausal woman. In Figure 7.74, a probe has been forcibly passed through where the cervical canal previously existed. In a meta-analysis in 2006, Kyrgiou et al. found that LLETZ or LEEP was significantly associated with preterm delivery (relative risk of 1.70; 11% in the treatment group compared with 7% in the control group), low birth weight <2500 g (relative risk of 1.82), and premature rupture of the membranes (relative risk of 2.69). Cold knife conization was significantly associated with preterm delivery (relative risk of 2.59) and low birth weight <2500 g (relative risk of 2.53). Interestingly, similar but marginally non-significant adverse effects were recorded for laser conization (preterm delivery). This meta-analysis did not detect significantly increased risks for obstetric outcomes after laser ablation. More worryingly, in another meta-analysis, Arbyn et al. reported in 2008 that cold knife conization was associated with a significantly increased risk of perinatal mortality (relative risk of 2.87), severe preterm delivery <32 weeks (relative risk of 2.78), extreme preterm delivery <28–30 weeks (relative risk of 5.33), and low birth weight <2000 g (relative risk of 2.86). LEEP and destructive therapy with cryotherapy and laser vaporization were not associated with a significantly increased frequency of serious adverse pregnancy outcomes. Whenever surgical treatment is performed, the body heals the wound by repairing the tissue with collagen; when this repair is excessive, scarring occurs. On the cervix, the result of such scarring over a long period, that is, 6–12 months or more, is a narrowing of the cervical os; in its most extreme form this will completely seal the cervix (Figures 7.73, 7.74). This problem tends to occur most frequently in postmenopausal and postpartum women, and may result in the development of a pyometra (Figure 7.73). As a long-term complication in the younger woman, stenosis may lead to pelvic endometriosis following on from a hematometra. This extreme enlargement may result in a diagnostic dilemma that can only be solved surgically, although, with modern ultrasound techniques, the diagnosis can now be readily made without surgery. Scarring and stenosis can occur following the use of any of the described conservative techniques (Figures 7.75–7.79). Figure 7.75 shows artificial twin orifices that have developed after cryosurgery. Residual glandular epithelium can produce an unusual appearance, as shown in Figure 7.76; dense white scarring is also seen in this case and this may confuse the clinician into believing that abnormal (atypical) epithelium exists. Figure 7.75 Artificial twin ora (1) produced by the center of the cervix healing over following cryosurgical treatment. The typical radial ridging (2) seen after cryosurgery is present on the posterior lip of the cervix. Figure 7.76 A narrowed and scarred external os (1) surrounded by curious puncta that are the result of residual foci of glandular epithelium (2), following laser therapy. This is an example of cervical endometriosis. Figure 7.77 (a) Severe constriction of the ectocervix existed until a cervical dilator (b) was used to break down a small membrane covering the os (1). A large cone biopsy had previously been performed; the edge of the scarred treatment area is at (2), the remaining cervix at (3). (b) A cervical dilator (1), developed by Dr B. Mansell of London, and an endocervical brush (2); both should be used to dilate the cervical constriction. Figure 7.78 A large area of very abnormal (atypical) (Coppleson grade 3) epithelium exists on the ectocervix (1) and extends toward the left vaginal fornix; the upper limit can be clearly seen at (2). A large excisional procedure was employed, resulting in constriction and deformity of the cervix as shown in Figure 7.79. Figure 7.79 The cervix shown in Figure 7.78 is seen 6 months later. A large area of the left-hand side of the cervix is missing, with the cervix close to the left vaginal fornix (1). The residual ectocervix on the left is at (2). The cervical canal is at (3). Although excision of this large area (which was found to contain an early microinvasive lesion) led to much deformity, the patient has had two normal vaginal deliveries since undergoing this procedure. Removal of large areas of the cervix can lead to much subsequent deformity (Figures 7.78, 7.79), with surprising preservation of function. When stenosis has occurred (Figures 7.75–7.77a), the patient often presents with symptoms of painful and prolonged menstruation. Probably the most simple management method is to perform a dilatation of the cervix under general anesthesia. However, it may be possible to temporize by using a small dilator and injecting local anesthetic into the cervix; this technique was developed by Dr B. Mansell of London (Figure 7.77b). Even the use of a narrow endocervical brush may relieve the problem. Rarely, it may be necessary to reconstruct the cervix when it has been severely distorted by scarring or when symptoms are such that severe discomfort is regularly felt by the patient. Figure 7.80 shows the operative appearance of a patient who required treatment for a high endocervical lesion. The results of this treatment were satisfactory with a deep central clearance for a microinvasive carcinoma. Unfortunately, some 6 months after treatment, the os had scarred to such an extent that the patient was experiencing severe discomfort during menstruation. The os was reduced to a mere pinhole (Figure 7.81). A commonly used technique for management is cervical dilatation at regular intervals (Figure 7.82). Figure 7.82 The cervical canal is dilated. In cases of complete stenosis (Figure 7.83), the cervix can be infiltrated with local anesthetic and gently probed with a 21G needle (Figure 7.84) in an attempt to aspirate retained menstrual blood, thereby indicating the direction in which entry should be made into the site of the obstructed endocervix (Figure 7.85). In Figure 7.85, an incision has been made exposing the endocervical canal and a cervical dilator (Hegar 4) has been inserted into the endocervix. Countertraction is provided with a pair of tenaculum forceps. During the early days of laser usage in the 1980s, a potent cause of recurrence was limited destruction of the CIN lesion. This resulted in recurrence of large areas of columnar epithelium on the ectocervix, which seemed to be prone to recurrent infection by the mutagenic agent that is responsible for CIN. This high rate of recurrence resulted in more radical destruction of the CIN and its surrounding tissue and this led to a dramatic improvement in results. To avoid the development of such areas of columnar epithelium, it is suggested that the “top hat” configuration be adopted when removing the abnormal (atypical) epithelium within the transformation zone. An excellent example of this is seen in Figure 7.68a. Comparison can be made with the cervices shown in Figures 7.86 and 7.87; both are within 6 months of treatment. Laser vaporization was used in the case seen in Figure 7.86 and large loop diathermy was used in the case shown in Figure 7.87. In both, the everted and exposed columnar epithelium is at (1) and the healed (treated) cervix at (2). It is suggested that women who would seem to be at increased risk for the development of a new CIN lesion should be kept under close surveillance. Although modern conservative therapies for the treatment of CIN are extremely successful, with clearance rates of the order of 95% or better, treated women are between two and five times more likely to experience cervical cancer than the general population. It is important to stress that the patient must take part in a regular surveillance program so that any residual or recurrent disease can be identified at an early stage and effectively dealt with. Six cases illustrating recurrent disease are presented in Figures 7.88–7.93. Follow-up of the patient is traditionally by cytology and, if necessary, colposcopy. The UK and European recommendation is to offer annual follow-up for at least 10 years after the treatment of CIN2 or worse before returning to the routine screening interval. Women treated for CIN1 can be returned to routine recall after 2 years of negative post-treatment cytology. Figure 7.88 An extremely scarred cervix, 5 years after a cold knife cone biopsy was used to treat cervical intraepithelial neoplasia 3. An area of atypical epithelium within the endocervix is seen at (1) and there is a further small area within the lateral scar at (2). Two Sturmdorf sutures were used and the deformity caused by them can be seen at (3). An excisional procedure will be needed to diagnose and treat this lesion. Figure 7.93 A high-grade lesion (1) within the endocervix; the top of the lesion cannot be seen. It occurred 2 years after laser vaporization. Excision biopsy revealed the presence of cervical intraepithelial neoplasia 3.
Management of cervical precancer
7.1 Introduction
7.2 Rationale behind treatment
7.3 Colposcopic and pathologic characteristics of cervical intraepithelial neoplasia lesions: a prerequisite to treatment
The limits and nature of the abnormal (atypical) epithelium
Glandular involvement
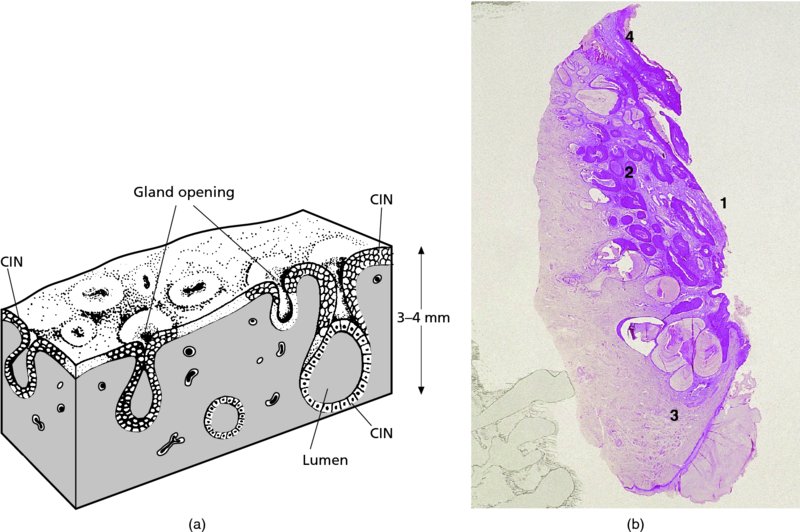
Endocervical extension
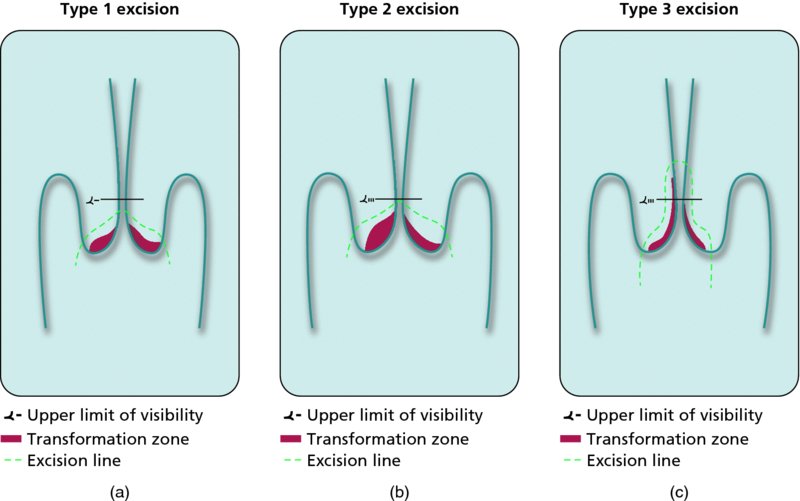
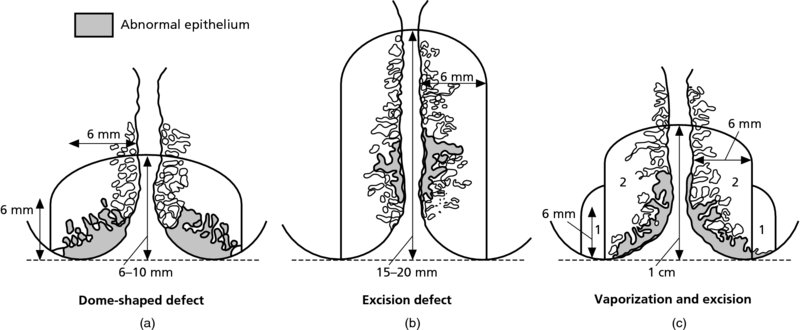
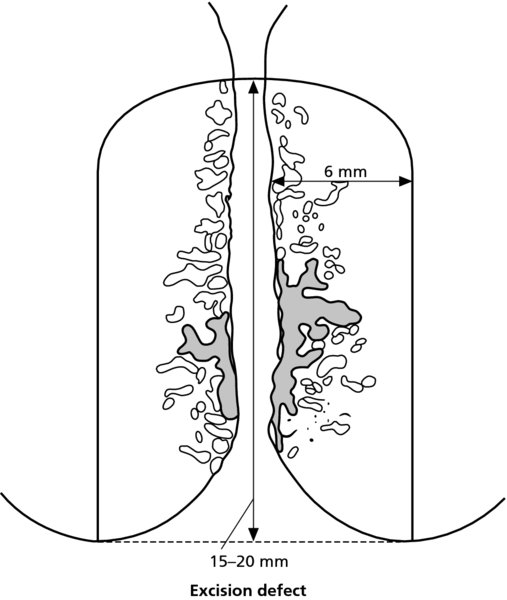
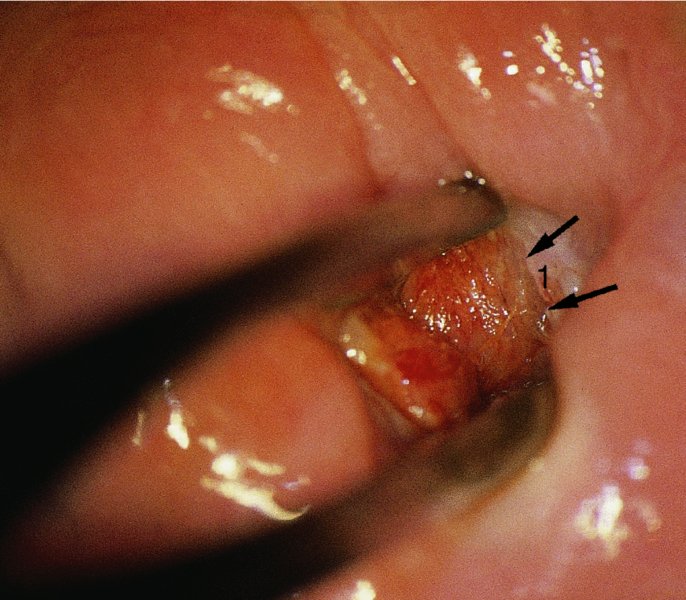
7.4 Colposcopically directed biopsy
Referral cytology
Colposcopy
Biopsy
ASCUS/LSIL
Low grade
Punch biopsy
ASCUS/LSIL
High grade
Punch biopsy
ASCUS/LSIL
Normal
ASC-?H
Treat as HSIL
HSIL
High grade
HSIL
Low grade
Punch biopsy
HSIL
Normal
MDT, examine vagina, ?HPV DNA
HSIL
Microinvasion
Always excision biopsy
HSIL
Invasion
Large biopsy
AGC
No obvious cancer
Extended excision
7.5 How to manage abnormal cytology?
Management of cytology with atypical cells of undetermined significance or borderline nuclear abnormality
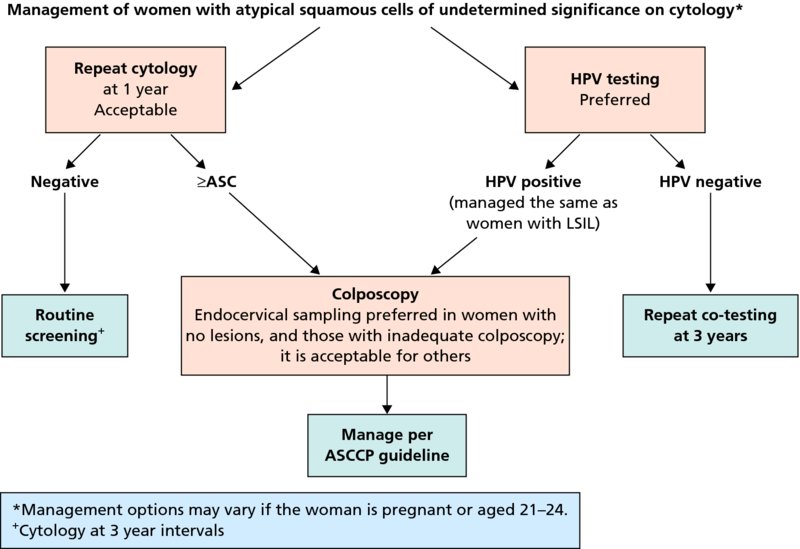
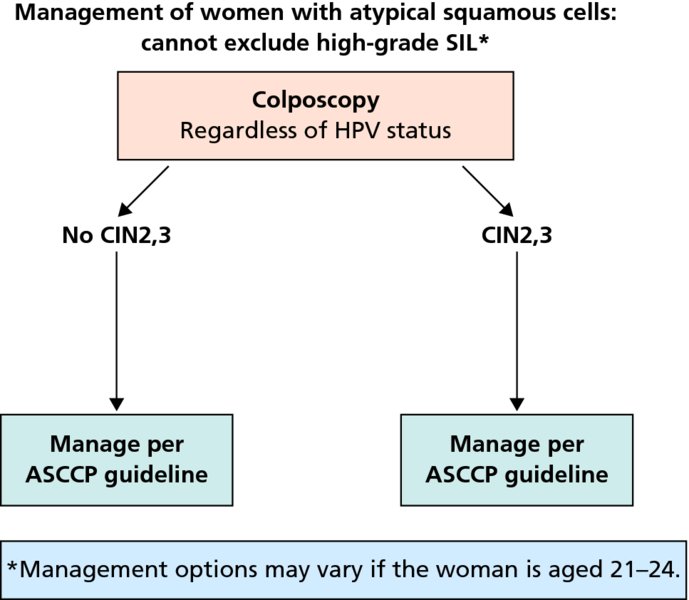
Management of low-grade squamous intraepithelial lesions (mild dyskaryosis)
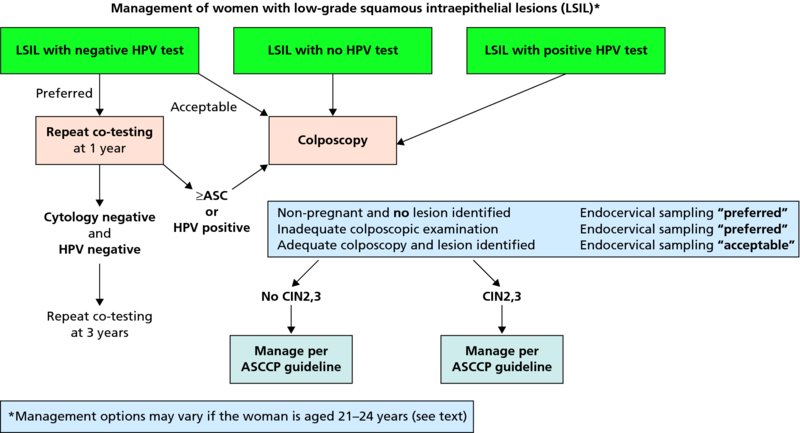
Management of high-grade squamous intraepithelial lesions (moderate and severe dyskaryosis)
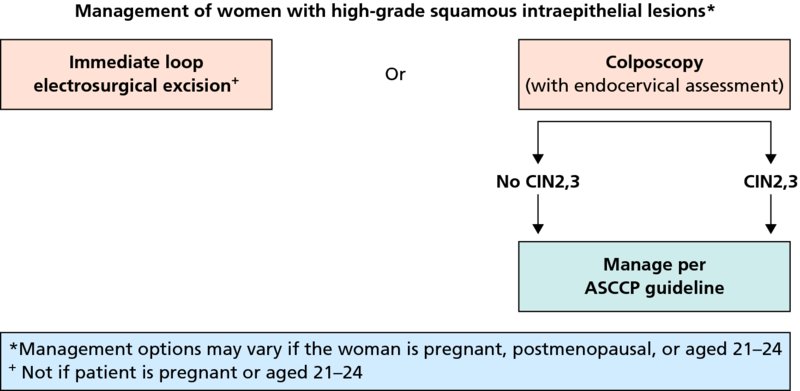
Management of cytology with atypical glandular dyskaryosis
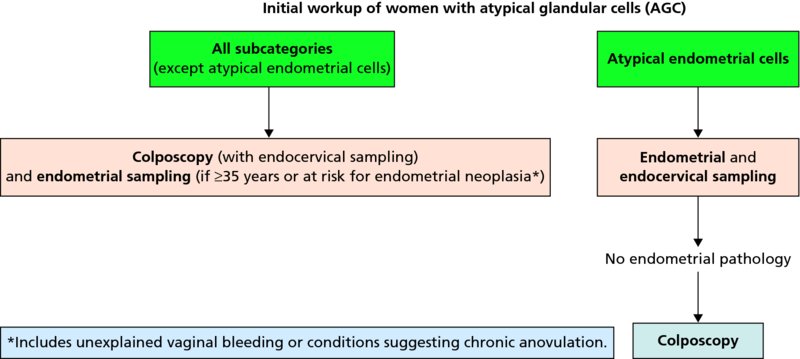
7.6 Which lesions to treat
High-grade lesions: cervical intraepithelial neoplasia 2–3/high-grade squamous intraepithelial lesion
Low-grade lesions: cervical intraepithelial neoplasia 1/low-grade squamous intraepithelial lesion
Glandular cervical lesions/adenocarcinoma in situ
7.7 Prerequisites for treatment
Type
Size
Site and visibility
Treatment options
Type 1
Small
Type 1
Large
Type 2
Small
Type 2
Large
Type 3
Small
Type
Large
7.8 Methods of treatment
Local destructive techniques
Cryotherapy
Principle
Equipment
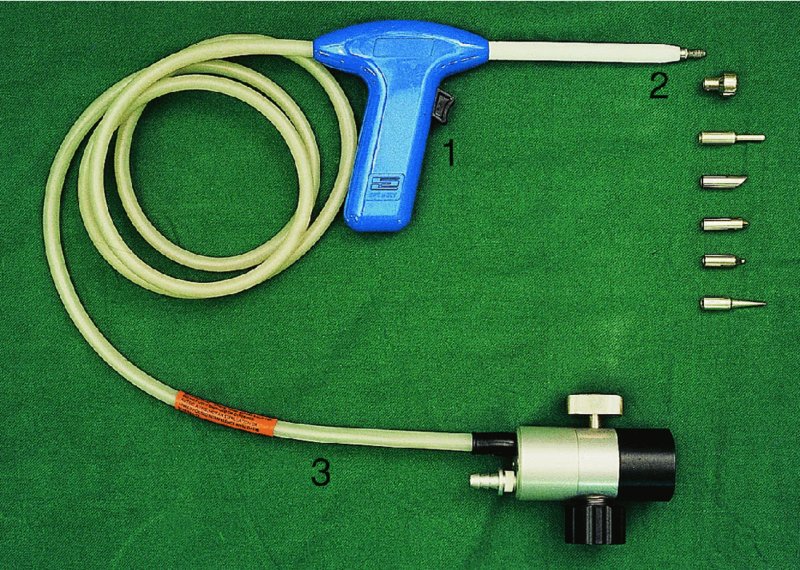
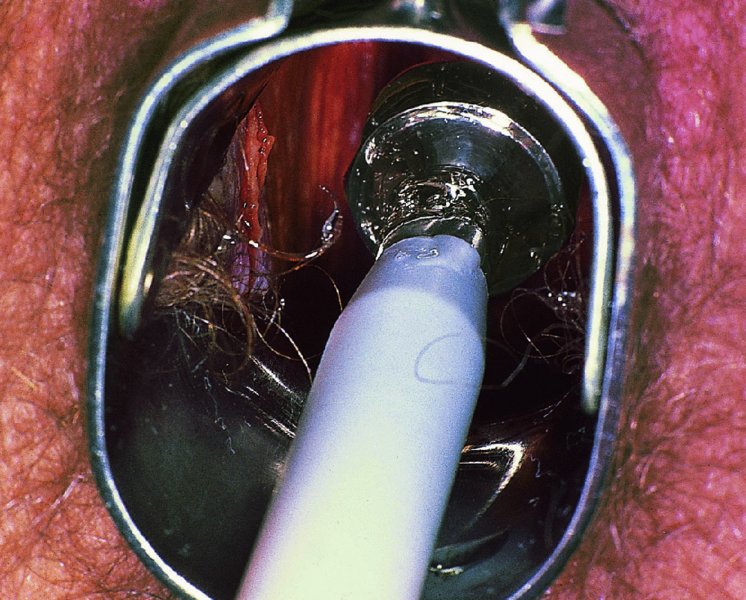
Technique
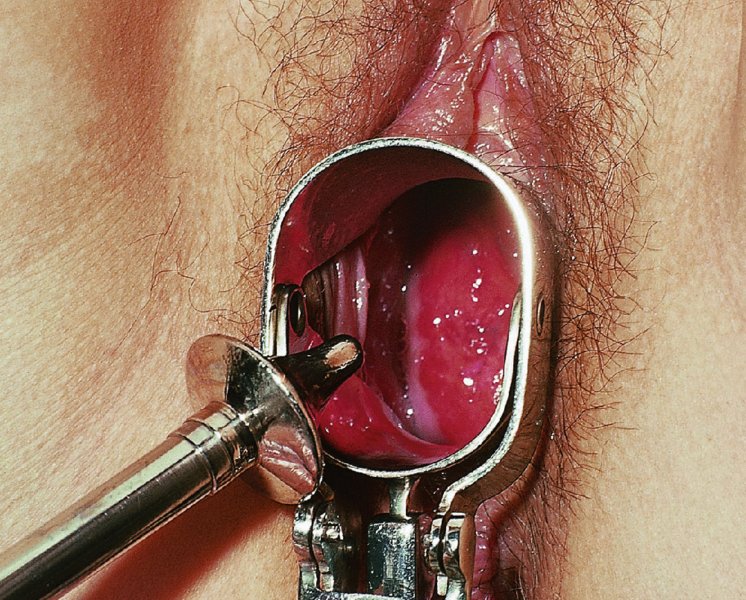
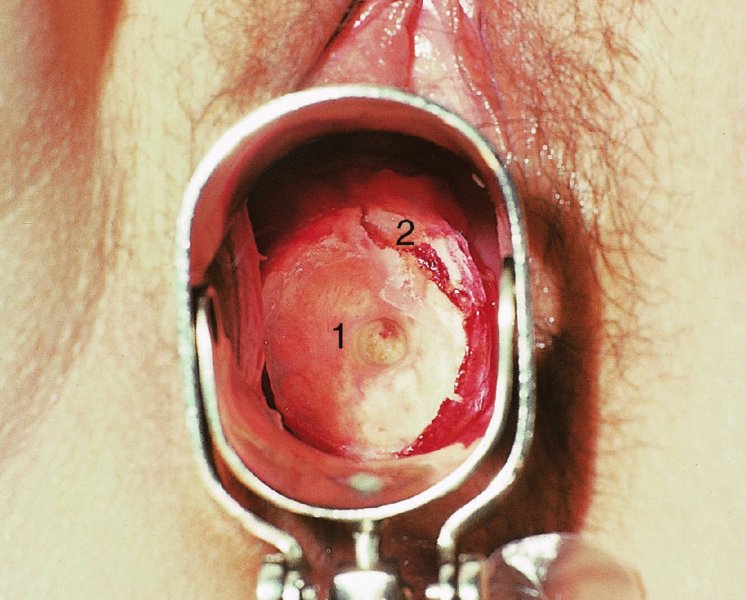
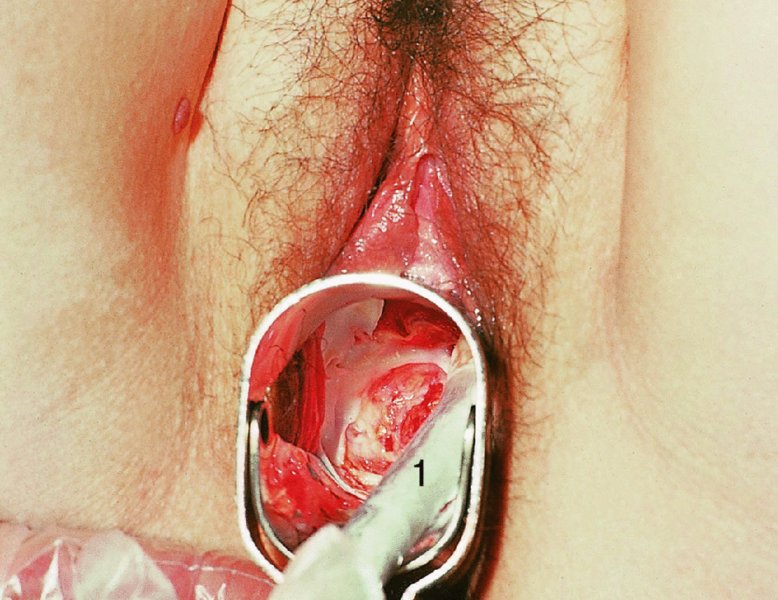
Healing
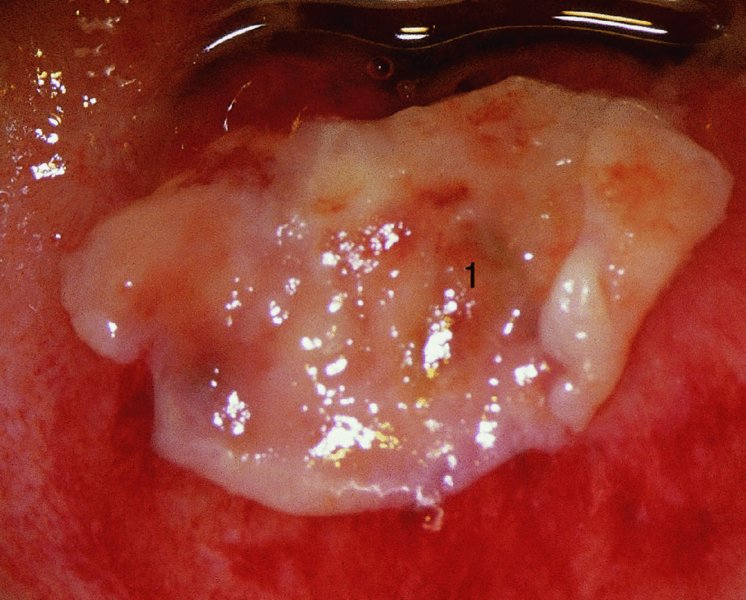
Post-treatment patient information and instructions
Complications
Cold coagulation
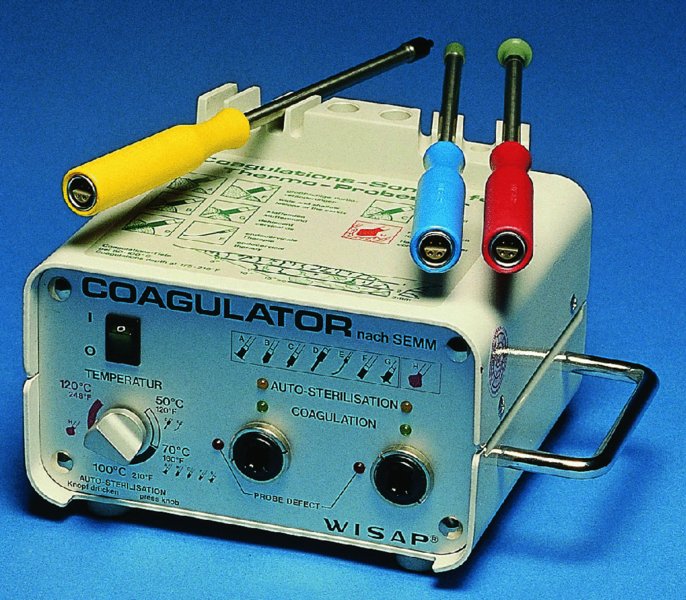
Technique
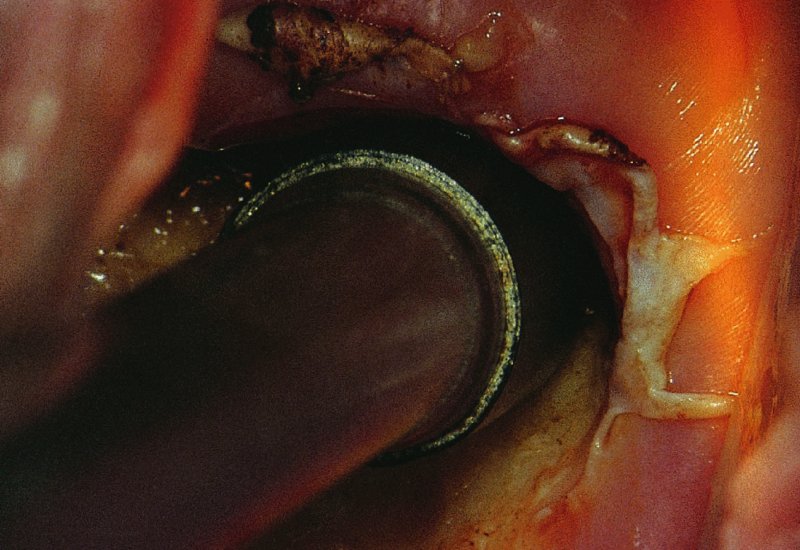
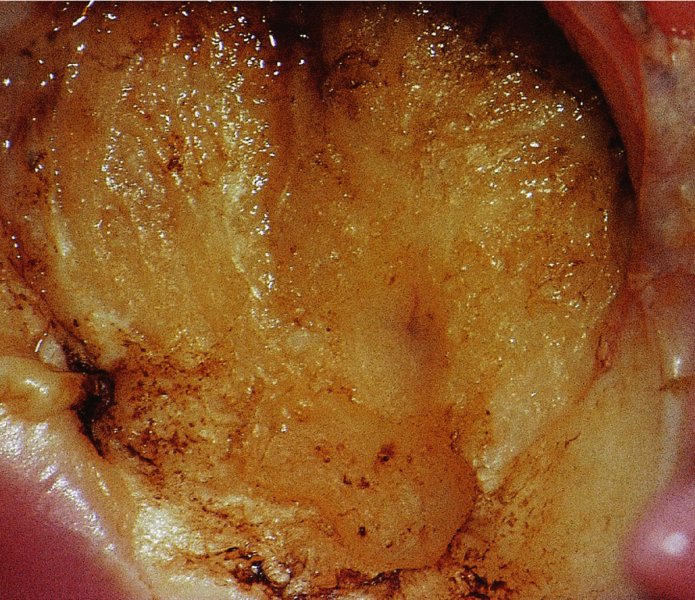
Follow-up
Pregnancy post-treatment
Radical electrocoagulation diathermy
Technique
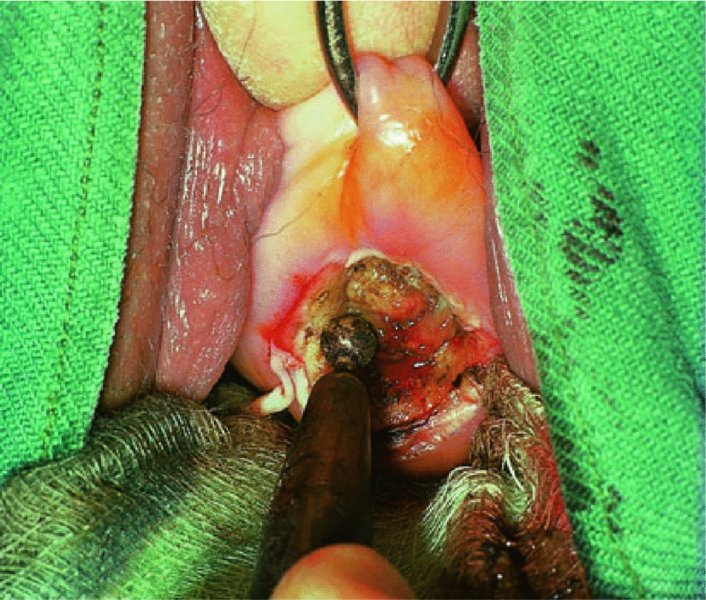
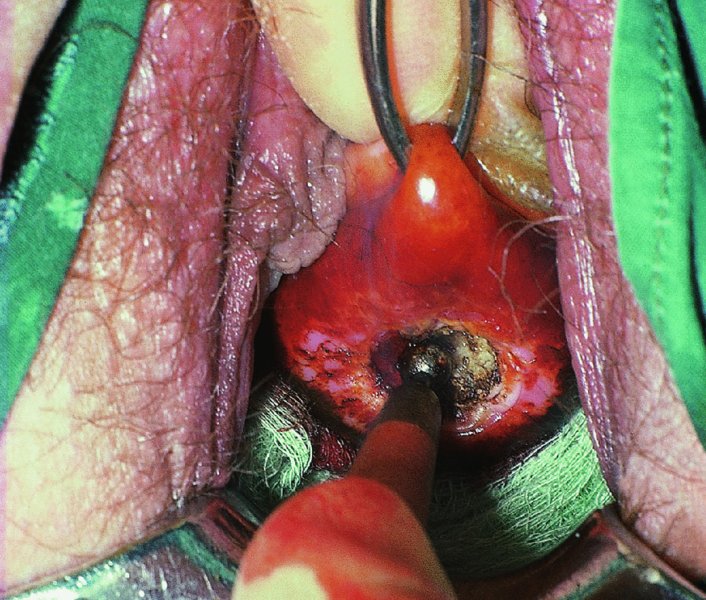
Postoperative course
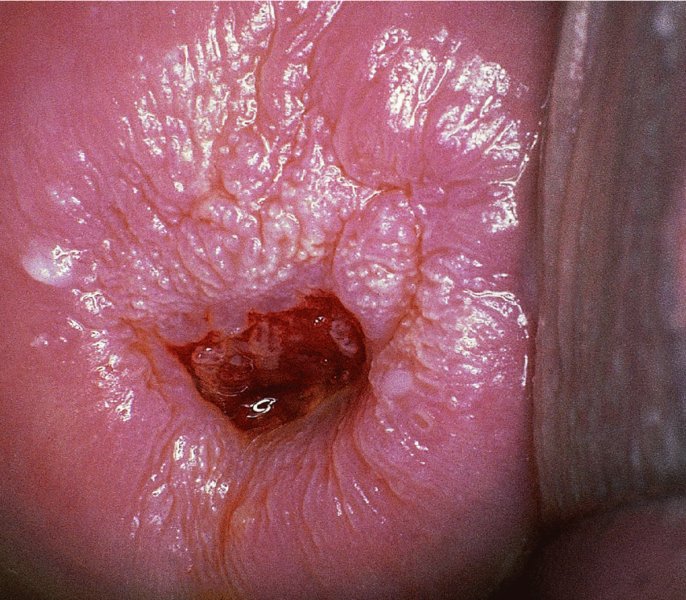
Carbon dioxide laser vaporization
Equipment
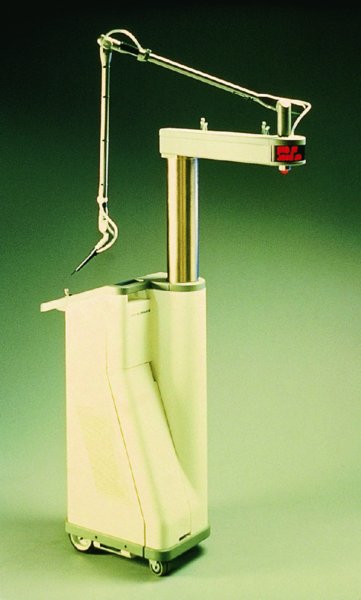
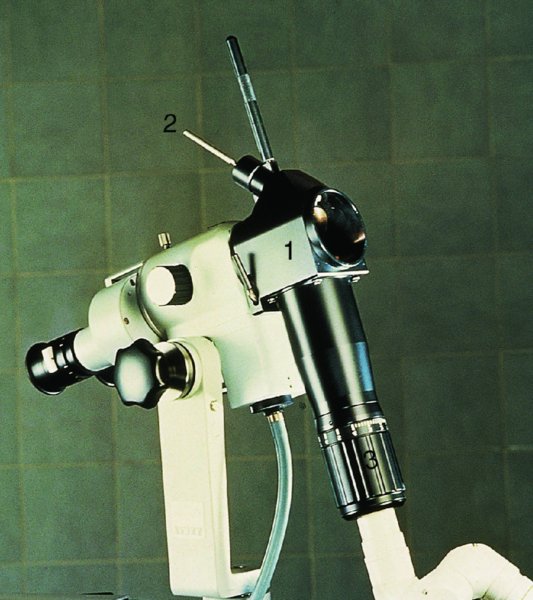
Procedure
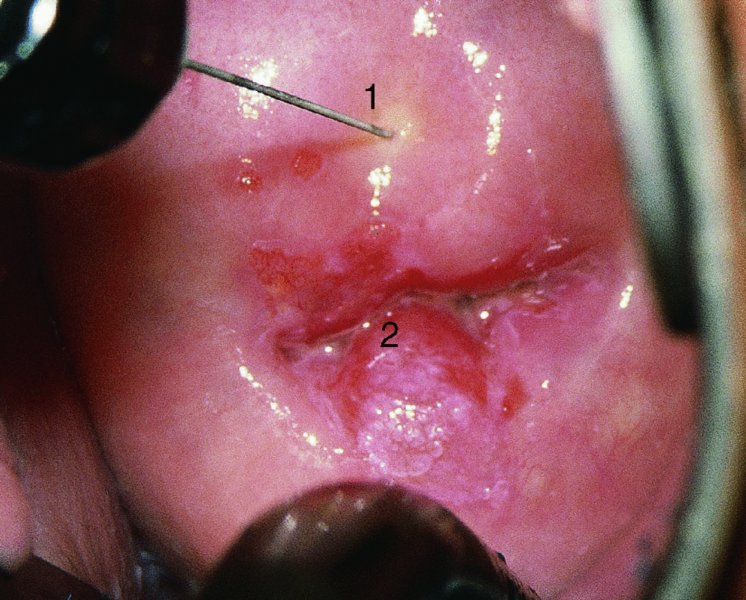
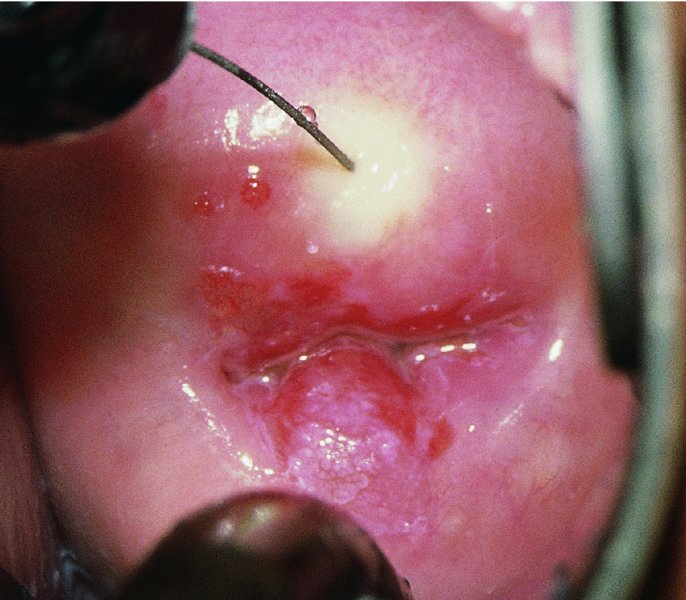
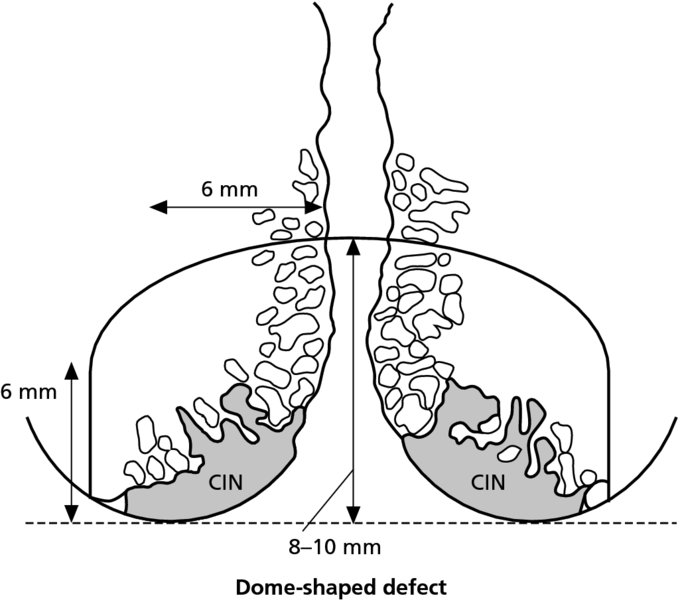
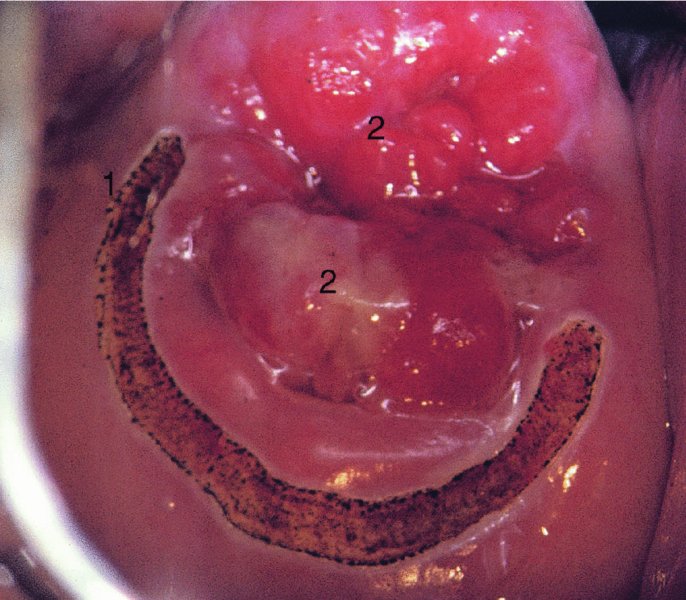
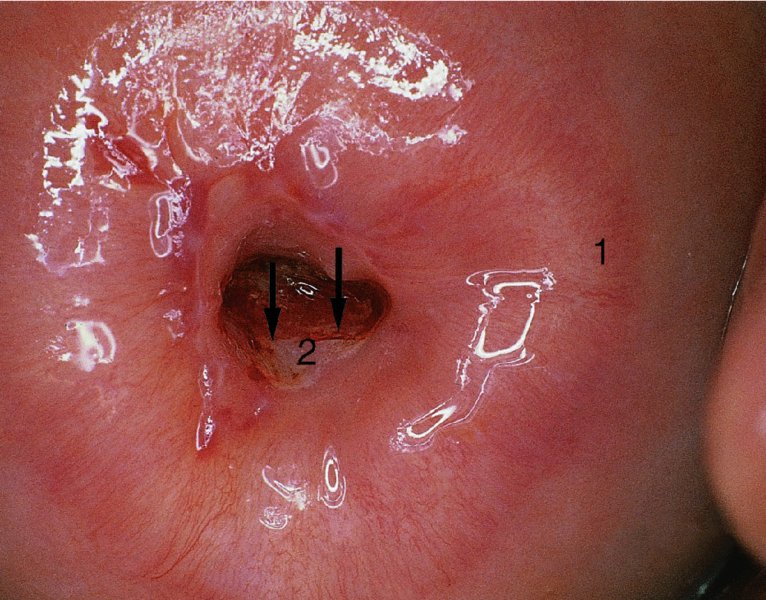
7.9 Excision techniques for treating cervical intraepithelial neoplasia
Patient assessment and the development of treatment protocols
Loop diathermy excision and cone biopsy
Equipment

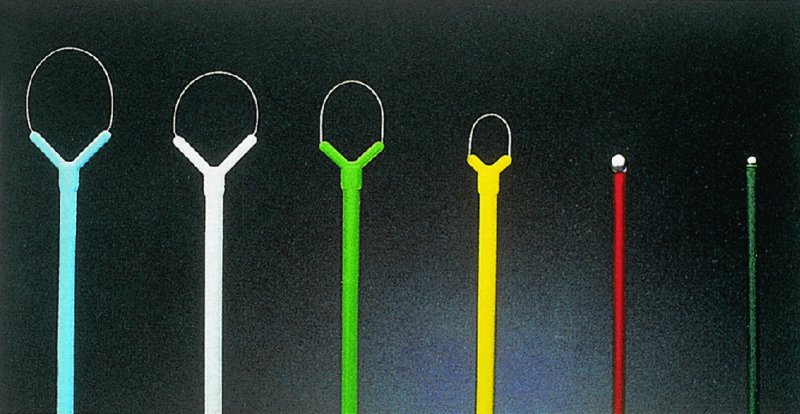
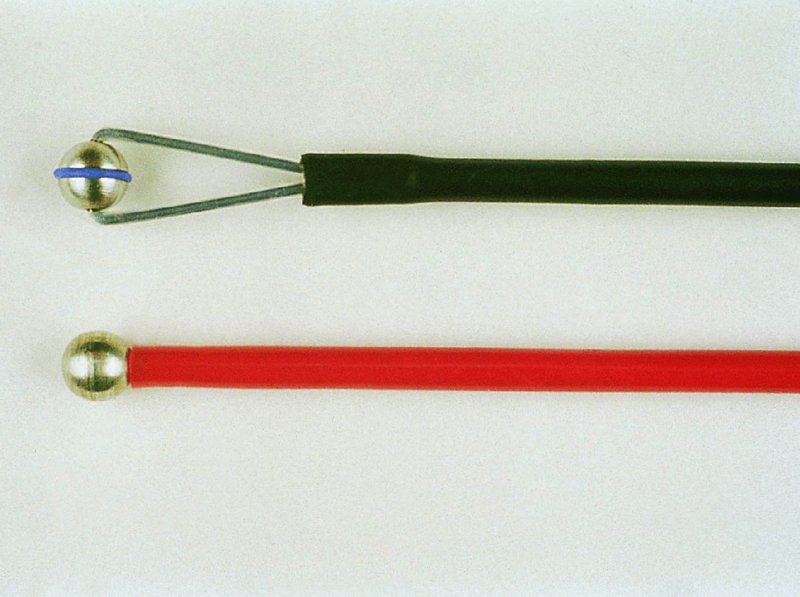
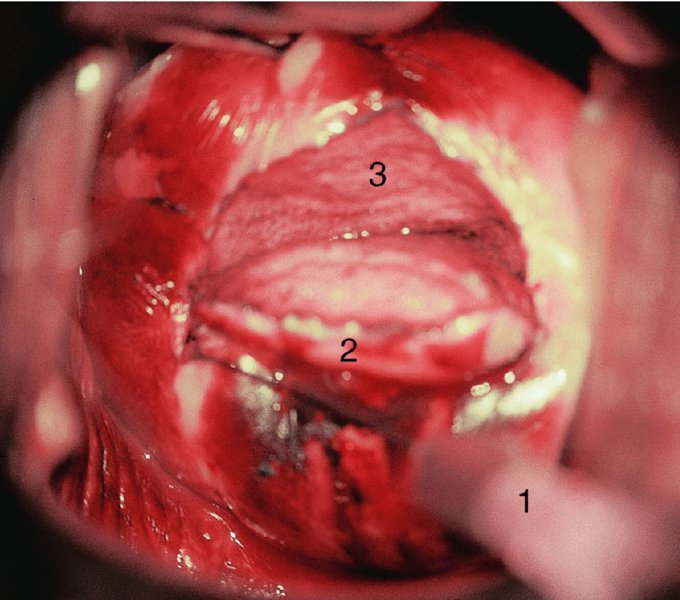
Technique
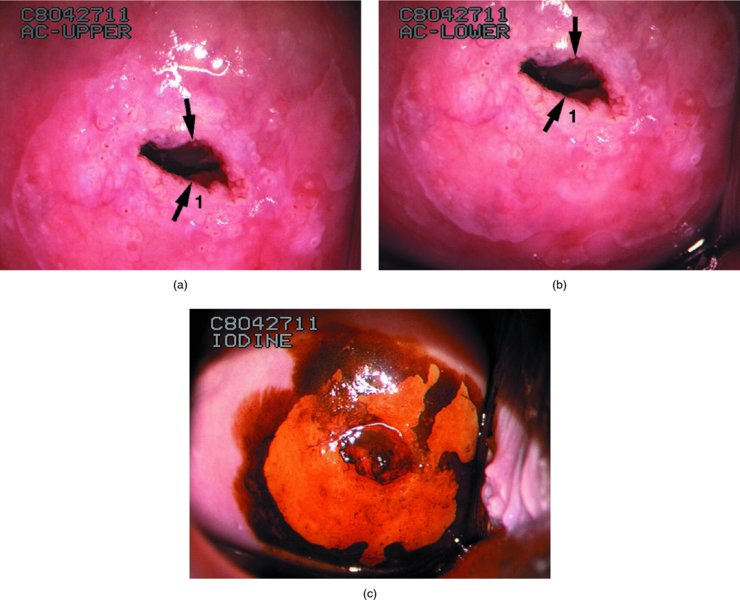
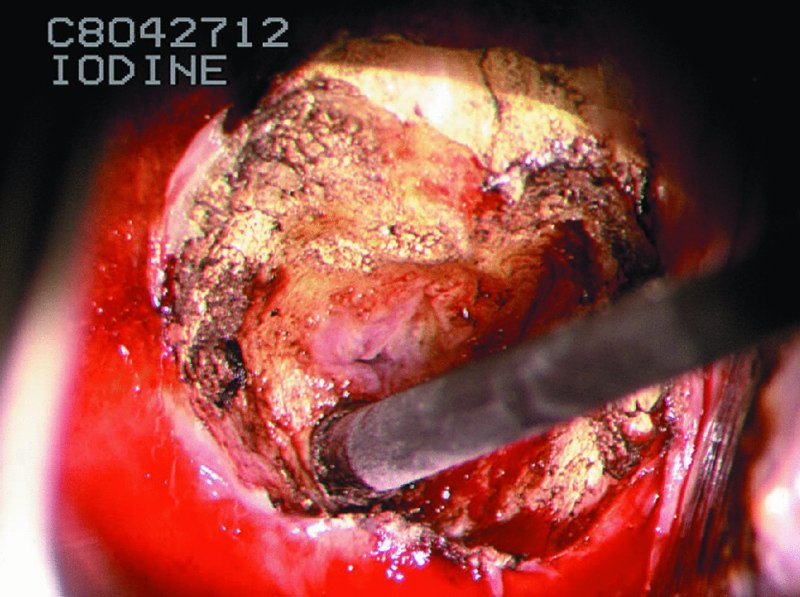
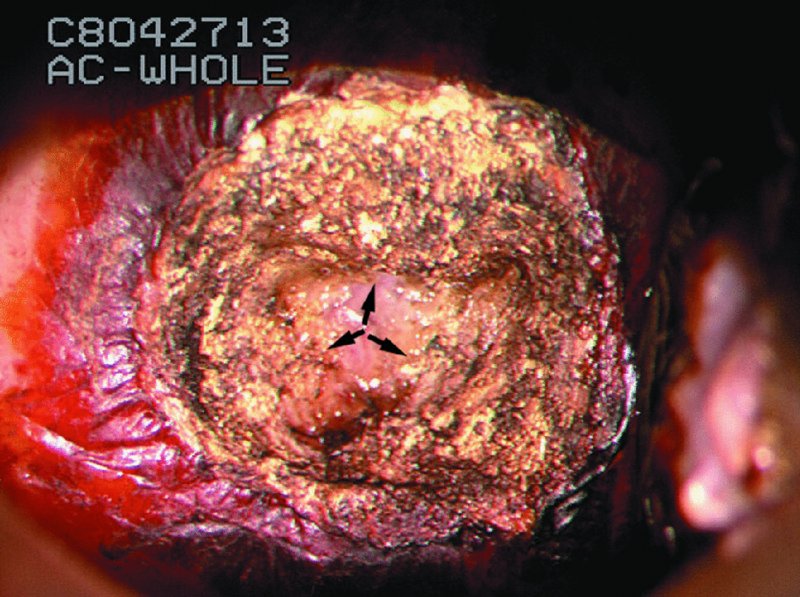
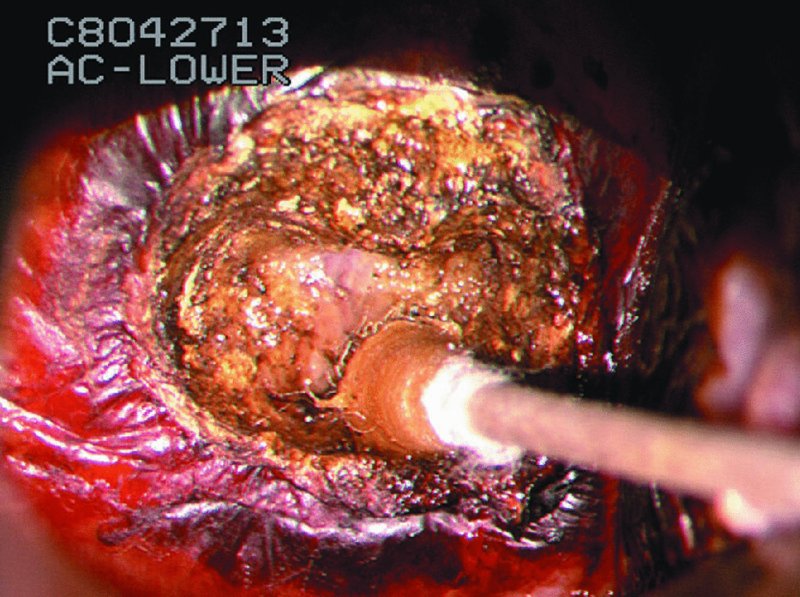
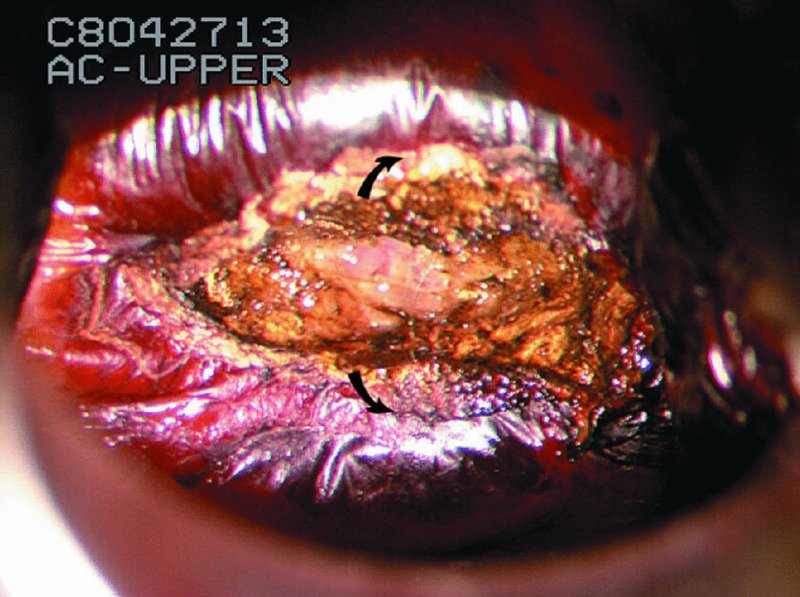
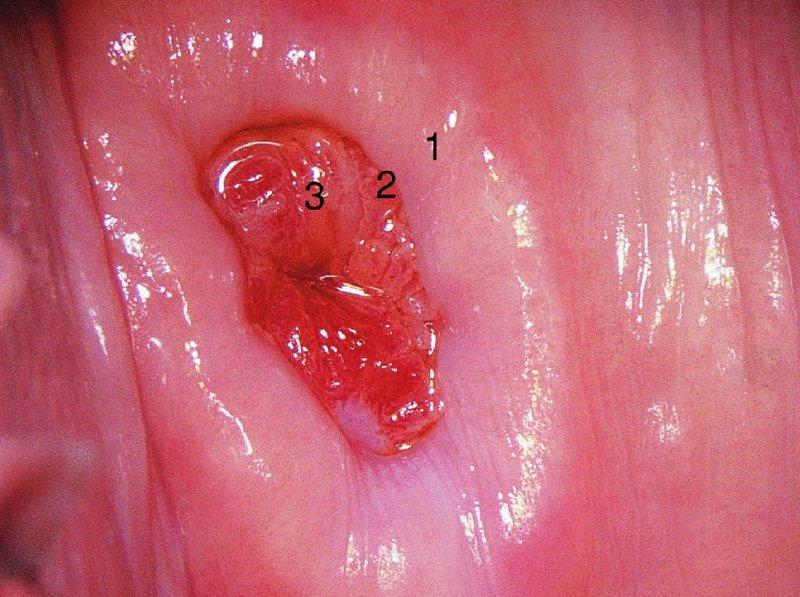
Laser cone biopsy
Equipment
Technique
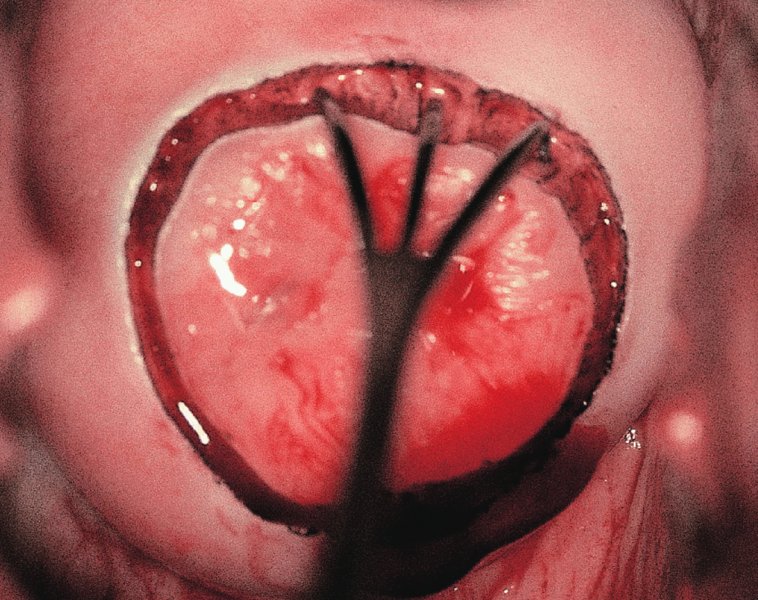
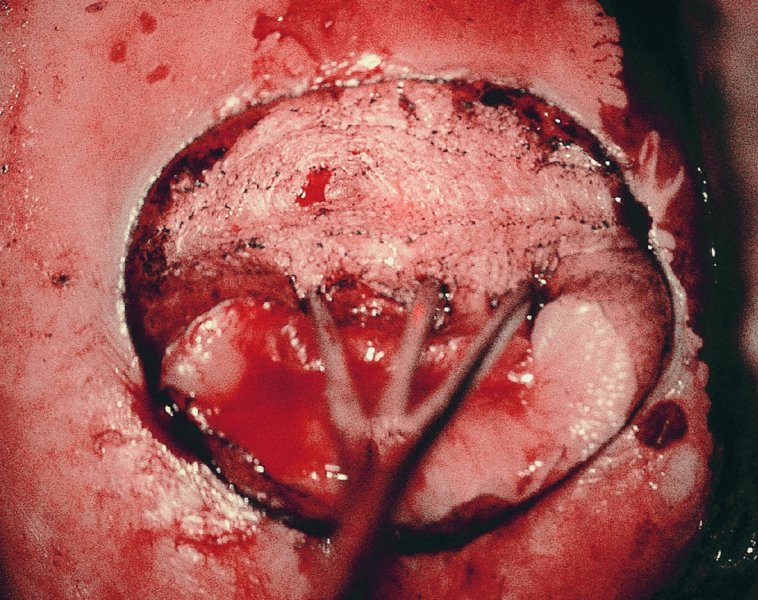
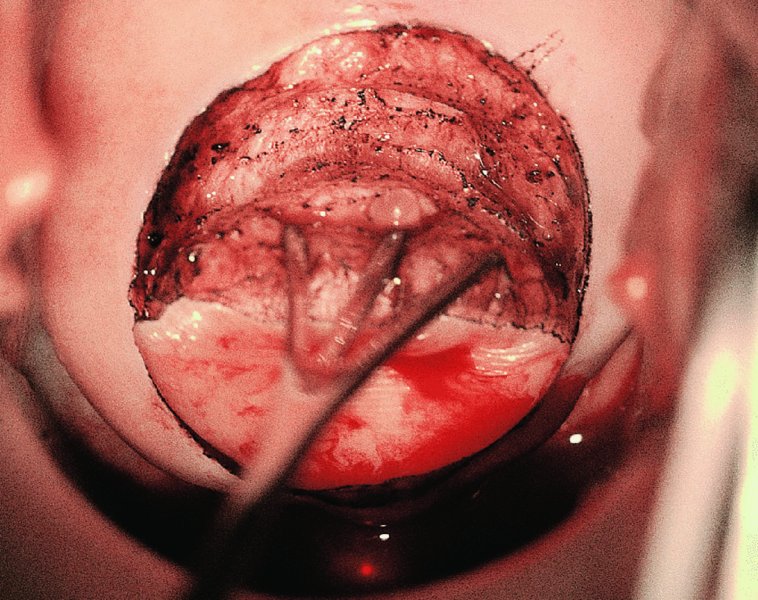
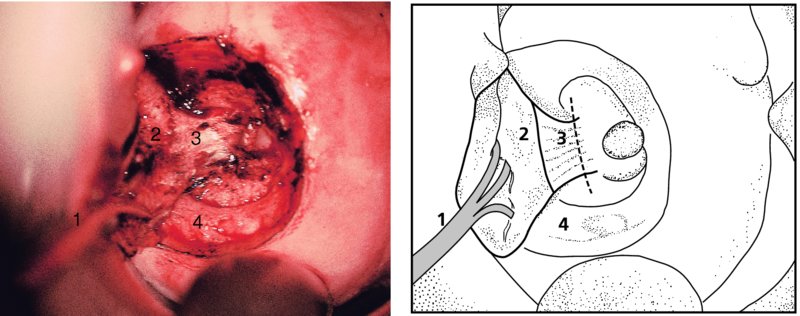
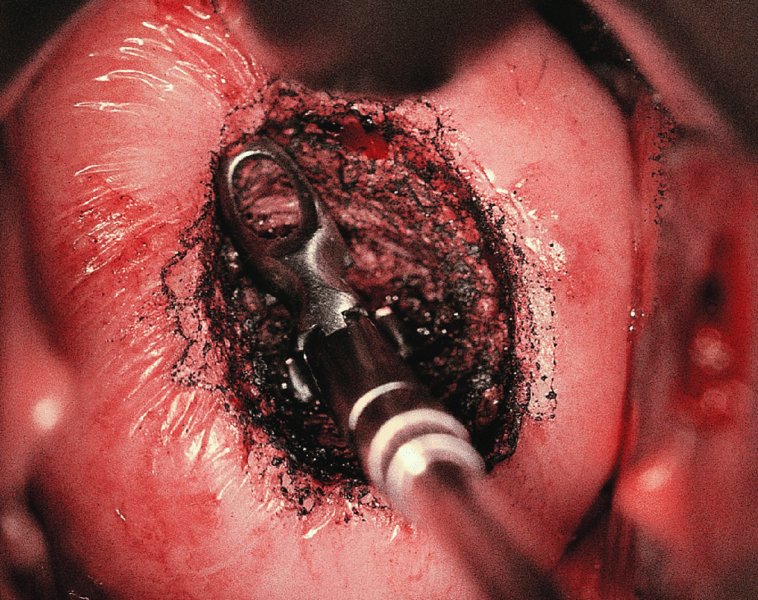
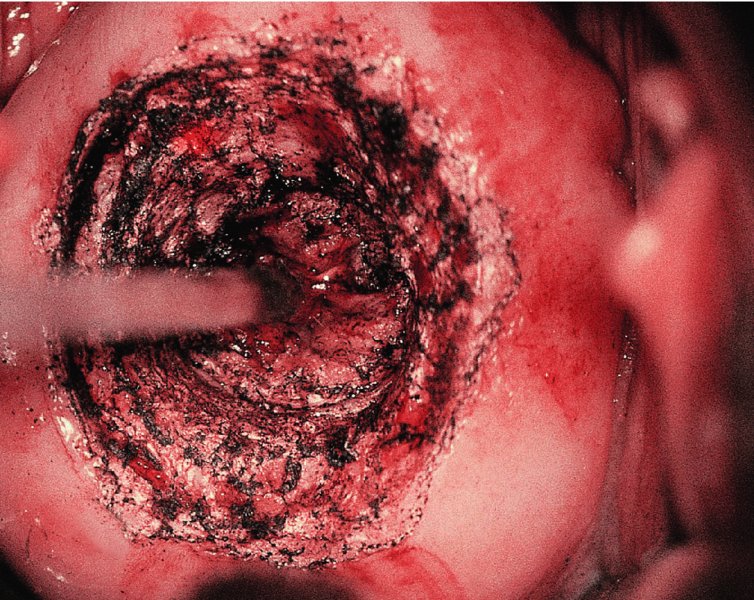
Needle excision of the transformation zone
Technique
Handling of the excised cone specimen
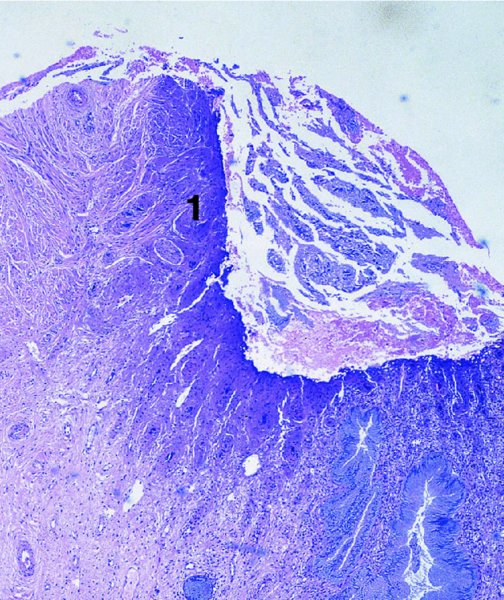
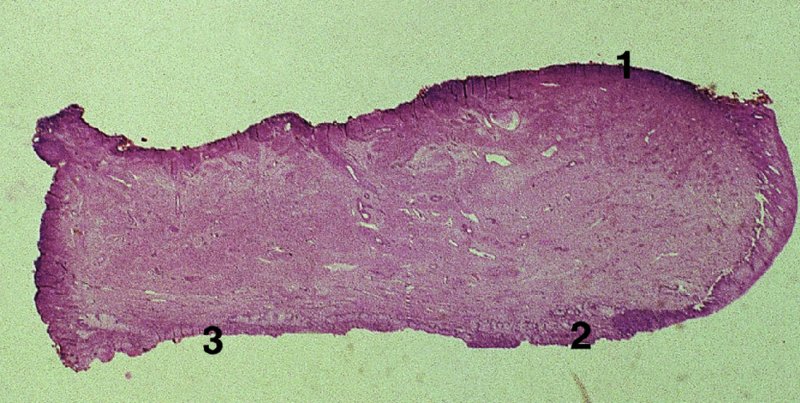
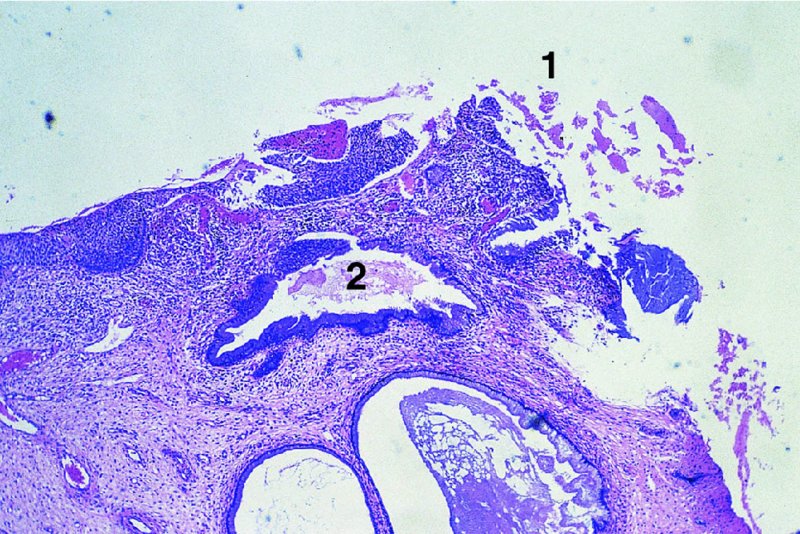
The cold knife cone biopsy
Indications
Technique

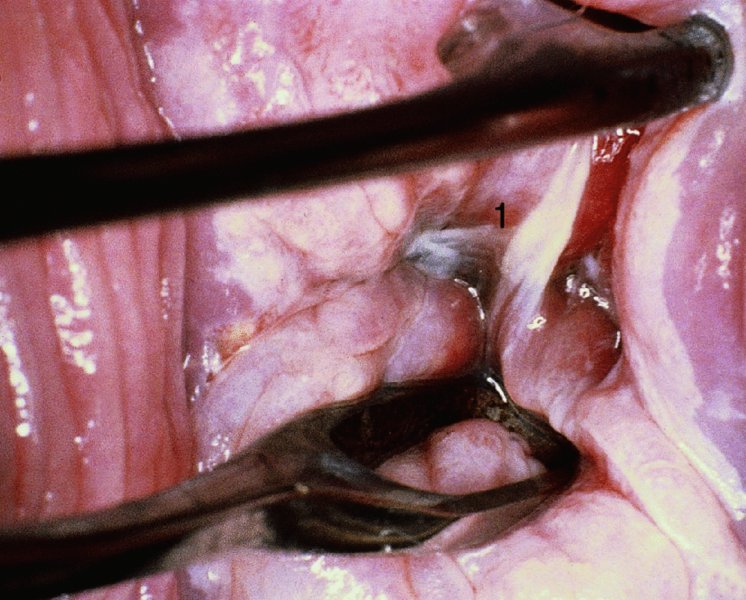
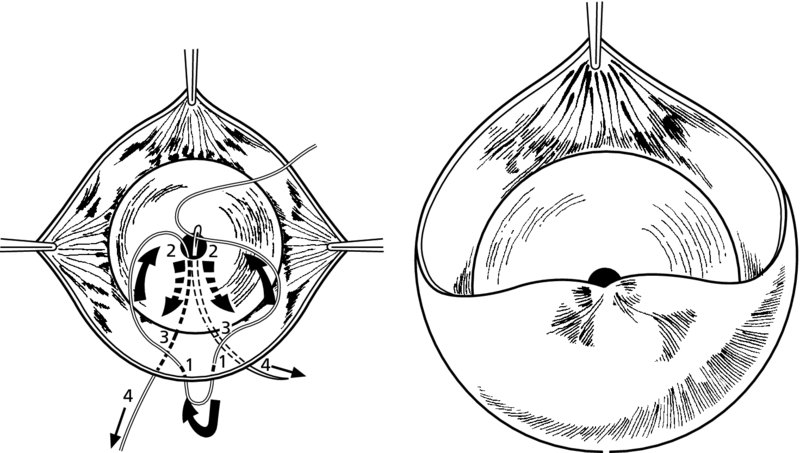
Repairing the defect in the cervix
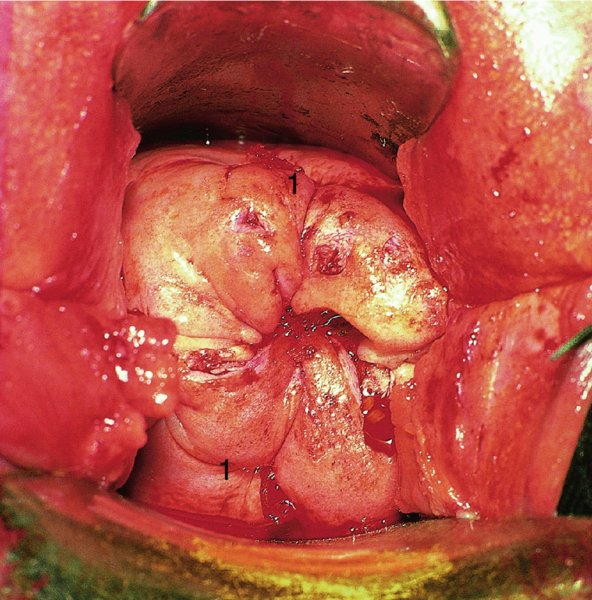
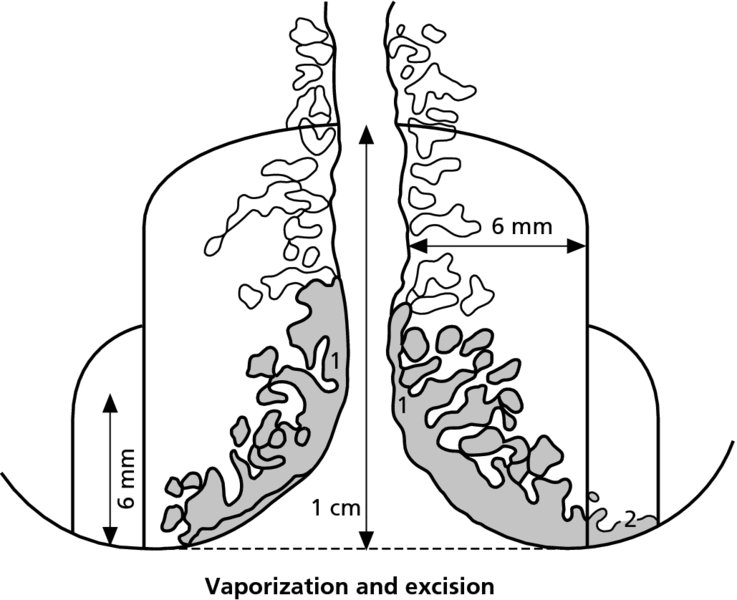
7.10 Management of extension of the abnormal (atypical) transformation zone
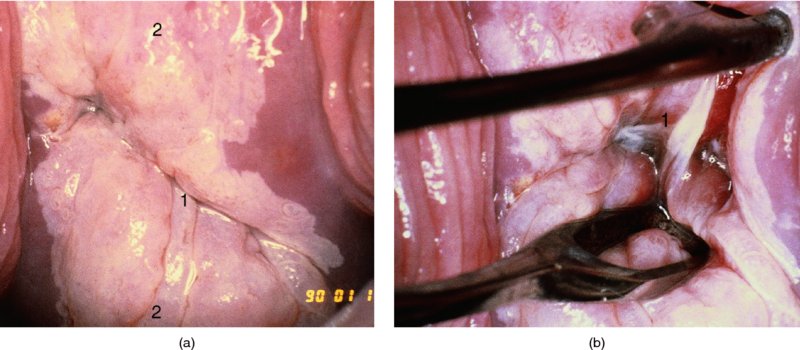
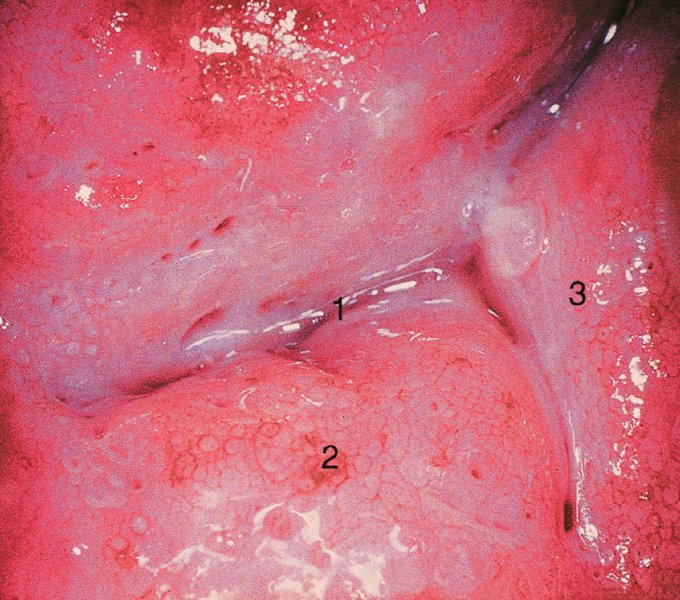
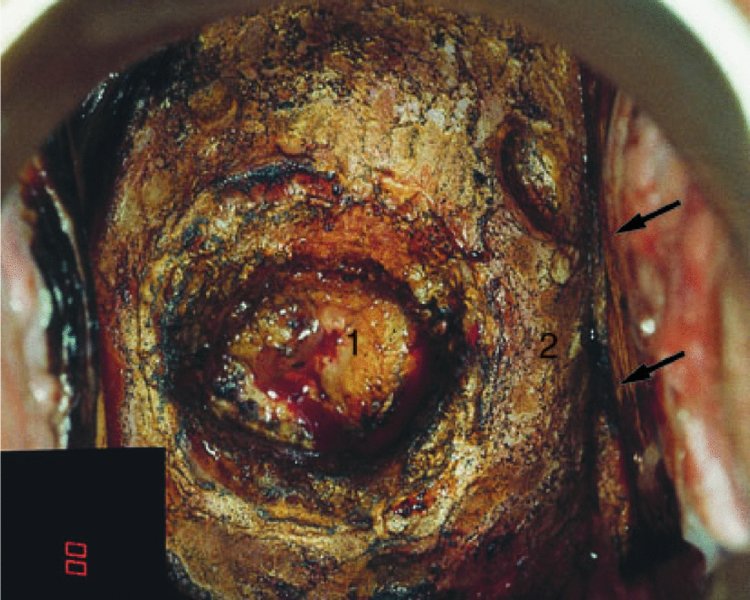
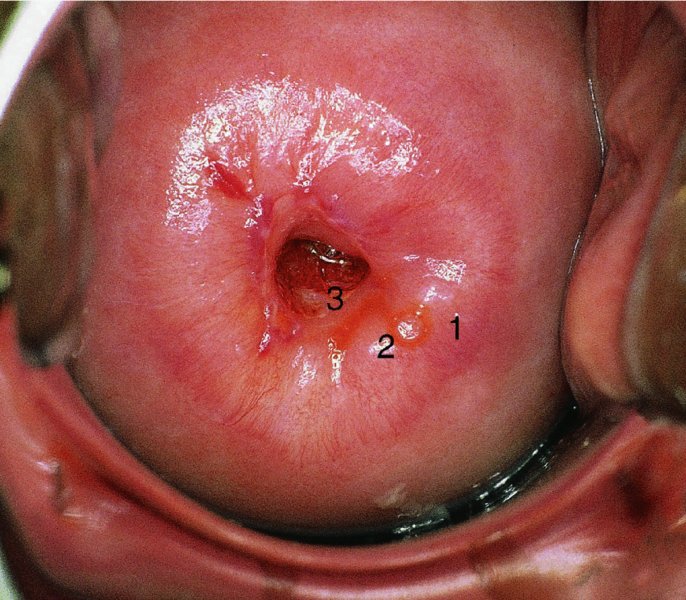
Technique
7.11 Hysterectomy in the treatment of cervical intraepithelial neoplasia
7.12 Immediate complications of cervical intraepithelial neoplasia treatment
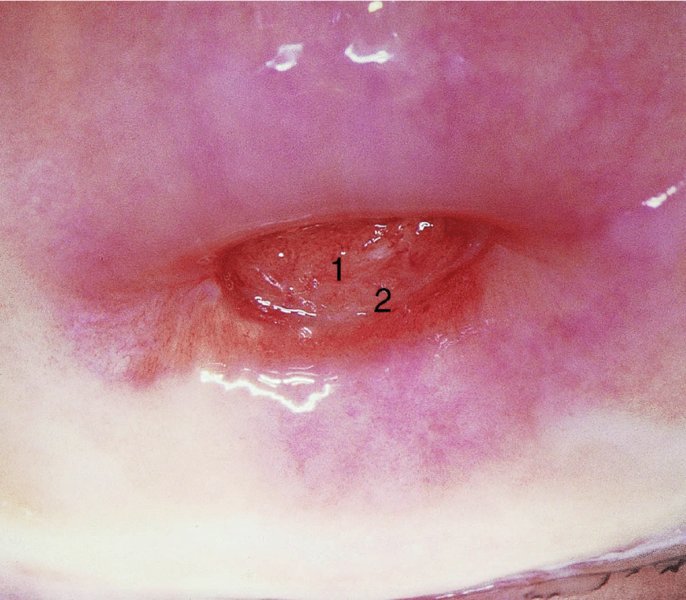
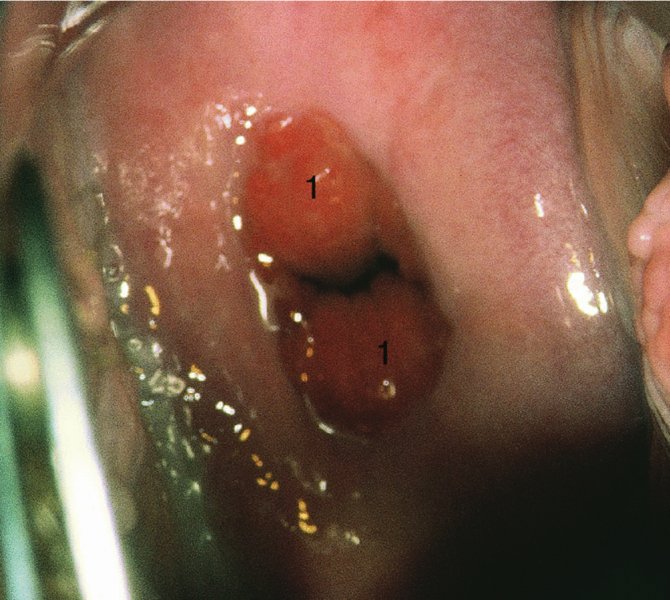
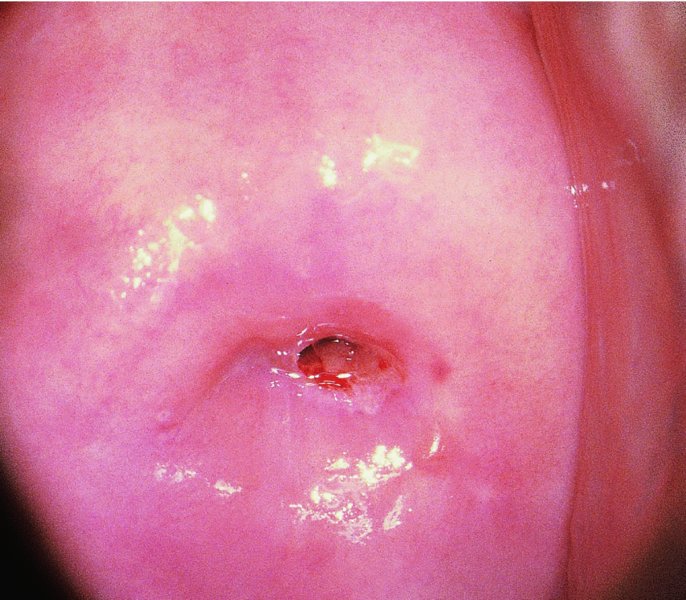
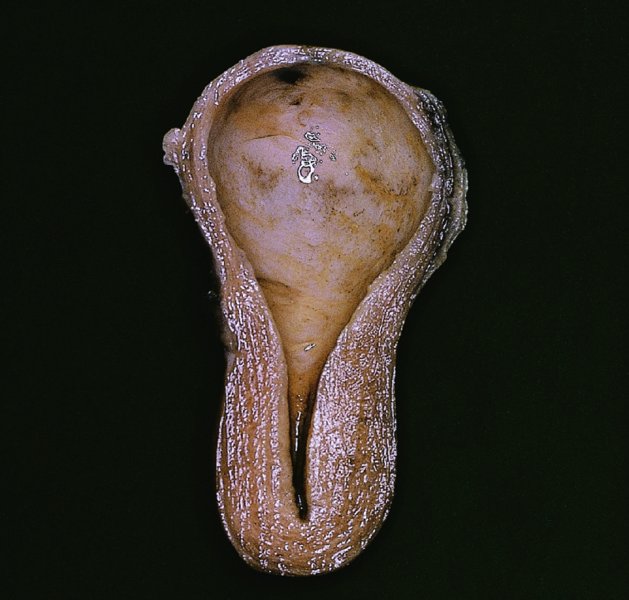
7.13 Long-term complications of cervical intraepithelial neoplasia treatment
Pregnancy-related complications
Stenosis and constriction
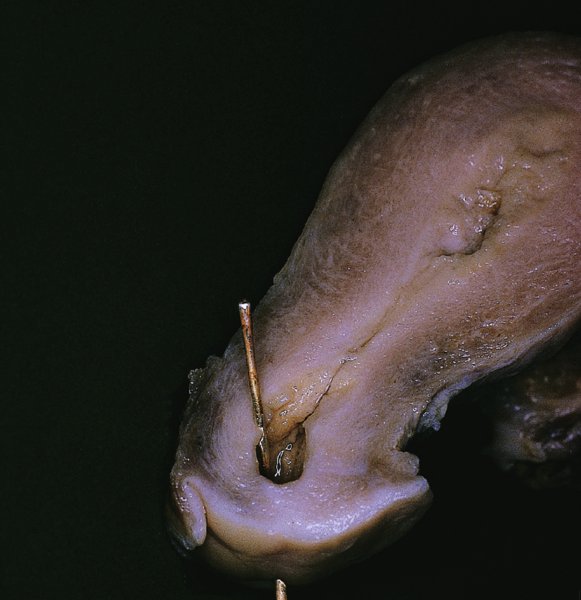
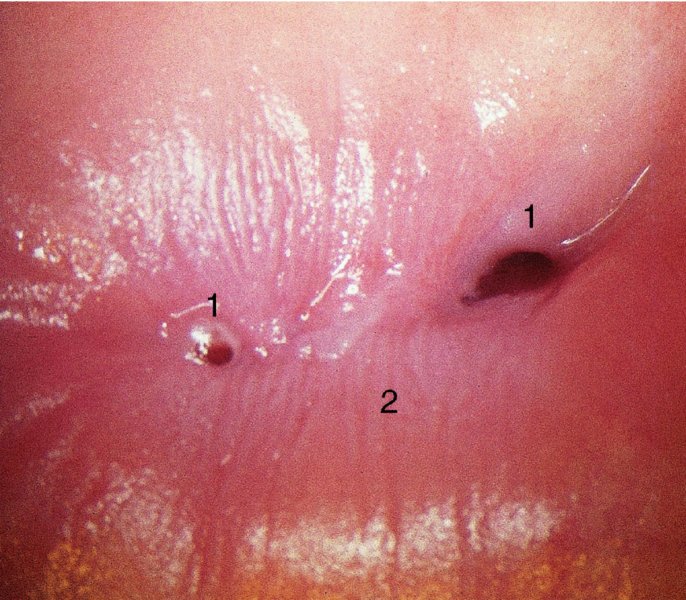
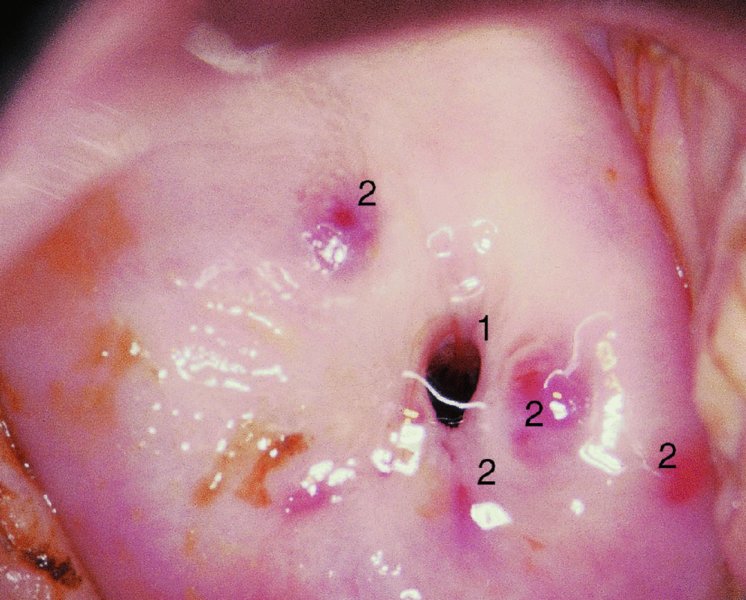
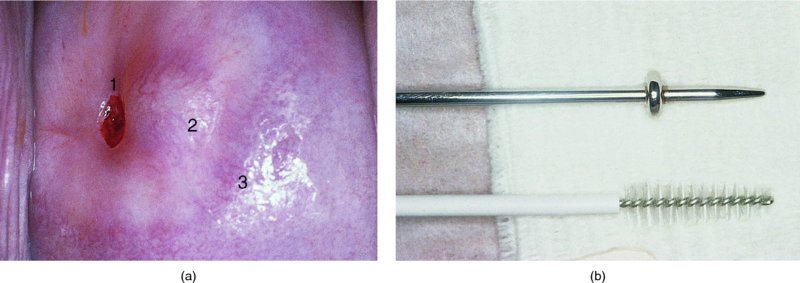
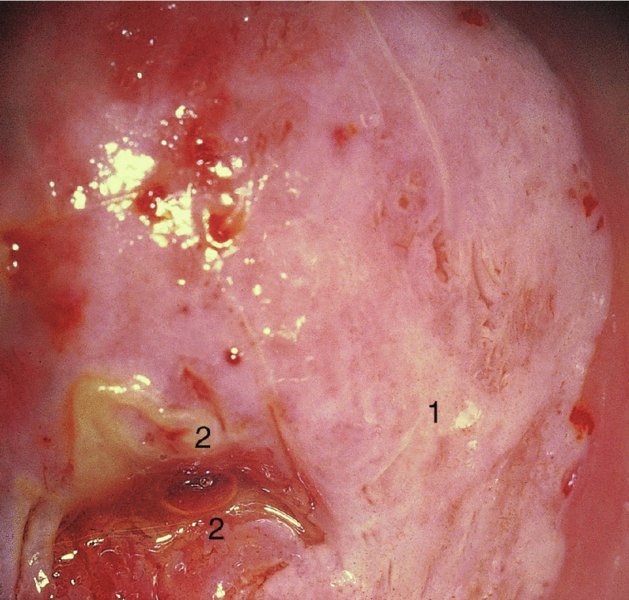
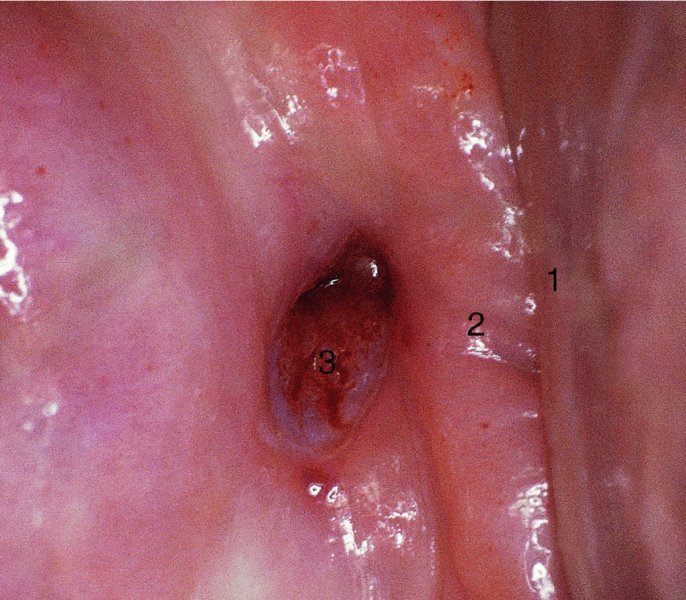
Surgical treatment and reconstruction following stenosis
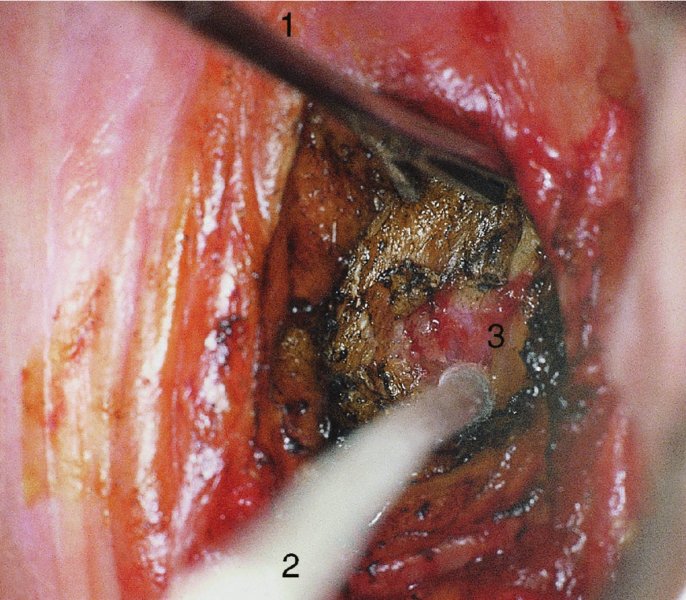
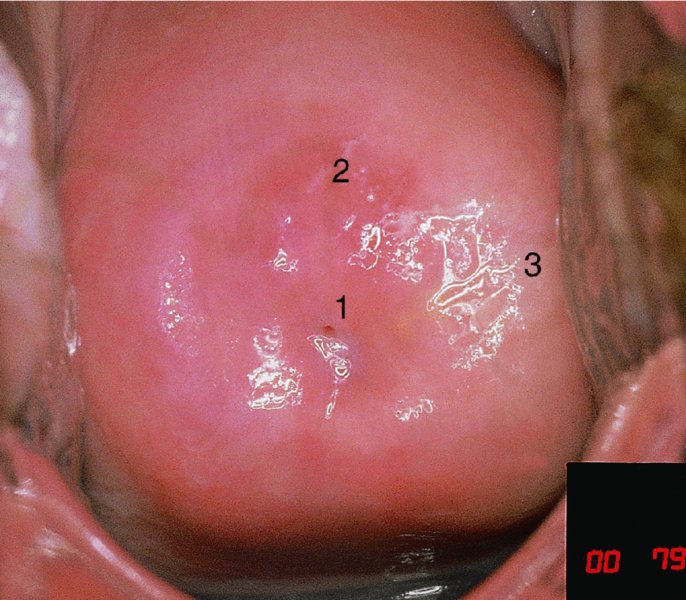
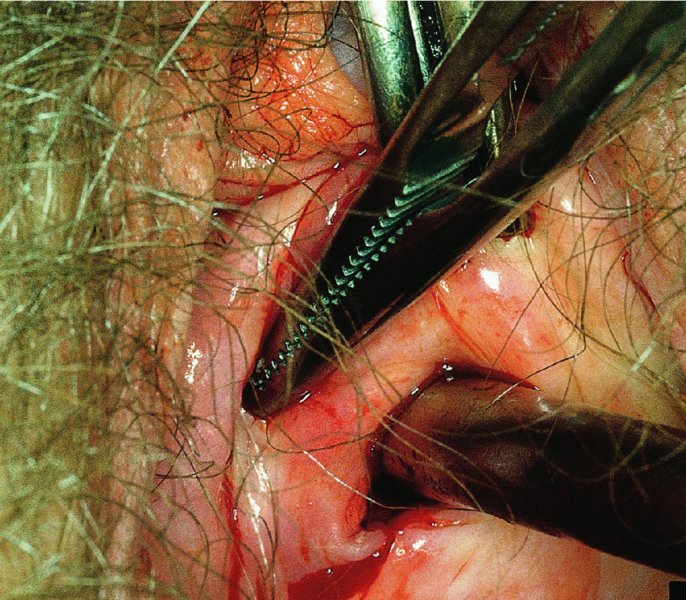
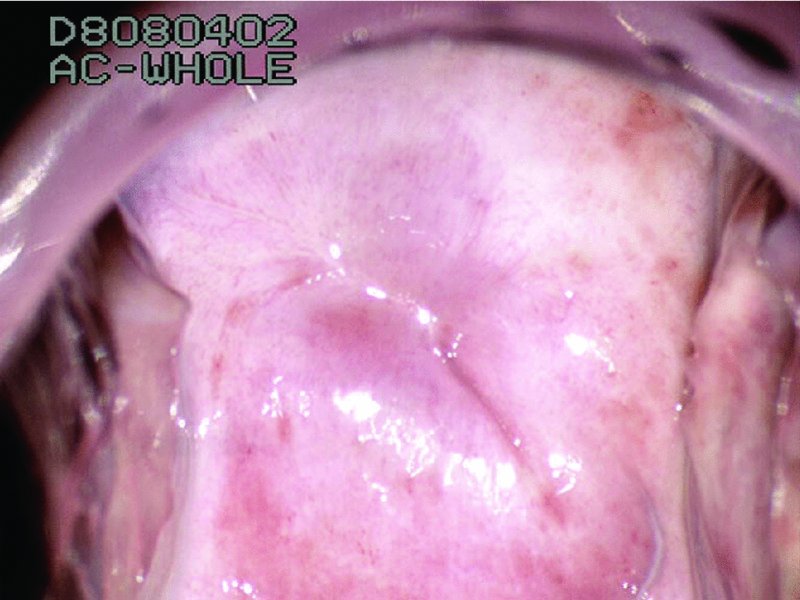
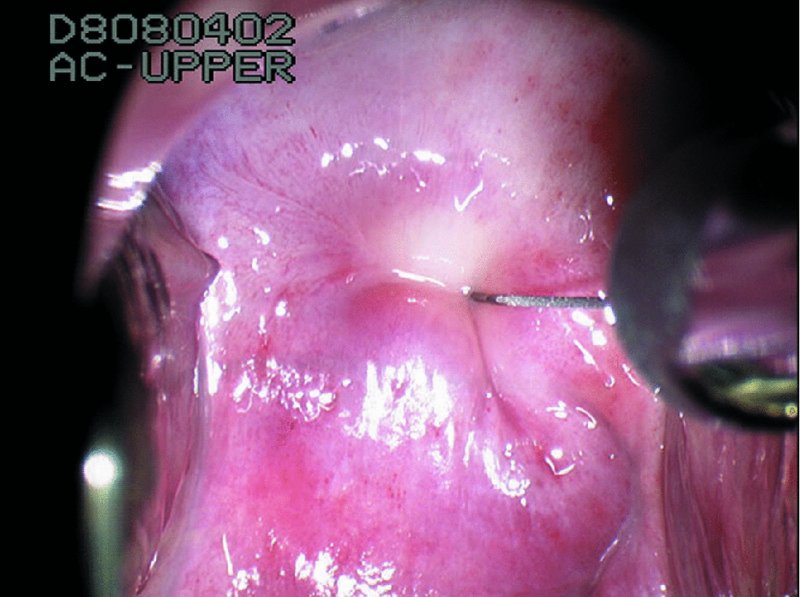
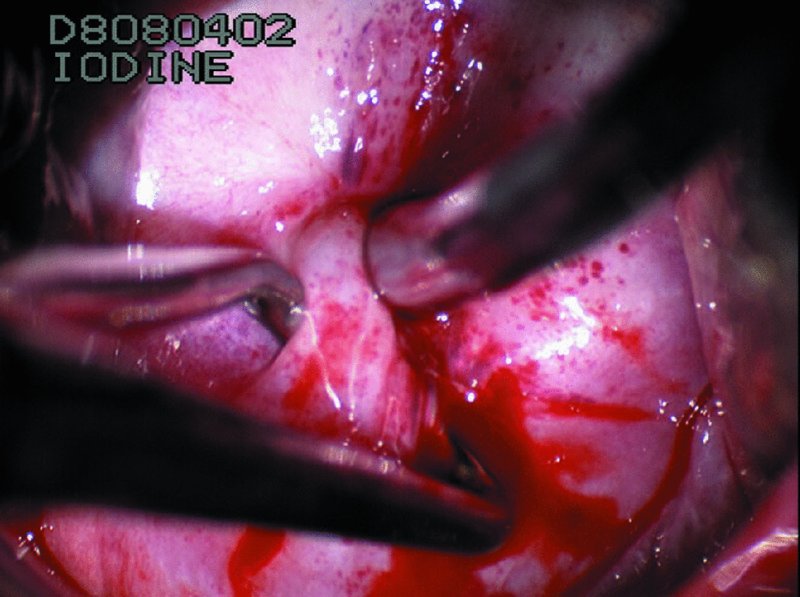
Excessive eversion of the columnar epithelium (post-treatment)
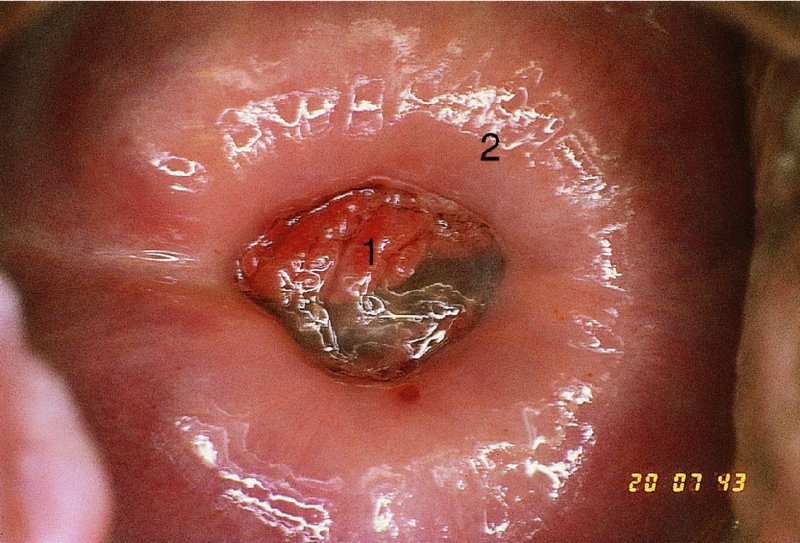
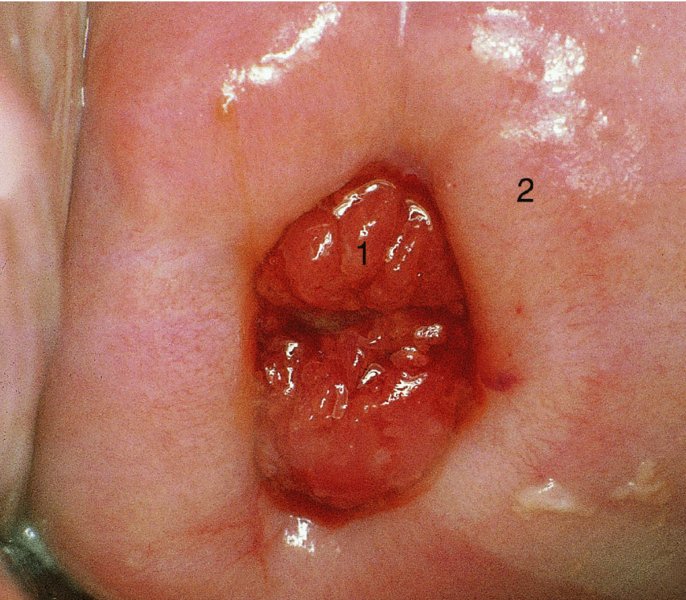
7.14 Follow-up after treatment of cervical intraepithelial neoplasia
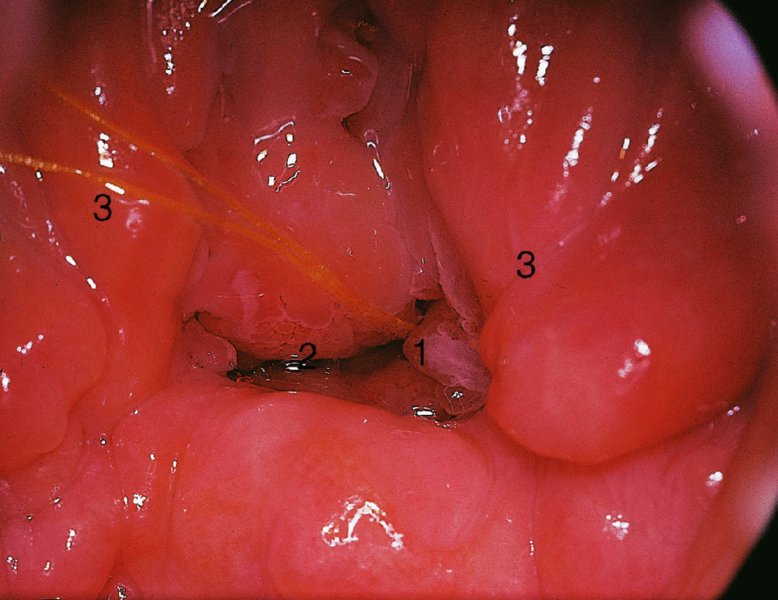
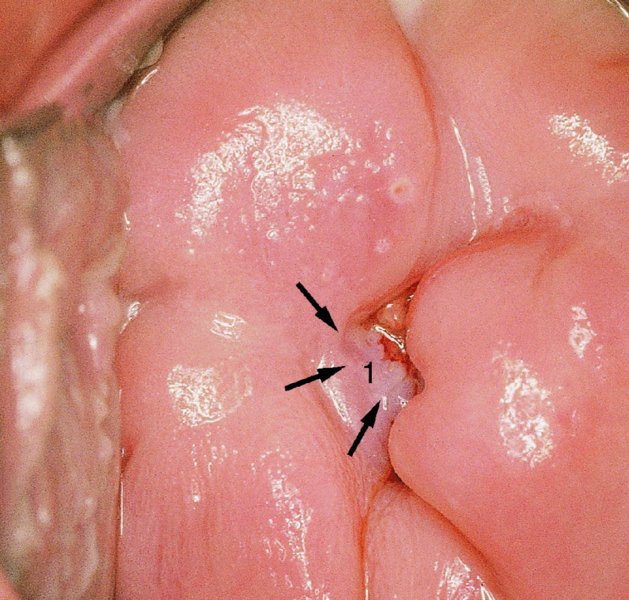
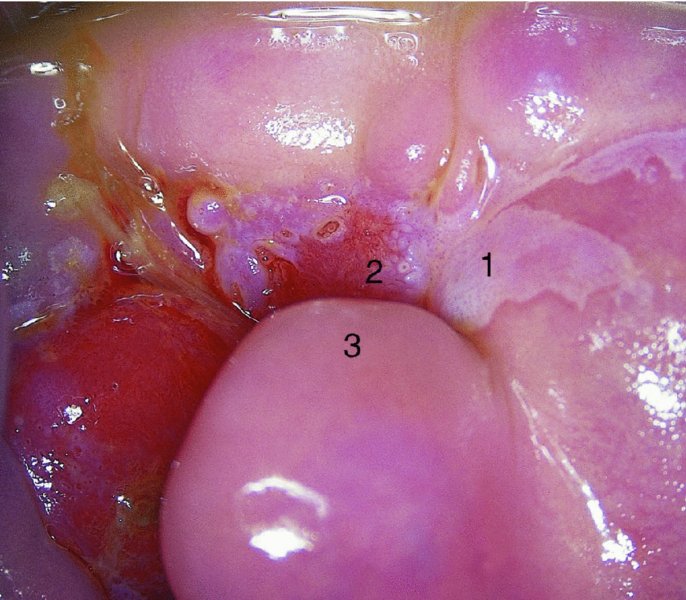
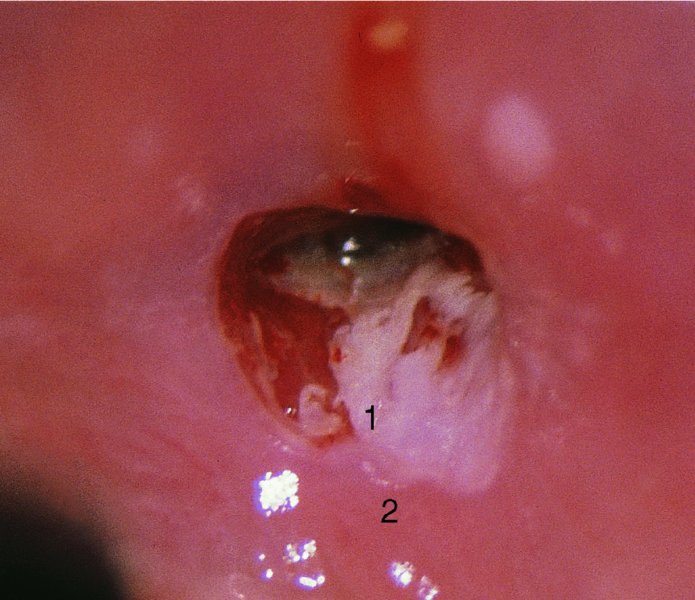
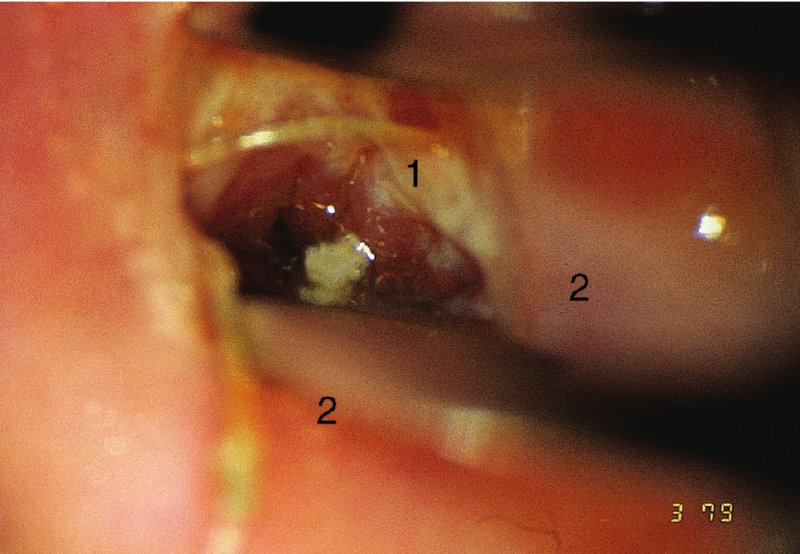
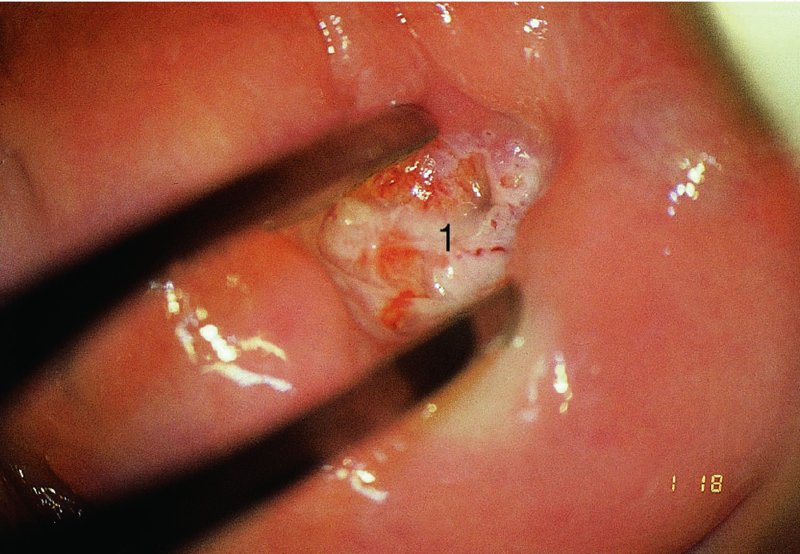
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


