Malrotation
The earliest descriptions of intestinal development were from Mall in 1898, and later expanded upon by Frazer and Robbins in 1915.1,2 Eight years later, Dott translated these preliminary embryologic observations into problems encountered clinically.3 In his 1932 landmark paper, Ladd described the evaluation and operative treatment of malrotation.4 He described a relatively simple solution to a complicated problem.5 Over 200 postmortem studies had been reported previous to Ladd’s paper, yet he was the first to emphasize the importance of placing the duodenum along the right abdominal wall, widening the mesenteric base, and moving the cecum to the left upper abdomen. With the exception of the laparoscopic approach, Ladd’s original technique has remained relatively unchanged.
Embryology
The development of the midgut begins with the differentiation of the primitive intestinal tract into the foregut, midgut, and hindgut at the fourth week of gestation.6 The mature alimentary tract and all associated digestive organs are formed from this primitive tube. The most accepted model of midgut maturation involves four distinct stages: (1) herniation; (2) rotation; (3) retraction; and (4) fixation. Normal fixation of the duodenum and colon is illustrated in Figure 31-1. The intestinal loop can be divided into the cephalic (duodenojejunal) limb and the caudal (cecocolic) limb, which rotate separately but in parallel. The SMA serves as the fulcrum with the omphalomesenteric duct at the apex. Due to the disproportional growth and elongation of the midgut during the fourth gestational week, the intestinal loop herniates into the extraembryonic coelom. Next, the bowel enters a critical period of rotation when the prearterial and postarterial limbs make three separate 90° turns, all in the counterclockwise direction around the SMA. The first 90° rotation occurs outside the abdomen. The second 90° turn commences during the return of the intestine into the abdominal cavity during the 10th gestational week. The duodenojejunal junction now passes posterior to the SMA. The last rotation occurs in the abdomen. The primitive intestine has thus completed a 270° counterclockwise rotation, allowing the duodenojejunal limb to be positioned to the left of the SMA while the cecocolic limb is on the right. Fixation of the ascending and descending colon then occurs. Disruption of any of these vital steps leads to the spectrum of malrotation encountered clinically.
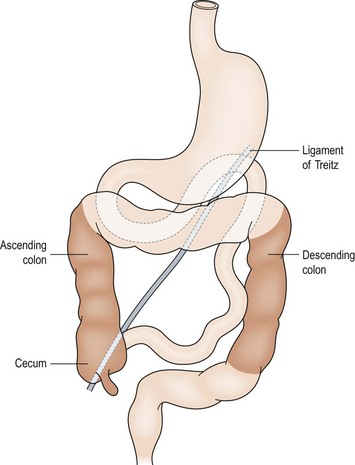
FIGURE 31-1 Normal intestinal anatomy results in fixation of the duodenojejunal junction in the left upper quadrant and the cecum in the right lower quadrant. This allows a wide breadth to the mesentery of the small bowel.
The most common forms of rotational disorders include nonrotation (Fig. 31-2), incomplete rotation (Fig. 31-3), and reversed rotation. Right and left mesocolic hernias can also occur. In nonrotation, there is failure of the normal intestinal 270° counterclockwise rotation around the SMA. Thus, the duodenojejunal limb lies in the right hemi-abdomen with the cecocolic limb in the left hemi-abdomen. Midgut volvulus due to a narrow mesenteric pedicle and extrinsic duodenal obstruction secondary to abnormally positioned cecal attachments are the most common symptomatic consequences. In cases of incomplete rotation, normal rotation has been arrested at or near 180°. The cecum will usually reside in the right upper abdomen. Obstructing peritoneal bands over the duodenum are present. With reversed rotation, an errant 90° clockwise rotation occurs, which leaves a tortuous transverse colon to the right of the SMA, passing through a retroduodenal tunnel dorsal to the artery and in the small bowel mesentery.7,8 The duodenum will assume an anterior position. Reverse rotation with volvulus may occur with obstruction of the transverse colon. Paraduodenal hernias are rare and result from failure of the right or left mesocolon to fuse to the posterior body wall. A potential space is created. Subsequently, the small intestine may become sequestered and potentially obstructed.
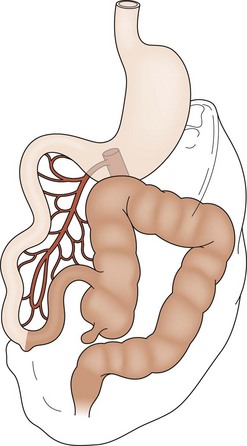
FIGURE 31-2 Nonrotation. The prearterial midgut (lightly shaded) is found on the right side of the abdomen, while the postarterial midgut (darkly shaded) remains on the left. Neither segment has undergone appropriate rotation. Volvulus is a risk.
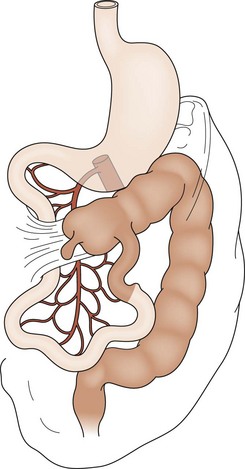
FIGURE 31-3 Incomplete rotation. Both the prearterial (lightly shaded) and postarterial (darkly shaded) segments have undergone partial, yet not complete, rotation. Ladd’s bands are seen attaching the cecum to the right posterior abdominal wall. The duodenum becomes compressed and possibly obstructed. Volvulus is a risk.
Presentation
The incidence of malrotation has been estimated at 1 in 6000 live births. An increased incidence of 0.2% has been found in barium swallow studies,9 whereas autopsy studies estimate that the true incidence may be as high as 1% of the total population.10 Associated anomalies are common (Table 31-1).11
Classic malrotation with midgut volvulus is often discovered in a previously healthy term neonate. Up to 75% of patients present during the first month of life, while another 15% will present within the first year.12–14 However, volvulus and mortality have been reported at all ages.15 Sudden onset of bilious vomiting is the cardinal sign of neonatal intestinal obstruction, and malrotation with volvulus must be the presumed diagnosis until proven otherwise. Physical examination findings will vary. Initially, the patient may have a scaphoid abdomen or only mild upper abdominal distension. However, if vascular compromise to the completely obstructed bowel develops, the abdomen will become progressively more distended and peritonitis will ensue. Late signs include abdominal wall erythema and shock. Similarly, laboratory data are often nonspecific and of limited diagnostic value. Thus, the clinician must have a high index of suspicion in a previously healthy baby who presents with bilious emesis. Furthermore, if signs of bowel ischemia are present, operative intervention must occur without delay.
Patients with chronic obstruction will present less dramatically. Nonspecific presenting problems such as failure to thrive, gastroesophageal reflux, early satiety, and mild abdominal discomfort are often seen. Partial volvulus can lead to mesenteric venous and lymphatic obstruction and subsequently impaired nutrient absorption. The diagnosis becomes even more challenging with the older child or teenager because the symptoms are often vague and may sometimes seem unrelated to the abdomen.16
Diagnosis
Radiologic studies play a critical role in establishing a diagnosis of intestinal malrotation. Initial evaluation will usually begin with a plain anteroposterior abdominal film combined with a lateral decubitus or upright view (Fig. 31-4). Nonspecific findings ranging from gastric distention to a gasless abdomen are common. Upper gastrointestinal contrast study remains the gold standard and is needed to document the position of the ligament of Treitz to the left of the spinal pedicles, and rising to the level of the gastric outlet. Additionally, the lateral film will show the duodenum in a retroperitoneal, posterior position.17 Findings of abnormal rotation include a low-lying ligament of Treitz or failure of the ligament of Treitz to be located left of the spine. If volvulus exists, the contrast study may show the ‘coil spring’ or ‘corkscrew’ configuration with incomplete obstruction and the “beak” appearance in the duodenum with complete obstruction (Figs 31-5 and 31-6).
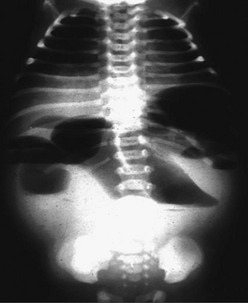
FIGURE 31-4 Upright abdominal film in an infant demonstrating proximal small bowel dilatation. This infant had a midgut volvulus.
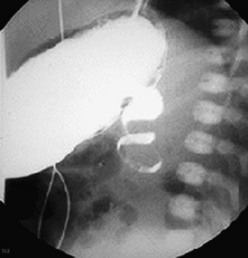
FIGURE 31-5 Lateral image on upper gastrointestinal series in an infant with malrotation and midgut volvulus showing the ‘corkscrew’ appearance of the obstructed duodenum.
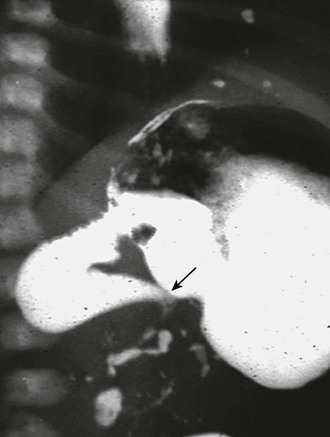
FIGURE 31-6 Lateral image on upper gastrointestinal series in infant with malrotation and midgut volvulus showing the ‘beak’ (arrow) appearance of the obstructed duodenum. Note that a small amount of contrast agent has progressed through the volvulus.
In some institutions, ultrasound (US) is being trialed to make the diagnosis of malrotation. Color Doppler ultrasound imaging may reveal a dilated duodenum with inversion of the SMA and vein (the whirlpool sign) in cases of acute volvulus.18–21 Additionally, Yousefzadeh and colleagues have proposed that ultrasound be used to diagnose malrotation without volvulus based on the position of the duodenum and the SMA. Because the third portion of the duodenum assumes a retroperitoneal position anterior to the aorta and posterior to the SMA in individuals with normal intestinal rotation, verification of this position by ultrasound potentially obviates the need for further imaging. This group prospectively validated this technique in 33 neonates at their institution.22 However, application of this technique prospectively in multiple institutions is likely needed prior to widespread acceptance.
Management
Open Approach
There has been little change from Ladd’s original description of the operative technique for correction of malrotation with or without volvulus. The six steps are summarized in Box 31-1. A right upper abdominal transverse incision is typically used. Chylous ascites secondary to lymphatic obstruction and rupture of mesenteric lacteals is often initially found. The bowel and mesentery should then be completely eviscerated. In patients with volvulus, the surgeon will commonly encounter two or three complete clockwise revolutions. Prompt, gentle counterclockwise detorsion is performed (Fig. 31-7). Once detorsion has been accomplished, warm soaked lap pads are placed on the bowel, and the surgeon should patiently observe for reperfusion. If perfusion remains in question, the surgeon has several options, including assessment of the antimesenteric vascular integrity with the use of a Doppler probe and intravenous administration of fluorescein with Wood’s lamp evaluation.




