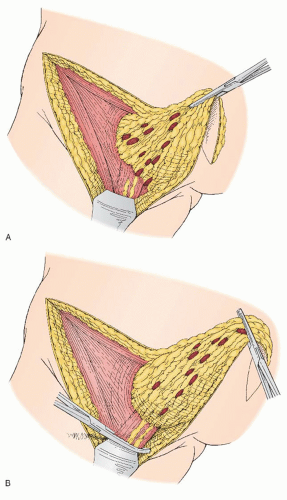
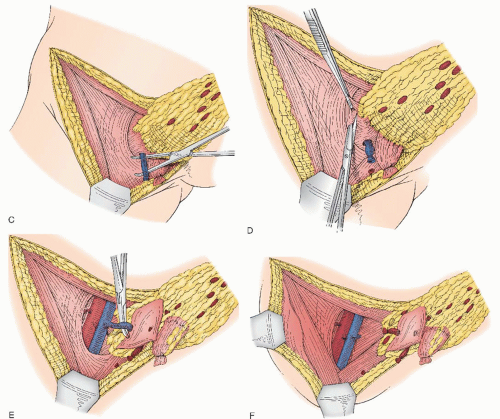
Appropriate Candidates for Primary Surgery
Certain tumor characteristics may preclude an otherwise medically fit patient from undergoing primary surgery. Proximity to important functional structures such as the urethra, clitoris, and anal sphincter must be considered. If adequate surgical margins cannot be obtained without sacrificing such a structure, neoadjuvant treatment with radiation and/or chemotherapy should be considered. Very large primary tumors may leave a large defect after primary resection, which may prove challenging to close. These patients may be candidates for neoadjuvant radiation plus chemotherapy to shrink the size of the primary cancer before surgical resection. The patient’s ability to tolerate a radical resection must also be taken into consideration. Some elderly patients or those with significant comorbidities who may not recover from a radical resection would tolerate a more limited resection after neoadjuvant treatment.
Modified Radical and Radical Vulvectomy
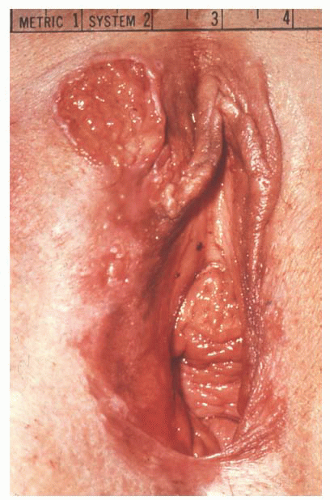
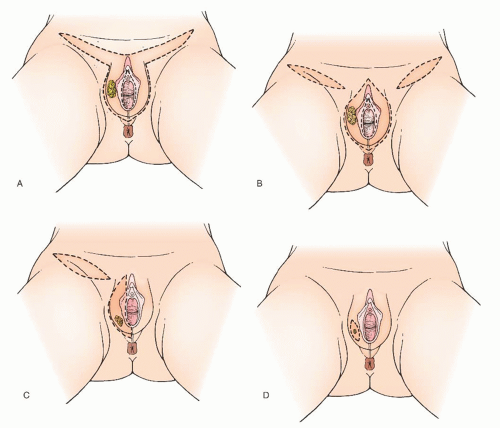
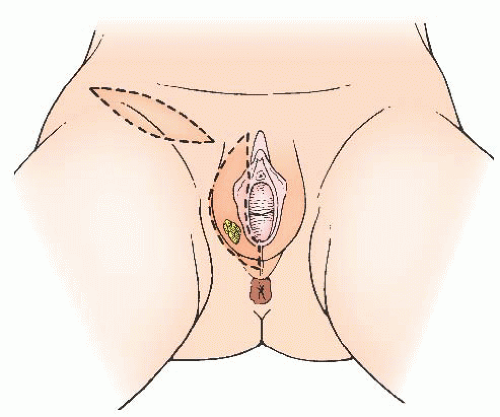
Modified radical vulvectomy is an ambiguously defined operation that generally refers to radical removal of the portion of the vulva containing the tumor (
Fig. 49.8). In recent
years, the extent of the radical vulvectomy has been modified to emphasize wide excision of the primary cancer with adequate skin and deep margins but not necessarily radically resecting the entire (uninvolved) vulva. Recommendations have included 1- to 3-cm skin margins for well-localized, unifocal lesions and to the depth of the inferior fascia of the urogenital diaphragm. The main type of morbidity relating to increasing the radicality of vulvectomy is subsequent sexual dysfunction and disfigurement. Modification of the surgical resection or the use of neoadjuvant chemoradiation should be considered to avoid compromising the function of the anus or urethra. In a 1979 study, DiSaia and colleagues reported complete preservation of sexual function in 17 of 18 patients who underwent wide local excision for early invasive tumors. They also reported that preservation of the mons pubis as well as the major portion of the superior aspect of the vulva resulted in an appreciably more satisfactory cosmetic result.
The chief concerns with decreasing the radicality of vulvectomy are the possibility of an increased risk of local recurrence and later an increased risk of a second primary vulvar cancer. Hacker and colleagues have demonstrated that the extent of the vulvectomy can be decreased as long as careful attention to the margins is observed. They compared radical vulvectomy (56 patients) with wide local excision (28 patients) in the treatment of superficially invasive squamous cell carcinoma of the vulva. The local recurrence rate was identical, 4% in both groups. A later review by Hacker and van der Velden of literature regarding patients with vulvar cancer 2 cm or less in diameter showed a local invasive recurrence rate of 7.2% for 165 patients treated with radical local excision versus 6.3% for 365 patients treated with radical vulvectomy. In a comparative study reported by Hoffman, 45 patients underwent radical vulvectomy and 45 patients were treated with modified radical vulvectomy. The local recurrence rates were 2.2% and 4.4%, respectively. Several additional studies have reported excellent local control with a modified radical vulvectomy (
Table 49.3).
In a GOG study, Stehman and colleagues analyzed recurrences following modified radical hemivulvectomy. The mean time to “relapse” on the vulva was 43.4 months, and 11 of 18 patients had recurrence on the contralateral side from the primary lesion. This suggests a second cancer rather than a true local recurrence. A study by de Hullu and associates also reported a significant number of late recurrences following modified radical vulvectomy. From these results, it is apparent that women undergoing a modified vulvar operation for cancer are at increased risk for later development of a new primary vulvar tumor and require long-term close follow-up.
It appears reasonable to conclude that modified radical vulvectomy is an efficacious treatment for well-localized invasive squamous cell carcinoma of the vulva. Attention should be focused on obtaining a 2-cm gross margin around the tumor while sparing as much vulvar tissue as possible. Most patients with squamous cell carcinoma of the vulva are candidates for a modified radical vulvectomy via a separate incision from the groin lymphadenectomy. A few patients, however, by virtue of disease extent, require a traditional radical vulvectomy with radical resection of the entire vulva. Whatever the vulvar phase of the operation is called, the aim should be to excise the tumor with a 2-cm gross margin.
Because of tumor proximity, it is occasionally necessary to remove a portion of the urethra to obtain an adequate resection of a vulvar carcinoma. Although several authors have stated that removal of the outer urethra does not result in significant problems with incontinence, there are no substantial confirming data. In one small study, Reid and colleagues did find urinary incontinence to be a problem after resection of the distal urethra or even an excision close to the urethra. If a portion of the urethra is resected, a Foley catheter should be left in place (carefully taped to the leg) for about 1 week postoperatively to facilitate healing and to splint the urethra. A surgical anti-incontinence procedure should also be strongly considered at the time of resection, especially if there is any preoperative stress urinary incontinence or if more than 1 cm of the urethra has to be removed. Increasingly, consideration has been given to treating these patients with neoadjuvant radiotherapy and chemotherapy to minimize or avoid urethral resection.
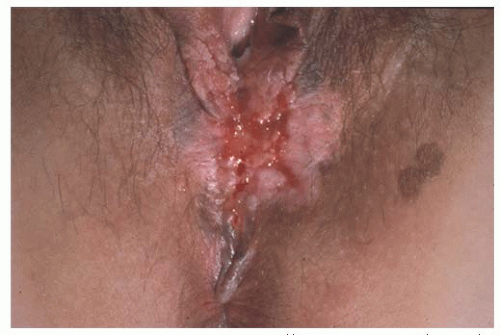
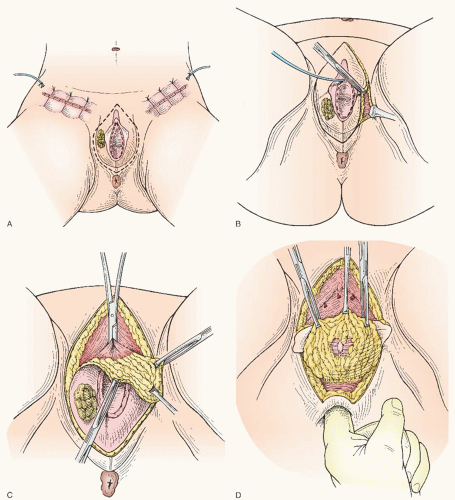
There is scant literature concerning the local management of vulvar cancer with perianal involvement (
Fig. 49.9). However, we have noted that about one third of our patients who are referred for vulvar cancer have lesions with perianal or anal involvement. The chief problems in the management of patients with these tumors are the difficulty in obtaining adequate surgical margins while attempting to preserve anal sphincter function and deciding on which patients would be better treated either with a more radical excision and colostomy or with neoadjuvant radiotherapy. In our experience with vulvar carcinoma, partial resection of the external anal sphincter in combination with radical local resection of perianal tissue is associated with a significant rate of subsequent fecal incontinence. Careful sphincter reapproximation and levator muscle plication are done in an effort to minimize incontinence. Other important measures include good bowel preparation preoperatively, prophylactic antibiotics, and careful postoperative bowel management. In addition, we have had
good results with the use of cutaneous rhomboid flaps in the reconstruction of the perineum and perianal area. These flaps allow for reconstruction of a perineal body, they bring tissue with a good blood supply into the area that promotes healing, and they allow closure of the wound without tension on the anus. However, if there is concern that these functional structures would be sacrificed in order to obtain margins, strong consideration should be given to neoadjuvant treatment as discussed later in the chapter.
Regional Lymph Nodes
It should be kept in mind that certain vulvar cancers, by virtue of their anatomic extent, may have access to lymphatics that bypass the groins. These include tumors that extensively involve the anus (particularly the anal canal or its surrounding tissue), the rectovaginal septum, the vagina above the lower third, and the proximal urethra. However, for the vast majority of vulvar cancers, the primary metastatic pathway is via the inguinal lymph nodes.
Sentinel Lymph Node Dissection
A large number of reports have been published over the past 25 years on sentinel lymph node detection in various cancers. The concept relies on the presumption that the sentinel lymph node is the initial site of metastatic disease and that lack of metastatic involvement of the sentinel lymph node reflects an absence of metastasis in the rest of the lymph nodes in the basin. Lymphatic mapping is the standard of care in the United States for the surgical treatment of patients with clinically early-stage melanoma and breast cancer.
In the case of invasive squamous cell carcinoma of the vulva, interest in sentinel lymph node was driven by the high incidence and significant morbidity of complications following full inguinal-femoral lymphadenectomy. Though the incidence of groin wound complications has been reduced by separate incisions, it still may occur in up to 30% of patients, and the risk is increased by patient comorbidities and adjuvant chemotherapy or radiation. Therefore, the use of a lesser surgical procedure such as sentinel lymph node dissection is particularly relevant to vulvar cancer.
The concept of sentinel lymph node dissection applies well to vulvar cancer as the tumor location is easily accessible to injection of both radiocolloid and blue dye, and the lymphatic drainage is predictable for most tumors. With lymphatic mapping, the pathologist has only a few lymph nodes to examine, allowing a more detailed examination. Techniques such as serial sectioning, immunohistochemical staining, and reverse transcriptase-polymerase chain reaction analysis can be applied, increasing the sensitivity of the examination and allowing the detection of micrometastases.
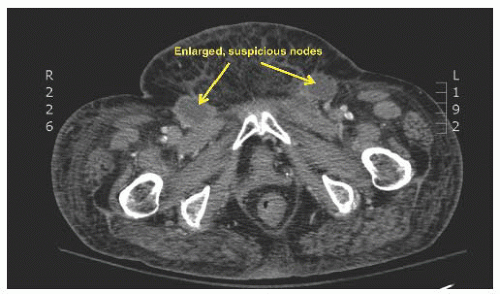
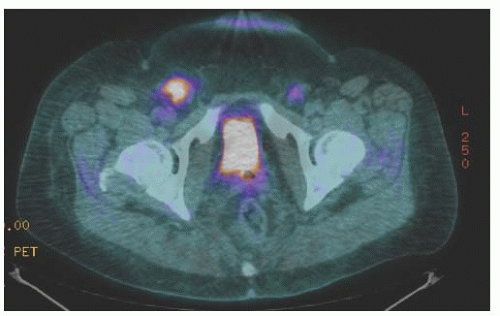
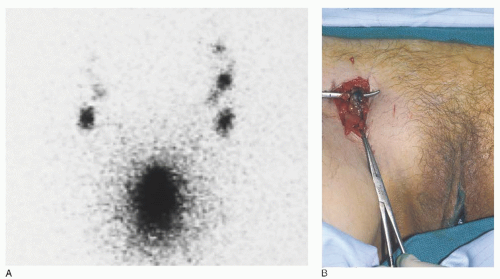
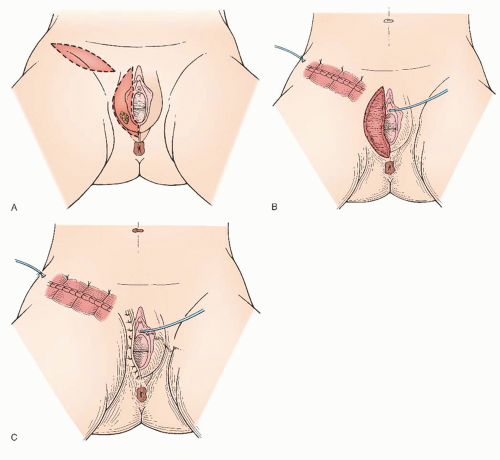
When using the combined technique for vulvar cancer patients, lymphatic mapping is nearly always successful in identifying sentinel lymph nodes (
Fig. 49.10). Further, the false-negative rate (based on standard pathology) is very low. A meta-analysis by Hassanzade and colleagues showed that pooled sensitivity of 27 studies was 92% and a negative predictive value of 97%. A phase III trial by the GOG including tumors from 2 to 6 cm showed a sensitivity of 92.1% and a negative predictive value of 97.4% when a combination of blue dye and radiocolloid was used. Another multiinstitutional observational study by van der Zee et al. revealed a sensitivity of 94.1% and negative predictive value of 97.1%. A full inguinal-femoral lymph node dissection is reserved for those patients in which the sentinel node is positive or has not been successfully identified.
Sentinel lymph node mapping is a promising method to substantially reduce the morbidity of inguinal lymphadenectomy. It is widely used in the management of vulvar cancer today. However, many unanswered questions remain, including who the best candidates are for the technique, the role of frozen section, the role of immunohistochemistry, the role of lymphoscintigraphy, the reliability of an isolated lymphoscintigraphy-directed unilateral negative sentinel node with a midline lesion, the role of ultrasound, management of a patient with a microscopically positive sentinel lymph node, the incidence of “skip” metastases, the reliability of lymphatic mapping after prior excisional biopsy, and the optimal lymphatic mapping methodology for vulvar cancer patients.
Modifications on the Extent of Inguinal-Femoral Lymphadenectomy
Various modifications on the extent of lymphadenectomy have been examined. Before the introduction of sentinel lymph node biopsies, a technique involving limited resection of the inguinal lymph nodes in the management of superficially invasive vulvar cancer was reported by DiSaia and coworkers in 1979. The dissection they described is aimed at removal of the superficial lymph nodes above the cribriform fascia, mainly associated with the great saphenous and superficial epigastric veins. These lymph nodes were sent for frozen section analysis. If results were positive, a complete bilateral inguinofemoral lymphadenectomy was performed. In the 1979 study, DiSaia and colleagues also reported 79 cases of invasive squamous cell carcinoma of the vulva treated with radical vulvectomy and bilateral inguinal-femoral lymphadenectomy. In these cases, it was noted that the deep femoral lymph nodes were never positive in the absence of positive superficial inguinal lymph nodes. The purpose of a superficial dissection only is to reduce the morbidity of the groin lymphadenectomy. The dissection is less radical and resulted in only one groin breakdown in the 18 patients in the series of DiSaia and associates. The series was updated in 1989, and they reported no groin recurrences in 50 patients, 42 of whom had been followed for a median of 36 months.
A GOG study specifically studied the issue of superficial inguinal lymphadenectomy in patients with early carcinoma of the vulva. The study group included clinical stage I patients with tumor invasion of 5 mm or less and no capillary or lymphatic space involvement. A modified radical vulvectomy
and ipsilateral superficial inguinal lymphadenectomy were performed, and 121 patients were evaluable. These were compared with a historical control group in the GOG registry who had undergone radical vulvectomy with bilateral inguinofemoral lymphadenectomy. Nine patients in this study, or 7.3%, experienced groin recurrences versus no recurrence in the control group. Six of the groin recurrences were in the ipsilateral groin, and 5 of the 9 patients died of the recurrent vulvar cancer. The interpretation from this study was that superficial inguinal lymphadenectomy may not be adequate treatment even for early vulvar carcinoma. However, in a number of patients in this study, the tumors approached the midline; there is evidence that more medial tumors may have direct drainage to the deep inguinal lymph nodes. Another area of concern in this study is the high percentage of poorly differentiated tumors—almost twice as many as in the control group. Six of the nine groin recurrences in this study were from the poorly differentiated tumors. Whether poorly differentiated tumors are more likely to metastasize to deep inguinal or contralateral inguinal lymph nodes deserves further study. Subsequent additional retrospective data from large cancer centers also report a 5% to 10% incidence of groin relapse in patients with negative nodes from superficial inguinal lymphadenectomy.
The technique of superficial lymphadenectomy of the inguinal nodes above the cribriform fascia has been largely replaced by sentinel lymph node biopsy, but the occurrences of groin recurrences after apparently negative superficial nodes in these studies remains concerning.
The benefit of sparing versus sacrificing the saphenous vein remains unclear. Various studies have found conflicting results on the reduction of the incidence of lymphedema. A randomized trial by the GOG showed that application of fibrin sealant at time of lymphadenectomy also did not reduce the incidence of lymphedema.
Unilateral Groin Lymphadenectomy
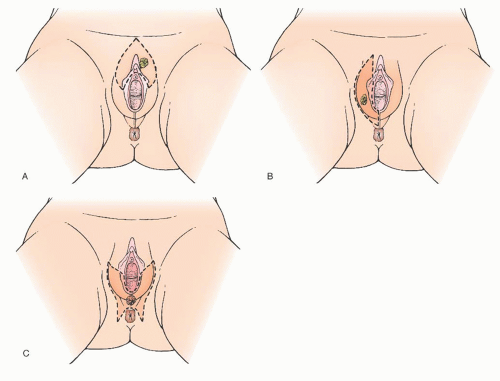
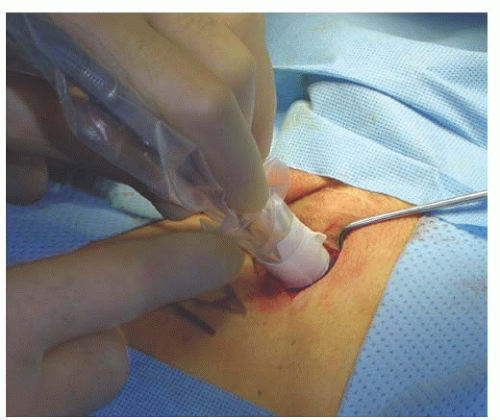
Unilateral lymphadenectomy only on the side of a unilateral vulvar tumor has been another modification of surgical treatment that has been used for selected patients (
Fig. 49.11). In 1981, Iversen reported on 53 women with unilateral tumors and lymph node metastases. Eighty-three percent of these patients had only one positive ipsilateral node, 15% had bilateral positive nodes, and one patient had contralateral positive lymph nodes only. Other retrospective studies have confirmed these results. It has been the opinion of a number of authors that capillary or lymphatic space involvement by tumor may increase the
risk of contralateral nodal metastases. Patients with tumors approaching the midline or involving more medial structures (perineum, clitoral hood or clitoris, vagina, and labia minora) are at increased risk for contralateral lymph node metastases. The issue of unilateral groin lymphadenectomy was studied to some extent in 1992 by Stehman and associates in a GOG study. Patients with early disease and negative ipsilateral superficial inguinal lymph nodes were treated with ipsilateral superficial inguinal lymphadenectomy and a modified radical vulvectomy. A few patients in this study did have a bilateral inguinal lymphadenectomy because of midline involvement. A total of 121 patients were in the study, and 3 experienced contralateral inguinal lymph node recurrences. The vulvar lesions of these 3 patients ranged from 0.6 to 2.5 mm in depth of invasion, and all were poorly differentiated. Although lesion location was not given for these 3 cases, a large percentage of patients included in this study had lesions approaching the midline, as defined by involvement of the labia minora. Tumors with capillary or lymphatic space involvement were excluded from this study.
At present, unilateral inguinal-femoral lymphadenectomy appears to be a reasonable approach in a patient with a welllateralized early tumor that is well differentiated, with no capillary or lymphatic space involvement and with negative ipsilateral inguinal lymph nodes.
Pelvic Lymphadenectomy
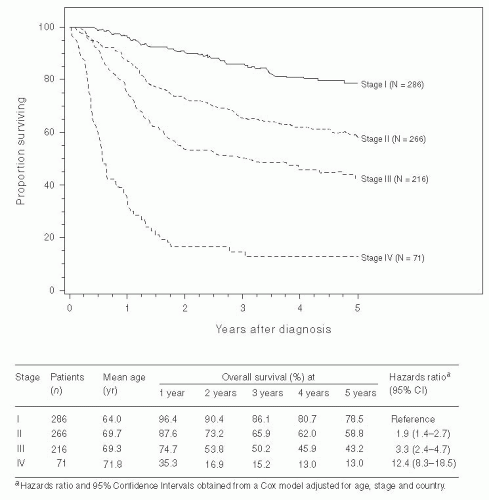
During the late 1970s and early 1980s, several studies were published showing that carcinoma of the vulva metastasizes to the inguinofemoral lymph nodes before spreading to the pelvic lymph nodes. Extension of the groin lymphadenectomy to include removal of the pelvic lymph nodes continued to be performed in selected patients with positive inguinofemoral lymph nodes. A number of studies also showed that the 5-year survival rate of vulvar cancer patients with positive pelvic lymph nodes is less than 20%. A 1986 study by the GOG directed by Homesley compared pelvic lymph node dissection with groin and pelvic radiotherapy in patients with positive inguinofemoral lymph nodes. The study included 114 patients and showed no difference in morbidity between the two treatment arms and a better 2-year survival rate in the radiotherapy group (68% vs. 54%). The improved survival was seen in those patients with suspicious or grossly positive lymph nodes or those with more than one positive groin lymph node. There was no evidence that groin radiation therapy was beneficial to those patients with occult metastases and only one positive groin node. Review of the pattern of recurrence in that study suggested that adjuvant radiation was more effective largely because groin recurrences were reduced. The value of removing positive pelvic lymph nodes before radiotherapy is unknown. Pelvic lymphadenectomy is now rarely indicated in the treatment of vulvar cancer.
Omitting Groin Dissection for Superficial Disease
Extensive data support the contention that a subset of early vulvar carcinomas (carefully studied pathologically) can be identified that have an extremely low risk of nodal involvement. In a GOG study reported by Sedlis and associates, a subgroup (63 of 272) of patients with early disease was identified as having a zero incidence of lymph node metastases. This subset included nonmidline tumors with no capillary or lymphatic space involvement that were well differentiated or were grade 2 and limited to 2 mm in thickness. Other factors that have been studied for the purpose of identifying a low-risk group include tumor volume (which does not appear to have received further attention since Wilkinson’s report in 1985), tumors that are largely carcinoma in situ with very early stromal invasion and with a pushing rather than an infiltrative pattern, tumor diameter, squamous cell type, the presence of an inflammatory response, and tumor ploidy.
There seems to be general agreement that the patients for whom it would be most reasonable to omit the lymphadenectomy are those with tumor invasion less than or equal to 1 mm and less than 2 cm in diameter. However, certain tumors with less than 1 mm of invasion should still be considered for lymphadenectomy. As reported in the literature, three of nine patients with nodal disease or nodal recurrence in association with tumor invasion less than or equal to 1 mm had poorly differentiated cancers (
Table 49.2). Thus, women with suspicious lymph nodes, poorly differentiated tumors, tumors with capillary or lymphatic space involvement, and perhaps those with multiple foci or broad areas of invasion or aneuploidy should still undergo lymphadenectomy. It is important to remember that even with superficial invasion, metastases to the nodes do occur. In the report by Sedlis and colleagues of 272 patients with invasion of 5 mm or less, the groin nodes were positive in approximately 20%.