Malaria
Chandy C. John
Malaria is among the leading infectious causes of morbidity and mortality in children worldwide. Each year, there are 300 to 500 million clinical cases, causing between 1.5 and 2.7 million deaths, most in sub-Saharan African children under the age of 5 years. Recent World Health Organization, governmental, and nonprofit foundation support for effective preventative measures—such as insecticide-treated bednets, indoor residual spraying, and the implementation of artemisinin combination therapy as first-line treatment for malaria in many sub-Saharan African countries—appears to have significantly reduced malaria incidence and deaths in some countries.1
More than 40% of the world’s population, or 2.5 billion people, are at risk for malaria in 90 countries in Africa, Asia, South and Central America, and Oceania (Fig. 352-1). For many years, it appeared that malaria in humans was caused by four species of Plasmodium: P falciparum, P vivax, P ovale, and P malariae. There is now evidence that P knowlesi, a Plasmodium species that usually infects monkeys, has crossed over to cause malaria in humans in Southeast Asia, notably in Malaysia4; it is now considered a fifth human malaria species. Plasmodium falciparum is found mainly in tropical areas, where warm weather ensures the relatively constant presence of the Anopheles vector. Plasmodium vivax has the widest geographic distribution of the four species and is found in both tropical and temperate areas. Plasmodium ovale is found primarily in sub-Saharan West Africa, where it appears to have almost completely replaced Plasmodium vivax. Plasmodium malariae can be seen in both tropical and temperate zones but is the least common of the malaria species.
Diagnostic and treatment approaches differ significantly in malaria endemic countries as compared to countries like the United States, where almost all malaria is imported. In the United States, all of the approximately 1500 cases of malaria that were reported to the Centers for Disease Control and Prevention (CDC) in 2005 occurred in travelers to or immigrants from malaria-endemic countries, with the exception of two cases of congenital malaria, in which the mothers were immigrants from malaria-endemic countries.2 Rare cases of local transmission have been reported in the United States.3
Malaria can be a life-threatening illness. Delay in seeking treatment, misdiagnosis, or both are often seen in individuals who die from malaria in the United States.2 Any febrile child who has been in a malaria-endemic area in the preceding year should be assessed for this illness.
 ORGANISMS AND LIFE CYCLE
ORGANISMS AND LIFE CYCLE
Plasmodium species can infect many different animals but most are host-specific. P falciparum is the only Plasmodium species that infects all ages of red blood cells, so it generally causes a much higher level of parasitemia than the other Plasmodium species. P vivax and P ovale preferentially infect reticulocytes and tend to cause a lower level of parasitemia than does P falciparum. P malariae preferentially infects senescent red cells and causes the lowest level parasitemia of the human Plasmodium species, but this low-level parasitemia may persist for decades. It is not clear at this point if P knowlesi preferentially infects a subset of red cells, but it multiplies rapidly and can cause very high levels of parasitemia. Morphologically, it can be confused with P malariae on microscopic examination.
Understanding the malaria parasite life cycle is crucial to understanding malarial infection and disease. The malaria life cycle is summarized in Figure 352-2. Sporozoites are inoculated into the bloodstream by the Anopheles mosquito and migrate within minutes to the liver, where they invade hepatic parenchymal cells. Here, the sporozoites undergo asexual multiplication (hepatic schizogony), forming schizonts that rupture the hepatic cells and release merozoites into the bloodstream. Very few hepatic cells are invaded by sporozoites, but multiplication within the hepatic cell produces thousands of merozoites from each sporozoite-infected hepatic cell. The process of liver schizogony lasts from 7 to 10 days for P falciparum, P ovale, and P vivax and 10 to 14 days for P malariae. P vivax and P ovale can also produce dormant liver stages (hypnozoites) that can reactivate weeks or months after the initial infection and can cause clinical relapse.
Merozoites released by ruptured hepatic cells invade red blood cells, where they may asexually multiply or undergo sexual differentiation into male and female gametocytes. Parasites established in the red blood cell (trophozoites) that asexually multiply form red blood cell schizonts. These schizonts eventually rupture the red cells containing them and release more merozoites, which continue the cycle of red cell invasion and multiplication.
Male and female gametocytes are ingested by mosquitoes with their human blood meal. In the mosquito, the male gametocyte exflagellates, releasing a microgamete that fertilizes the female macrogamete, producing a zygote. The elongated zygote, or ookinete, penetrates the mosquito’s stomach wall and forms an oocyst behind it. The oocyst grows and eventually ruptures to release numerous sporozoites, which migrate throughout the mosquito. Those that enter the salivary glands can then infect humans the mosquito bites, thus renewing the cycle of infection.
Malaria can also be acquired by direct blood exposure through blood transfusions. With current blood-screening procedures, such cases are rare in the United States. Congenital malaria, with passage of infection from mother to newborn, can also occur, though it is relatively infrequent in endemic areas. It is seen more frequently in nonimmune women and in women who have an overt attack of clinical malaria during pregnancy. In areas where malaria is endemic, infection during pregnancy, even among semi-immune women, can lead to low birth weight and an increased risk of perinatal mortality.
FIGURE 352-1. Worldwide distribution of malaria. (Courtesy CDC.)
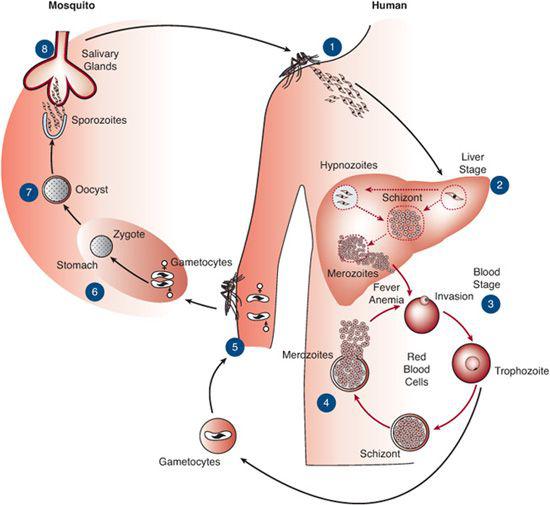
FIGURE 352-2. Life cycle of the malaria parasite in humans and in Anopheles mosquitoes. For description, see text.
 PATHOPHYSIOLOGY OF MALARIA INFECTION
PATHOPHYSIOLOGY OF MALARIA INFECTION
Malarial disease is caused by the blood stages of the parasite. Rupture of red cells and release of merozoites into the blood leads to the fever, chills, and malaise seen in all forms of malaria. Plasmodium-infected erythrocytes, opsonized with antibodies or complement, are less deformable than uninfected erythrocytes and are consequently trapped in the spleen, leading to splenomegaly. Anemia and thrombocytopenia are due primarily to splenic consumption of erythrocytes and platelets, but autoimmune hemolysis plays a role in the continued destruction of erythrocytes that can occur for weeks after appropriate treatment. In addition, bone marrow suppression occurs in severe malarial anemia, so the anemia seen is due to both erythrocyte destruction (by autoimmune hemolysis and spleen removal of infected erythrocytes) and impaired erythropoiesis.
The pathogenesis of organ dysfunction in P falciparum malaria is complex, and precise mechanisms are still being worked out, but it clearly originates in the interactions between infected red blood cells (iRBCs) and the endothelial cells lining vascular organ beds. Cytoadherence of iRBCs in capillaries leads to sequestration of red cells and parasites in microvascular beds, and the resultant local tissue ischemia and hypoxia likely contributes to the renal, gastrointestinal, pulmonary, and central nervous system complications seen in falciparum malaria. However, several additional factors likely contribute to pathogenesis of P falciparum complications. Endothelial cell damage from activation by iRBCs may cause impairment of the blood-brain barrier (in cerebral malaria) or vascular damage in other organs and may lead to local release of cytokines and other inflammatory factors.5 In support of this hypothesis, several cytokines, notably TNF-α, are present in higher amounts in the cerebrospinal fluid of children with cerebral malaria than in control children.6 Animal models also strongly support the role of proinflammatory cytokines, particularly TNF-α and IFN-γ, in the pathogenesis of severe malaria.5 Regulatory polymorphisms of cytokine genes also appear to play a role in the development of disease.
 HOST FACTORS
HOST FACTORS
Numerous host genetic factors can affect susceptibility to malarial infection and disease. Protective factors against disease with P falciparum include hemoglobinopathies (hemoglobin S [sickle cell trait], hemoglobin C and E, a and b thalassemia, blood group 0, and G6PD deficiency), and specific HLA class I and class II alleles; Duffy blood group antigens and hereditary ovalocytosis have been associated with protection against P vivax. 
 DEVELOPMENT OF IMMUNITY
DEVELOPMENT OF IMMUNITY
Individuals living in malaria-endemic areas never develop complete immunity to the illness. However, with repeated exposure to a variety of different malaria strains over several years, they become relatively tolerant to infection. These “semi-immune” individuals often have asymptomatic parasitemia, and when malarial disease does occur, it is generally much milder than that seen in nonimmune persons.
The major factors in acquiring immunity to malarial disease are repeated and frequent exposure to P falciparum, exposure to multiple strains of P falciparum, and age. Acquiring immunity to malarial disease occurs during childhood in malaria-endemic areas, but the pattern of acquisition differs in areas of differing endemicity. In areas of low- and midlevel endemicity, children acquire immunity more slowly than in areas of high-level endemicity. The primary manifestations of disease also tend to differ in areas of varying endemicity. In areas with high endemicity, children develop severe anemia, which is seen most commonly in those ages 6 months to 3 years. In areas with low- or midlevel endemicity, cerebral malaria is more common and occurs in a broader age range (6 months to 6 years). In malaria-endemic areas, children less than 6 months old, and especially those less than 3 months old, are protected from malarial infection and disease by fetal hemoglobin and passively transferred maternal antibodies.
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
The clinical presentation of malaria depends on the infected individual’s age and level of immunity and on the Plasmodium species causing the illness. In the United States, most children who present with malaria are nonimmune. More than 90% of those with P falciparum infection (the most common Plasmodium species in imported malaria) present within 3 months of travel to or immigration from a malaria-endemic area,2 while less than 50% of those with P vivax or P ovale infection present within 3 months; rarely, P vivax and P ovale may present more than a year after exposure.
Nonimmune individuals, whether children or adults, tend to present with more severe signs and symptoms than semi-immune individuals and may develop severe disease with relatively low-level parasitemia. Prodromal, flulike symptoms occur during the early cycles of erythrocytic infection and may include fever (with no specific pattern), headache, malaise, myalgias, arthralgias, abdominal pain, and diarrhea. Children, especially infants, may not exhibit the classic “febrile paroxysm” seen in adults. In infants, more nonspecific symptoms such as fever, lethargy, decreased appetite, and listlessness may continue to predominate. Vomiting, loose stools, and abdominal pain are very common complaints in both infants and children. Many infants and older children will also have intermittent fevers without a clear pattern, rather than the 48-hour (P vivax, P ovale, P falciparum) or 72-hour (P malariae) fever patterns classically described with these infections. Children with P falciparum in particular may exhibit very irregular fever patterns. Up to 10% of children with malarial disease may not have documented fever during the illness. Seizures are common in severe malaria. Nonimmune adults frequently exhibit the classic febrile paroxysm, which consists of three phases: a brief “cold” phase, with chills and sometimes rigors; a hot phase, with high fever, dry, flushed skin, tachypnea, and thirst; and a sweating stage, with defervescence accompanied by diaphoresis and a feeling of great relief but also great weakness. The paroxysms coincide with the rupture of infected erythrocytes and the release of merozoites, pigment, and cell debris into the circulation.
The physical signs most frequently seen in malaria are hepatomegaly and splenomegaly, which occur in about half of all children with acute malarial disease. In areas where malaria is highly endemic, a large percentage of children develop palpable splenomegaly over time, and the prevalence of splenomegaly in children ages 2 to 9 years has been used to define an area’s malaria-endemicity pattern. In areas of unstable transmission and in nonimmune individuals, it is less common.11 Although malaria often leads to some degree of anemia, particularly when not treated immediately, pallor is seen in only 25% of children with malaria in endemic areas and jaundice in only 10% to 15% of children. Scleral icterus may be seen in children. Jaundice is more common in nonimmune adults. Other physical exam findings relate to complications of malaria, such as coma or posturing in children with cerebral malaria or chest indrawing and respiratory distress in children with lactic acidosis.
 COMPLICATIONS FROM P FALCIPARUM MALARIA
COMPLICATIONS FROM P FALCIPARUM MALARIA
Nonimmune children with P falciparum malaria often develop complications from the disease. The World Health Organization (WHO) lists 10 defining criteria for severe malaria, as listed in Table 352-1.12 The most common of these complications in children are severe malarial anemia, respiratory distress, and impaired consciousness. Each of these complications can contribute to and exacerbate the others, and mortality increases as the number of malarial complications increases.
Cerebral Malaria
By WHO definition, cerebral malaria is present in a patient who (1) cannot localize a painful stimulus, (2) has peripheral asexual P falciparum parasitemia, and (3) has no other causes of an encephalopathy. The pathophysiology of impaired consciousness in a child with severe malaria is likely the same as that of coma. Recurrent convulsions are a frequent antecedent to subsequent impaired consciousness and coma: according to strict WHO criteria, 50% to 80% of African children with cerebral malaria have a prior history of convulsions.13
Table 352-1. World Health Organization Criteria for Severe Malaria
Impaired consciousness |
Prostration |
Respiratory distress |
Multiple seizures |
Jaundice |
Hemoglobinuria |
Abnormal bleeding |
Severe anemia |
Circulatory collapse |
Pulmonary edema |



