D. Ware Branch, MD
T. Flint Porter, MD
• Effect of Pregnancy on Lupus
• Intrauterine Growth Restriction
• Neonatal Lupus Erythematosus
• Drugs for Use in SLE and Pregnancy Implications
1. Definite Antiphospholipid Syndrome
2. Catastrophic Antiphospholipid Syndrome
3. Possible or Probable Antiphospholipid Syndrome and Equivocal Cases
5. Refractory Obstetric Antiphospholipid Syndrome
6. Catastrophic Antiphospholipid Syndrome
SYSTEMIC LUPUS ERYTHEMATOSUS
Introduction
Systemic lupus erythematosus (SLE) is a chronic, multiorgan, autoimmune condition characterized by intermittent periods of increased disease activity (“flares”) interspersed with periods of remission. SLE has an estimated prevalence of 20 to 150 per 100,000 individuals in the United States.1–3 The incidence of SLE has nearly tripled in recent decades, most likely because of an increased diagnosis of mild cases.4 There are important gender differences in the burden of disease, with 88% of affected individuals being women.3 African American women are affected approximately 2.5 times more commonly than white women.1 SLE most often presents during the reproductive years with initial symptoms that include fatigue, joint pain, fever, and rash.
The cause of SLE appears to be multifactorial, with genetic, hormonal, and environmental factors all contributing to susceptibility to the disease. High concordance for the disease (~25%) has been reported among monozygotic twins.5 Genome-wide association studies have identified multiple single nucleotide polymorphisms (SNPs) that appear to increase susceptibility to SLE. Those with the strongest association include deficiencies in complement components (C1q, C4A, C4B, C2) and a TREX1 gene mutation (both of which are relatively rare).6 More commonly identified SNPs associated with SLE include those within the major histocompatibility complex.6 Not surprisingly, however, such common SNPs confer only a modestly increased risk, emphasizing the complex and multifactorial nature of SLE pathogenesis. Recently, epigenetic modification (such as CpG dinucleotide methylation, histone modifications, and micro-RNAs) of SLE-predisposing genes has received attention as a potentially important mechanistic contributor to disease.7,8
These epigenetic modifications (and the associated changes in gene expression) likely mediate some of the effects of environmental exposures on the development of SLE.9 Exposures identified as having an association with SLE include Epstein-Barr virus, ultraviolet light, and silica dust.9
As evidenced by the dramatically increased prevalence of SLE in women as compared with men, hormones appear to play an important role in the development of SLE.10 Data from the Nurses’ Health Study indicate that factors such as earlier menarche, oral contraceptives, and postmenopausal hormone replacement all increase the risk for SLE.11 Because of the fact that SLE is often diagnosed during a woman’s reproductive years, clinicians will frequently be presented with the challenge of managing a pregnancy complicated by SLE.
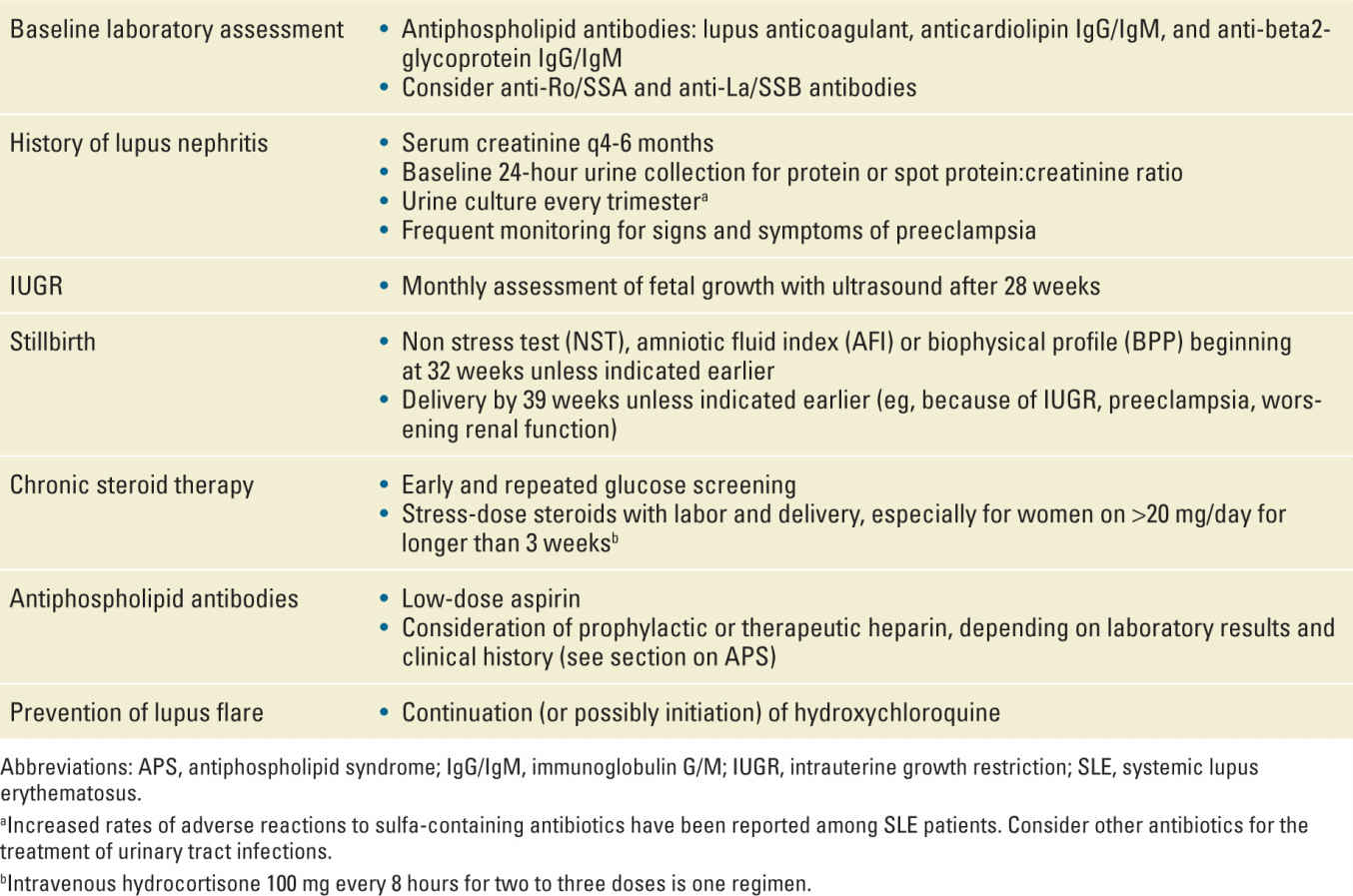
Pregnancy can affect the management of SLE (particularly with regard to choice of medications) and SLE increases the risk of multiple adverse pregnancy outcomes, including preterm birth (PTB), preeclampsia, and intrauterine growth restriction (IUGR). Women with more severe SLE manifestations, such as those with lupus nephritis (LN), are at particularly increased risk for complications.12 Women should be appropriately counseled (ideally preconceptionally) regarding these risks, and increased maternal and fetal surveillance is warranted to optimize outcomes.
Clinical Presentation
Although lupus can affect virtually any organ, the most common presenting symptoms include fatigue, fever, joint pain and stiffness, Raynaud phenomenon, and a photosensitive rash (typically over the malar region). The arthritis of SLE is usually migratory in nature and most frequently affects the proximal interphalangeal (PIP) and metacarpophalangeal (MCP) joints, knees, and wrists. More severe organ manifestations include nephritis, pleuritis, pericarditis, and cerebritis (manifested by seizures or psychosis).
Diagnosis
Once a clinical suspicion for SLE is raised, the diagnosis is made by a combination of clinical and laboratory criteria. The American College of Rheumatology has sensitive and specific diagnostic criteria for SLE (most recently revised in 1997) (Table 18-1). To be diagnosed with SLE, an individual must have at least 4 of the 11 clinical and laboratory criteria, either simultaneously or serially. The laboratory criteria include an abnormal titer of antinuclear antibody (ANA), anti-dsDNA antibodies, anti-Sm antibodies, or antiphospholipid antibodies. As nearly all individuals with SLE have an abnormal ANA titer, this is a reasonable initial screening test. Although a negative ANA test makes it very unlikely that the patient has SLE, a positive ANA is not specific for SLE and may be seen in association with other autoimmune disorders such as scleroderma, rheumatoid arthritis (RA), or Sjogren syndrome. Anti-dsDNA and anti-Sm antibodies are both highly specific but less sensitive for SLE. Although only 30% to 40% of SLE patients have anti-Sm antibodies, they are highly specific for the disease and associated with LN.13 Some patients may have antiribonucleoprotein antibodies, which have been associated with myositis and Raynaud phenomenon.14 Both patients with SLE and Sjogren syndrome may have anti-Ro/SSA and anti-La/SSB antibodies, which are particularly relevant for obstetric care as they are associated with the development of neonatal lupus and congenital heart block.
TABLE 18-1 | Revised American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus (1982 and 1997) |

Elevated anti-dsDNA titers are frequently seen in the setting of active SLE and elevated levels may precede disease flares.15 In a study from the Hopkins SLE cohort, low complement levels and/or positive anti-dsDNA in the second trimester were associated with a higher rate of pregnancy loss and PTB (independent of clinical disease status).16 However, women with high clinical activity in addition to these serologic markers of disease activity were at far greater risk. Routinely assessing complement levels and autoantibody titers in women with clinically inactive disease is of uncertain clinical utility, as these assessments do not highly correlate with clinically relevant outcomes such as PTB.16,17
Although LN may be suspected based on hematuria, proteinuria, and an active urine sediment, the diagnosis requires a renal biopsy. Biopsy results are divided into six classes according to the International Society of Nephrology and the Renal Pathology Society.18 The most common (and most severe) form is class IV, or diffuse LN. Patients with class IV disease usually have hematuria and proteinuria and may have nephrotic syndrome, hypertension, and renal insufficiency. Renal biopsy in pregnancy is not absolutely contraindicated but is usually avoided because of concern for potential bleeding complications (less common with the use of ultrasound guided biopsy).
Effect of Pregnancy on Lupus
It remains controversial as to whether pregnancy increases the risk for SLE flare. Studies from the 1960s and 1970s suggested a significant risk for increased disease activity in pregnancy with high rates of maternal and fetal morbidity and even mortality. However, most of the subsequent studies suggest that pregnancy does not significantly increase the risk of severe SLE flare.19–22 If flares do occur, they appear to be generally mild to moderate in severity and are relatively easily treated.21,23
The best determinant of the course of SLE in pregnancy is disease activity at the onset of pregnancy.24–26 In one report, approximately one-third of women who were in remission for 6 months or more before pregnancy suffered an SLE flare during pregnancy as compared with two-thirds of women with active disease at the onset of pregnancy.25 Women who stop SLE maintenance therapy at the onset of pregnancy are also more likely to have an SLE flare.26
Lupus Nephritis
Women with LN (particularly active disease) are an especially high-risk group for adverse pregnancy outcomes.12,27 Women with a history of LN are at greater risk for pregnancy-induced hypertension, preeclampsia, SLE and LN flares in pregnancy, and low-birth-weight infants.27–29 A recent review identified 13 studies that reported on 17 deaths in the 6-week postpartum period in women with SLE and LN.30 In all such cases, women had active LN during pregnancy. In the majority of cases, death was attributed to infection (mostly opportunistic) or disease activity.
Similar to SLE in general, status of LN activity at conception is a critical predictor of outcome. Inactive LN and normal baseline renal function are predictive of a favorable maternal outcome.28,31
The amount of baseline proteinuria in early pregnancy should be measured among all women with a history of LN. An increasing level of proteinuria across gestation is common in this group of women, related in part to increased glomerular filtration. As a general rule, an isolated increase in proteinuria (outside of other manifestations such as hypertension or a significantly rising serum creatinine) should not be used as an indication for early delivery. In fact, in recently revised guidelines, the American Congress of Obstetricians and Gynecologists (ACOG) now states that the diagnosis of preeclampsia is not incumbent upon a diagnosis of proteinuria.32 Serum creatinine should be assessed every 4 to 6 weeks. Moderate renal insufficiency (baseline serum Cr >1.5-2.0) is considered a relative contraindication and severe renal insufficiency (Cr >2.0), an absolute contraindication to pregnancy. Although results from existing studies are not entirely consistent, women with LN (particularly those with more severe disease) should be advised of the potential (~5%-10%) risk of an irreversible decline in renal function during pregnancy.33,34
Pregnancy Loss
Although women with SLE do not appear to be less fertile than those without the disease, they are at greater risk for pregnancy loss, particularly if their disease is more severe or less well controlled, or they have antiphospholipid (aPL) antibodies. The risk of pregnancy loss appears to be declining, however, because of improved treatment and pregnancy surveillance.35 Studies of lupus pregnancies from the 1960s and 1970s reported fetal loss rates as high as 50%. A more recent study found that among women with SLE in remission at the onset of pregnancy, the risk of miscarriage or stillbirth was 17% as compared with 5% for women without SLE.36 A subsequent meta-analysis reported a spontaneous abortion rate of 16% and a stillbirth rate of 3.6% among women with SLE (after excluding those who had an induced abortion).12 Women with higher levels of disease activity are more likely to suffer a pregnancy loss.37,38 Clowse et al., in a study of 267 pregnancies among women with SLE, found that 88% of pregnancies with low-activity SLE resulted in a live birth compared with 77% pregnancies complicated by high-activity SLE.38 Women with LN, and those with hypertension, aPL antibodies, and thrombocytopenia are at greater risk for loss.37,39
Women with SLE and aPL antibodies deserve particular attention due their elevated risks not only of pregnancy loss but also of preeclampsia and thrombosis. Even among those who do not meet clinical criteria for aPL syndrome (APS), it is reasonable to consider thromboprophylaxis in pregnancy and/or the postpartum period for women with SLE who test positive for lupus anticoagulant (LA) or moderate-to-high titers of anticardiolipin (aCL) immunoglobulin G (IgG) or anti-beta2-glycoprotein IgG antibodies.
Preterm Birth
Women with SLE are at an approximately threefold increased risk of PTB as compared with women without SLE.35 The risk of PTB is particularly pronounced among those with greater disease activity or more severe disease manifestations, such as LN. In one study, only 26% of pregnancies with high-activity disease resulted in a full-term delivery as compared with 61% of those with mild or no disease activity.38 Most PTBs in this setting are not spontaneous, but indicated because of concern for maternal or fetal well-being in the setting of SLE flare, preeclampsia, fetal growth restriction, or deteriorating renal function.
Intrauterine Growth Restriction
Multiple studies have found an association between SLE in pregnancy and IUGR.12,40 However, recent studies suggest lower rates of IUGR than previously reported, perhaps secondary to modern management schemes. In a study from the National Inpatient Sample that included 16.7 million admissions for childbirth and more than 13,000 women with SLE, only 5.6% of SLE patients had a diagnosis of IUGR, not a statistically significant difference from the 1.5% rate among non-SLE patients.41 It is probably not SLE itself that confers the increased risk for uteroplacental insufficiency and IUGR but rather associated complications such as hypertension, LN, and APS. It is common practice in the United States for pregnant women with SLE to undergo serial fetal growth assessment beginning in the mid-second trimester.
Preeclampsia
Preeclampsia is a frequent complication among women with SLE, occurring overall in 10% to 30% of cases.19,41 Women with LN are at particularly high risk of developing preeclampsia. In a recent study of women who were primarily in SLE remission at the onset of pregnancy, 28% of pregnancies in women with a history of LN were complicated by preeclampsia as compared with 16% of pregnancies in women without a history of LN.42 Among the pregnancies complicated by preeclampsia, the condition developed at an earlier median gestational age in those with a history of LN as compared with those without (37.5 vs 34.5 weeks). A sometimes difficult clinical challenge in caring for the pregnant woman with LN is distinguishing between preeclampsia and an SLE flare. Both conditions can present with symptoms of hypertension and proteinuria. However, the treatments for the two conditions are different, especially at very early gestational ages. An SLE flare may be successfully treated with immunomodulatory drugs, thus allowing continuation of the pregnancy in favor of improved neonatal outcome. In contrast, preeclampsia requires timely delivery. Assessing anti-dsDNA titers and complement levels may provide helpful information. However, the interpretation of these serologic tests is not straightforward, as complement levels (which usually rise in pregnancy because of increased liver synthesis) have been found to decrease in the setting of preeclampsia as well as SLE flare.43 A renal biopsy should be considered in difficult cases at very early gestational ages in which fetal-neonatal survival is in question. Beyond the early third trimester, delivery may be the most prudent approach in confusing cases. Postpartum resolution of hypertension and proteinuria suggests preeclampsia.
Among women with LN or more severe disease conditions, such as active SLE or APS, more frequent prenatal visits and consultation with a rheumatologist are indicated to monitor for signs and symptoms of flare or preeclampsia. Home blood pressure monitoring can provide helpful clinical information.
Neonatal Lupus Erythematosus
Neonatal lupus erythematosus (NLE) is an acquired autoimmune disease related to the transplacental passage of anti-Ro/SSA and anti-La/SSB antibodies. The most common manifestation of NLE is an erythematous, scaling, plaque-like rash that begins soon after birth and may last up to 1 to 2 months. A smaller proportion of infants with develop hemolytic anemia, leukopenia, thrombocytopenia, or hepatosplenomegaly. The most serious manifestation of NLE is complete heart block (CHB).
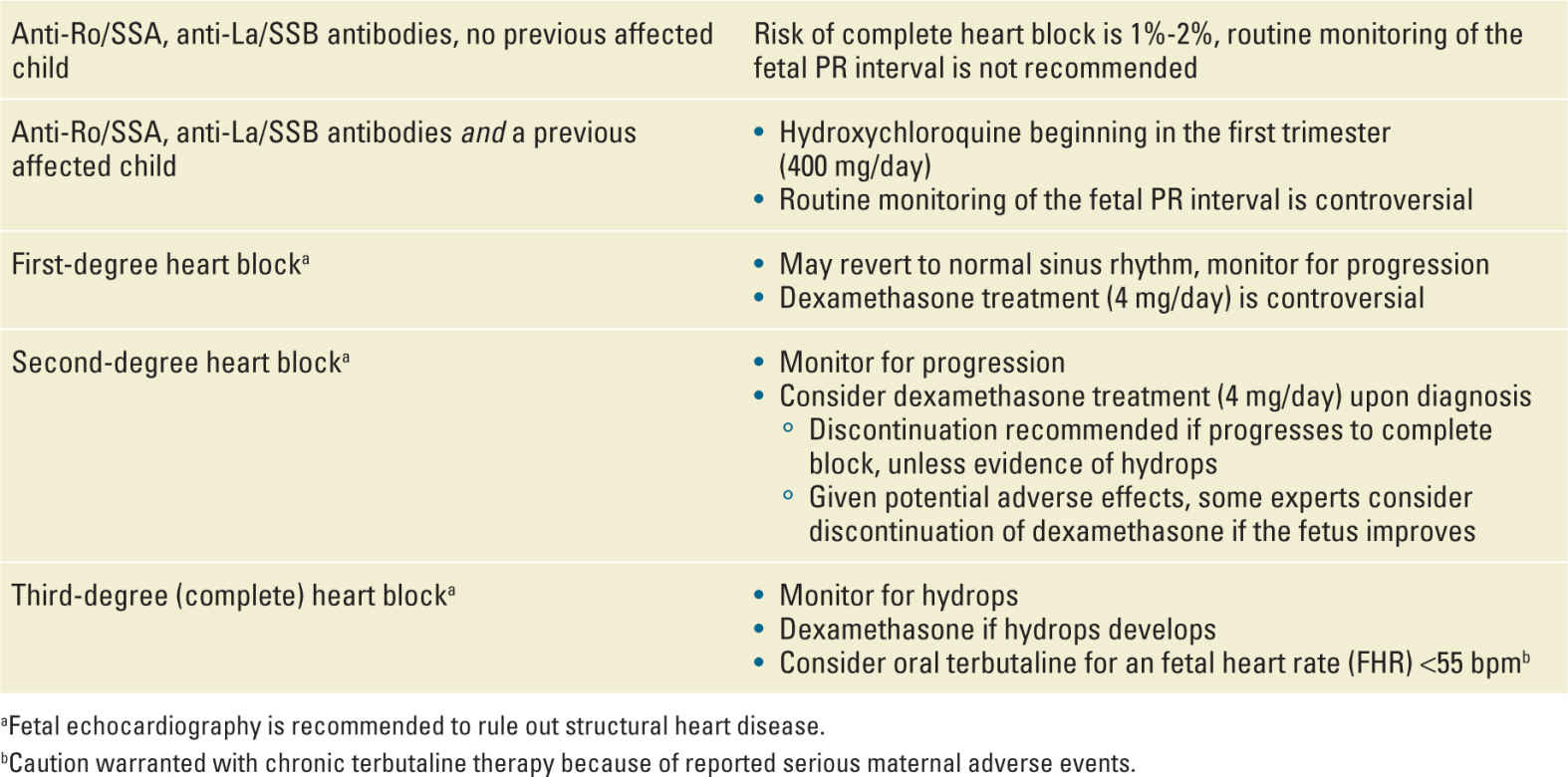
Though one-third of women with SLE have detectable anti-Ro/SSA and anti-La/SSB antibodies, no more than 20% of these will have a fetus-neonate with a manifestation of NLE. Among all mothers with SLE, the risk of NLE is less than 5%. Only 1% to 2% of women with anti-Ro/SSA and anti-La/SSB antibodies will have a fetus affected with CHB. The most important predictor of having a fetus with CHB is a prior affected baby. Among these women, the recurrence risk is as high as 15% to 20%.44 In one study, moderate-to-high titers of anti-Ro/SSA antibodies also appear to correlate with a higher risk of NLE cardiac manifestations.45
Many of these women have not been diagnosed with an autoimmune condition such as SLE or Sjogren syndrome but develop one of these conditions in the future.
CHB is perhaps most frequently diagnosed at a routine prenatal visit when a fixed fetal bradycardia of 50 to 80 bpm is detected, most commonly between 16 and 24 weeks. CHB can result in endocardial fibroelastosis and fetal hydrops, conditions that carry a high risk of fetal or neonatal mortality. Overall mortality associated with CHB is at least 20% (5% in utero), and in one series of 102 cases, an in-utero diagnosis of CHB carried a 43% risk of mortality in the first two decades of life.46
Given its serious nature, investigators have sought to determine how to improve CHB outcomes or to prevent it in pregnancies at increased risk. Weekly monitoring of the fetal PR interval with echocardiography in high-risk women (those with anti-Ro/SSA antibodies and a prior affected fetus) is controversial. Progression to CHB can occur rapidly and without detectable progression through first- and second-degree blocks,47 and earlier detection of CHB has not been convincingly shown to improve outcomes. Once CHB is present, it is irreversible.
Not surprisingly, well-designed trials to establish the best treatment of fetuses with CHB are lacking. Several case series have suggested a potential benefit of the use of prenatal fluorinated steroids for the treatment of CHB.48–50 Jaeggi et al. reported improved survival (95% at 1 year) in cases of CHB treated with a regimen of dexamethasone and beta-stimulation for a fetal heart rate <55 bpm.49 Saleeb et al. reported on four fetuses with second-degree block that reverted to first-degree block after treatment with steroids, as compared with two untreated fetuses with second-degree block that progressed to complete block.50 The PR Interval and Dexamethasone Evaluation (PRIDE) study investigators enrolled 40 women with anti-Ro/SSA antibodies and a fetus with CHB.48 Total 30 women were treated with dexamethasone and 10 declined treatment. There was no reversal of any case of CHB (treated or untreated). Among the six treated fetuses with second-degree block, three remained in second-degree block, two reverted to normal sinus rhythm, and one progressed to third degree block. However, steroid treatment was associated with more preterm and small for gestational age (SGA) infants. Dexamethasone has been associated with the improvement or resolution of hydrops associated with complete block and may be beneficial in this specific circumstance.50 Oral terbutaline has been used in cases of CHB with very low fetal heart rates (ie, <55 bpm) in an attempt to increase the fetal heart rate and prevent hydrops. However, caution is warranted as the use of chronic terbutaline for tocolysis is contraindicated because of reported cardiovascular complications and pulmonary edema.51,52
Glucocorticoids are not recommended as preventative treatment for women at high risk of CHB because of the lack of proven benefit, the fact that most women will not have a fetus with CHB, and the potential adverse maternal and fetal effects. Intravenous immunoglobulin has been studied as a preventative agent, but two multicenter prospective observational studies have not shown a benefit and it is thus not recommended.53,54 Recent analysis of retrospective data suggests a benefit for hydroxychloroquine (HCQ) in decreasing the risk of NLE cardiac manifestations.55,56 In an international historical cohort of 257 pregnancies in women with anti-Ro/SSA antibodies and a prior affected child, HCQ use starting in the first trimester was associated with a significantly decreased risk of cardiac NLE (odds ratio, 0.23; 95% confidence interval, 0.06-0.92). Given the low risk of fetal harm, initiation of HCQ in the first trimester should be considered for women with anti-Ro/SSA antibodies who have had a prior affected child. Table 18-2 details a management scheme for CHB.
TABLE 18-2 | Management of Congenital Heart Block |
It should be noted that screening all women with a diagnosis of SLE for anti-Ro/SSA and anti-La/SSB antibodies is not without controversy. Although the results may aid in counseling women regarding the risks of NLE, severe manifestations such as CHB are rare enough that a positive test may cause unnecessary anxiety in most women.
Management
Women with SLE should ideally have a preconceptional consultation with a rheumatologist and a perinatologist. Their history can be reviewed, disease activity determined, and medications assessed for safety. They can be counseled regarding the risks of pregnancy in their specific situation. Clinicians should advise women to postpone pregnancy until they have been in sustained remission for at least 6 months. Table 18-3 summarizes recommendations for maternal and fetal surveillance as presented in the preceding sections.
TABLE 18-3 | Recommendations for Management of Pregnancy Complicated by SLE |
Drugs for Use in SLE and Pregnancy Implications
The most frequently used drugs used in the treatment of SLE during pregnancy are HCQ, azathioprine (AZA), and glucocorticoids.
Several decades ago, concern was raised regarding HCQ (US Food and Drug Administration [FDA] category C) use in pregnancy and risk of fetal ocular toxicity and ototoxicity. Subsequent studies, however, have not confirmed these risks.57 In a recent study comparing 114 HCQ-exposed pregnancies (most in the first trimester) with 455 unexposed pregnancies, there was no significant difference in the rate of congenital anomalies.58 Recent data also suggest that the continuation of HCQ during pregnancy is actually beneficial, resulting in a decrease in SLE flares and less need for glucocorticoids.59 Many experts now feel that women who become pregnant on HCQ should continue on the medication. Because of the potential benefit and lack of proven harm, some experts also consider initiation of HCQ in early pregnancy (or preconceptionally) in women with SLE.
AZA (FDA category D) is a frequently used medication for women who require immunosuppression during pregnancy. Women with SLE who has been treated with mycophenolate mofetil (MMF), which is contraindicated in pregnancy, are frequently switched to AZA before attempting to conceive or when an unplanned pregnancy is confirmed. A recent retrospective study of women with LN desiring pregnancy found that the risk of renal flare with transitioning from MMF to AZA was low.60 The placenta metabolizes most of AZA to an inactive metabolite. Although there have been reports of increased rates of IUGR, PTB, and impaired neonatal immunity with AZA use in pregnancy, the data are difficult to interpret because of the use of multiple medications by women and variability in the severity of disease which may impact on pregnancy outcome.61 In general, AZA (often in combination with glucocorticoids) is preferred over other immunosuppressants for the treatment of severe or active SLE in pregnancy.
Glucocorticoids are a frequent first-line treatment for SLE flare in pregnancy. Prednisone is usually the preferred choice because the placenta metabolizes most of the drug into an inactive metabolite (in contrast to fluorinated steroids such as dexamethasone and betamethasone). Prednisone is FDA category C. First trimester use has been associated with a small increased risk of cleft lip and palate.62 Prolonged use is associated with an increased risk for bone loss, gestational diabetes, preeclampsia, premature rupture of membranes (PROM), and IUGR.63 Women on chronic glucocorticoids should be screened early for gestational diabetes, with the test repeated at the usual 24 to 28 weeks if normal. Glucocorticoids at significant doses may be difficult for many women to tolerate because of side effects such as fluid retention, striae, acne, and weight gain. Patients who have taken greater than 20 mg/day of prednisone for at least 3 weeks are at greatest risk of hypothalamic-pituitary-adrenal (HPA) axis suppression and should receive stress-dose steroids during labor and delivery. One regimen is 50 to 100 mg of intravenous hydrocortisone every 8 to 12 hours for two to three doses. For women taking less than 5 mg/day of prednisone for any length of time or any dose for less than 3 weeks, the risk of HPA suppression appears to be very low and stress-dose steroids are unnecessary. For women taking between 5 and 20 mg/day, the risk of HPA axis suppression is variable and management is less clear. However, the risk of adrenal insufficiency in this group is probably very low with continuation of the usual daily steroid dose.
Cyclosporine inhibits the production and release of interleukin-2. It is FDA category C and may occasionally be used for the treatment of severe SLE in pregnancy. Data from its use primarily in organ transplant patients indicate there is a low risk of teratogenicity,64 but there may be an increased risk for PTB and SGA infants.63 However, it is difficult to determine whether these effects are due solely to the use of cyclosporine or are in part related to the underlying maternal disease.
Several drugs used in the treatment of SLE are absolutely contraindicated in pregnancy because of significant risk for fetal harm. Cyclophosphamide (FDA category D) is an alkylating agent with a significant risk for teratogenesis and should not be used at all in the first trimester. It may be considered in the second and third trimesters in the infrequent patient with very severe and progressive disease manifestations. Methotrexate (FDA category X) is a folate antagonist used commonly to provide long-term, maintenance immunosuppression in patients with autoimmune conditions, including SLE. Its potent teratogenicity and embryo toxic effects are well known.63,65,66 The drug is widely distributed in maternal tissues and may persist for up to 4 months in the liver. All women taking methotrexate should be warned of its teratogenic risks and maintain adequate contraception. For those considering pregnancy, conception should be delayed for at least three menstrual cycles after discontinuation of methotrexate. MMF (FDA category D), an inhibitor of purine biosynthesis, is also teratogenic and can induce miscarriage. It is absolutely contraindicated in pregnancy, and women should be advised to use effective contraception until they have been off MMF for at least 6 weeks before conceiving.
Biologic agents may be used for the treatment of SLE that is not controlled with traditional therapies alone. This group of agents includes belimumab (a monoclonal antibody which inhibits a B-cell survival factor, FDA category C) and rituximab (monoclonal anti-B lymphocyte antibody, FDA category C). Data regarding the use of these medications in pregnancy are extremely limited and they should generally be avoided unless maternal health is critically dependent on their continued use. Rituximab has been associated with B-cell depletion in exposed neonates.67
Conclusion
SLE is an autoimmune condition that occurs most often in women during their reproductive years. Pregnancy outcomes for women with SLE appear to have improved with modern management. The greatest predictor of how SLE will do in pregnancy is the status of disease activity at conception. Women with more severe disease manifestations (such as LN), active disease, or APS are at greatest risk for adverse outcomes including pregnancy loss, IUGR, preeclampsia, and PTB. Anti-Ro/SSA and anti-La/SSB antibodies are associated with the development of NLE. A rare manifestation of NLE is CHB, which carries a high rate of perinatal morbidity and mortality. CHB is irreversible. Any potential benefit of treatment of first- and second-degree blocks with dexamethasone must be weighed against the adverse effects of chronic steroid therapy and the limited data to support its efficacy. Recent data suggest that HCQ may be useful for prevention of CHB in high-risk women. As disease activity is an important determinant of outcomes, women should not cease all medications with pregnancy, but if possible avoid those with moderate-to-high risk of fetal harm while continuing medications such as HCQ, which may decrease the risk of SLE flare.
Careful and frequent maternal/fetal surveillance is indicated to optimize outcomes for women with SLE and their offspring.
ANTIPHOSPHOLIPID SYNDROME
Introduction
APS is a multisystem autoimmune condition well known to obstetricians because of its association with adverse pregnancy outcomes, including pregnancy loss, preeclampsia, and placental insufficiency. APS is also associated with arterial and venous thrombosis. When APS is suspected, the diagnosis is confirmed by the finding of persistently positive aPL antibodies in the patient’s circulation. aPL comprise a heterogeneous group of autoantibodies directed against either negatively charged phospholipids or glycoproteins bound to these phospholipids. Though numerous aPL antibodies have been described, the diagnosis of APS rests on the detection of one or more of three aPL antibodies: LA, aCL, and anti-β2-glycoprotein-I (aβ2-GP-I) antibodies. As with many other autoimmune conditions, experts have developed classification criteria for APS; these were most recently revised in 2005 (Table 18-4).68
TABLE 18-4 | Revised Classification Criteria for the APS |
Abbreviation: APS, antiphospholipid syndrome.
Note: Definite APS is diagnosed if at least one clinical and one laboratory criteria are met.
aSuperficial venous thrombosis is not included in the clinical criteria.
bGenerally accepted features of placental insufficiency include: (1) abnormal or nonreassuring fetal surveillance test(s), eg, a nonreactive nonstress test, suggestive of fetal hypoxemia, (2) abnormal Doppler flow velocimetry waveform analysis suggestive of fetal hypoxemia, eg, absent end-diastolic flow in the umbilical artery, (3) oligohydramnios, eg, an amniotic fluid index of 5 cm or less, or (4) a postnatal birth weight less than the 10th percentile for the gestational age.
cInvestigators are strongly advised to classify APS patients in studies into one of the following categories: (1) more than one laboratory criteria present (any combination); (2a), LA present alone; (2b), aCL antibody present alone; (2c), anti-β2 glycoprotein-I antibody present alone.
Adapted with permission from Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). Journal of thrombosis and haemostasis, J Thromb Haemost. 2006 Feb;4(2):295-306.68
Clinical Presentation
APS may occur alone or in combination with another autoimmune disease, most commonly SLE. The prevalence and incidence of APS is not known, though definite APS in the absence of other autoimmune conditions is likely no more common than SLE. Including low titers, positive tests for one or more of the three aPL, usually aCL or aβ2-GP-I, are found in a small percentage (less than 5%) of healthy controls (including healthy pregnant women)69,70 and in up to 40% of patients with SLE.71 In the absence of a prior history of thrombosis or pregnancy morbidity, the risks associated with an incidentally discovered positive test for aPL among otherwise healthy women are not well understood.
APS presents with either pregnancy morbidity or thrombosis. According to international consensus, APS may present as any one of the following:
• Three or more otherwise unexplained recurrent early miscarriages (pre-embryonic or embryonic losses <10 weeks of gestation).
• One or more otherwise unexplained fetal deaths (≥10 weeks of gestation).
• One or more PTBs occurring at less than 34 weeks of gestation secondary to severe preeclampsia or placental insufficiency.
Given that APS is a relatively infrequent condition in the otherwise healthy female population, a diagnosis of definite APS according to international consensus laboratory criteria68 is likewise infrequent for any of the accepted (and somewhat common) pregnancy-related presentations. Although it is commonly quoted that up to 15% of women presenting with recurrent early miscarriage have positive aPL results,72 critical analysis suggests that the existing studies are flawed by poor standardization of assays, inclusion of women with other causes of recurrent miscarriage, inconsistent selection of controls, variability of aPLs or isotype tested, and varying definitions of recurrent miscarriage (differing numbers and gestational ages of losses).73 Thus, the prevalence and incidence of APS among women with recurrent miscarriage remain uncertain.
The association of fetal death with APS is somewhat more clear because of the recently published results from the Stillbirth Collaborative Research Network’s multicenter, population-based case-control study of stillbirths and live births.74 Studying more than 500 fetal deaths at or beyond 20 weeks of gestation and more than 1500 live births, the investigators found positive tests for aPL (aCL or aβ2-GP-I antibodies) in a statistically significant 9.6% of fetal death cases, albeit compared with 6% among live birth cases. IgG isotype aCL or aβ2-GP-I antibodies were found in 3.8% and 1.9%, respectively, of fetal death cases compared with 1.1% and 0.6%, respectively, of live birth cases. Among otherwise unexplained cases, for example, excluding fetal anomalies, IgG aCL and IgM aCL antibodies were associated with a fivefold odds and twofold odds of fetal death, respectively, whereas IgG aβ2-GP-I antibodies were associated with a threefold odds of fetal death. After thorough systematic evaluation of possible causes of fetal deaths, the authors concluded that 14% of otherwise unexplained fetal deaths were attributable to APS.
As with recurrent miscarriage, studies of the association between aPL and preterm delivery <34 weeks because of severe preeclampsia or placental insufficiency are flawed by poor standardization of laboratory tests, concerns related to patient and controls selection, and the definitions of preeclampsia and placental insufficiency. These issues notwithstanding, data from four studies that delineated severe preeclampsia from milder forms suggest that a median of 7.9% of women with severe preeclampsia have positive tests for aPL, compared with 0.5% for controls,75–78 though this likely varies with the population being studied. Placental insufficiency has only been studied as intrauterine fetal growth restriction. The association between preterm delivery <34 weeks because of severe preeclampsia or placental insufficiency and APS was recently reviewed by experts at the 14th International Congress on Antiphospholipid Antibodies.79 In short, these experts concluded that the true association of aPL with PTB <34 weeks of gestation because of severe preeclampsia or placental insufficiency is in much need of well-designed, confirmatory studies.
APS presentation with arterial or venous thrombosis is also recognized by the international consensus criteria (Table 18-4). The most common thrombotic presentation is venous thrombosis of the lower extremity, representing about two-thirds of thrombotic APS cases.80 Recent estimates suggest that 9% to 10% of deep venous thrombosis (DVT) cases are because of APS.81 The most common arterial presentation is stroke; positive aPL results are found in up to 20% of ischemic stroke patients <50 years of age.82 Small vessel thrombosis may present as nephropathy.83 Unlike heritable thrombophilias, APS is notable for thrombosis manifesting in virtually any vascular bed, and clinicians should consider APS in patients presenting with events as seemingly diverse as intracranial venous or arterial thrombosis, hepatic venous thrombosis, and intra-abdominal venous or arterial thrombosis.
An infrequent but very serious thrombotic presentation is that of catastrophic APS (CAPS), a condition of multiple, often small vessel thromboses. CAPS typically demonstrate rapid onset thromboses resulting in multiple organ dysfunction, evidence of a systemic inflammatory response, involvement of unusual organ systems (eg, renal or hepatic), and a high mortality rate.
Though not features of the international diagnostic criteria, experts recognized that aPL are also associated with “noncriteria” clinical features such as immune thrombocytopenia, autoimmune hemolytic anemia, cardiac valvular disease, chronic skin ulcers, myelopathy, chorea, migraine, epilepsy, and cognitive impairment, particularly among patients with SLE.71
Diagnosis
Definite Antiphospholipid Syndrome
The diagnosis of definite APS rests on at least one clinical criterion and at least one positive aPL, as defined by the international criteria, repeatedly positive at least 12 weeks apart (Table 18-4).68 Because the clinical criteria are modestly common and relatively nonspecific for APS, the final diagnosis of APS ultimately rests on the laboratory criteria. Specifically, the laboratory criteria require that a patient have medium-to-high titers of aCL antibodies, anti-β2-GP-I IgG or IgM antibodies, or LA. Because other conditions can result in transiently positive aPL, persistently positive results should be present on two or more occasions at least 12 weeks apart (Figure 18-1).
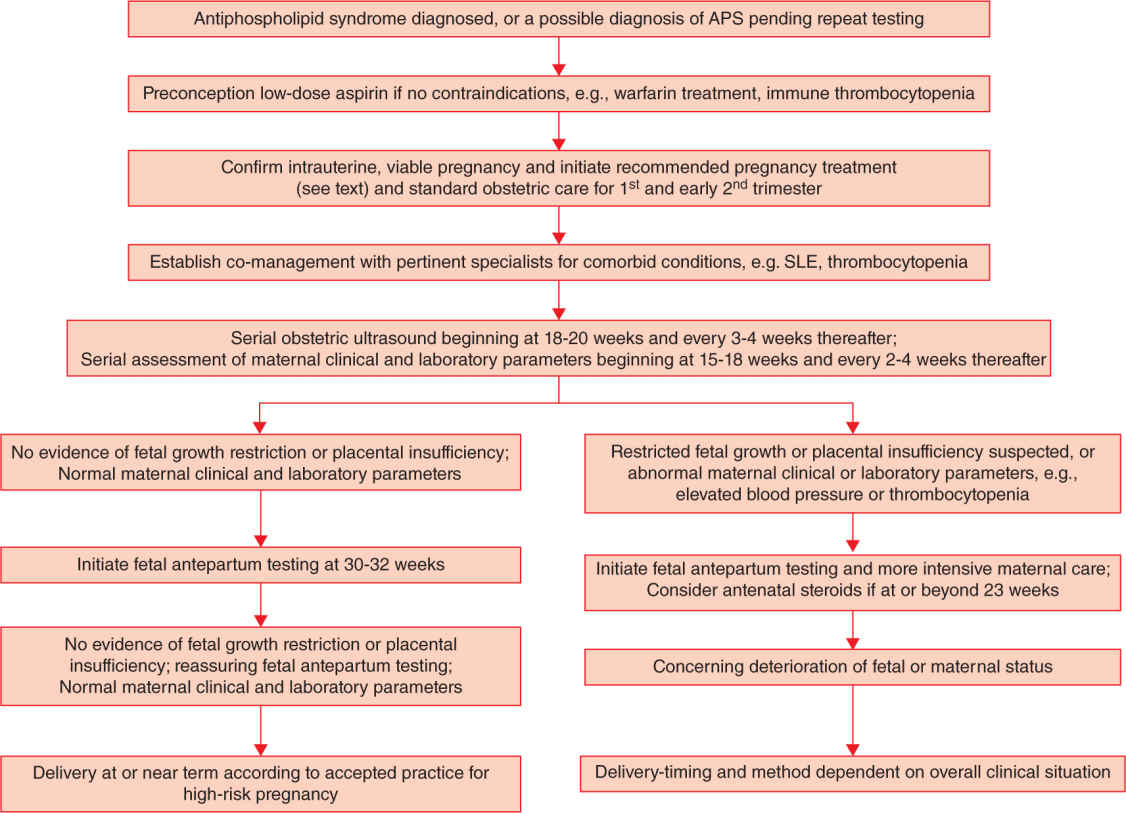
FIGURE 18-1. Algorithm for the diagnosis of antiphospholipid syndrome (APS).
The international guidelines emphasize several important points regarding aPL laboratory testing.68 Because other conditions can result in transiently positive aPL, APS is diagnosed by the finding of persistently positive results on two or more occasions at least 12 weeks apart. Performance characteristics of the aPL immunoassays and the widely recognized variability in aPL immunoassay require that the laboratory identify medium or high titer results for aCL and >99th percentile results for anti-β2-GP-I antibodies. The development of standard calibrators and international “units” for the aCL assay have established >40 IgG units (“GPL”) or IgM units (“MPL”) as being medium or high titer. The status of IgA aCL or anti-β2-GP-I antibodies is a matter of ongoing investigation, but currently IgA aCL or anti-β2-GP-I antibodies are not recognized as diagnostic of APS.
Clinicians should recognize several other points regarding aPL laboratory testing. First, LA is a better predictor of pregnancy morbidity or thrombosis than aCL or anti-β2-GP-I antibodies.84,85 False-positive LA results may occur because of anticoagulant agents; thus, LA testing is ideally performed with the patient off of anticoagulants. The specificity of aCL and aβ2 GPI antibodies for aPL-related clinical events increases with higher titers and with IgG isotype. Finally, some experts hold that triple aPL positivity (LA, aCL, and aβ2 GPI) is of greater clinical significance than double or single aPL positivity.86
Catastrophic Antiphospholipid Syndrome
CAPS has been reported in pregnancy87 and should be considered in the differential that includes hemolytic uremic syndrome and thrombotic thrombocytopenic purpura. International criteria have been developed for the diagnosis of CAPS (Table 18-5).88 CAPS is diagnosed when a patient develops thrombosis in three or more organs in less than a week, microthrombosis in at least one organ, and has persistent aPL positivity. In practice, features of microthrombosis vary from biopsy-proven thrombosis in small vessels to those typical of microangiopathies, with ischemia because of occlusion of arterioles and capillaries. Given the sometimes vague features of microthrombosis and the substantial mortality rate associated with CAPS, a high index of suspicion is warranted in patients presenting with less than three organ systems involved.
TABLE 18-5 | Preliminary Classification Criteria for CAPS |



