Background
Uterine fibroids are a significant health problem. These common benign tumors occur in 70–80% of women before age 50 years and often cause bleeding and pain and can interfere considerably with daily life. Current treatment options are limited. Fibroids contain substantial amounts of altered and disordered collagens, which contribute to their bulk. Targeting these collagens directly presents a novel treatment approach.
Objectives
We sought to test the hypothesis that a highly purified collagenase Clostridium histolyticum will digest interstitial collagen in uterine fibroids and reduce their stiffness and thereby evaluate the feasibility that this collagenase C histolyticum can be developed into an alternative treatment for fibroids. A secondary objective was to describe the collagen content of the fibroid tissue.
Study Design
Fibroid tissue cubes (1 cm 3 ; n = 154) were cut from 17 uterine fibroids that were obtained from 7 consented subjects undergoing scheduled hysterectomies. Tissue cubes were injected with diluent, placebo, or highly purified collagenase C histolyticum (0.05, 0.1, or 0.2 mg/cube) and incubated at 37°C for 24, 48, 72, or 96 hours. At each time point, 6 noninjected control cubes were also evaluated. Tissue cubes were photographed before and after incubation. Myometrial samples (n = 21) were also evaluated. Stiffness was quantified through rheometry by measuring complex shear moduli of the tissues. Percent fibrosis was determined by computerized analysis of Masson-trichrome–stained slides. Digestion of collagen fibrils was confirmed by transmission electron microscopy.
Results
Fibrosis in untreated fibroids ranged from 37% to 77%, reflecting the collagen-rich nature of these tumors. After treatment with collagenase for 96 hours, fibrosis ranged from 5.3% to 2.4%. Transmission electron microscopy confirmed complete digestion of collagen fibrils. Tissue stiffness was reduced with all 3 doses of collagenase treatment and at all 4 time points. Longer incubation times with collagenase caused greater reduction in stiffness, and treated cubes lost their cuboidal shape and had gelatinous/liquefied centers. At 96 hours the stiffness in tissues treated with the lowest dose was reduced to 966 ± 106 Pascal compared with the diluent-treated control at the same time (5323 ± 903 Pascal; P < .0001; by analysis of variance with Tukey-Kramer).
Conclusion
Uterine fibroids have a high content of collagen that can be effectively digested by highly purified collagenase C histolyticum , resulting in reduced tissue stiffness. Loss of stiffness may decrease bulk symptoms in vivo and possibly lead to shrinkage of fibroids through changed mechanotransduction, leading ultimately to reduced fibroid symptoms of pain and bleeding. Clinical trials are necessary to evaluate the safety and efficacy of collagenase C histolyticum including the rate of regrowth of fibroids. The data of this study provide a strong rationale for using this purified collagenase in clinical trials as a local treatment for women with fibroids.
Uterine fibroids develop from smooth muscle cells. Whereas cell proliferation contributes to tumor growth, the bulk is due to deposition of excessive extracellular matrix, mostly altered, disorganized, highly cross-linked interstitial collagens. This extracellular matrix gives fibroids their characteristic stiffness and renders them a fibrotic disease.
It is well established that mechanotransduction affects cell behavior in fibrotic tissues. Mechanotransduction is the conversion of physical forces into biochemical signals. Forces that stretch or compress cells in vivo as well as ex vivo activate cellular receptors and stimulate intracellular pathways. This leads to the production of biochemical signals that affect gene and protein expression and cell function.
Cells that are exposed to the mechanical forces of collagen-rich fibrotic tissue are known to secrete more collagen and other components of the extracellular matrix and develop resistance to programmed cell death (apoptosis), leading to the persistence of cells. This concept of mechanotransduction in reproductive tissues is discussed in detail in 3 recent reviews. Evidence shows that these mechanotransduction pathways occur in uterine fibroids. This supports the concept that stiffness is contributing to their continued enlargement by preventing apoptosis and stimulating continued collagen secretion.
Current treatment options are limited and targeting the bulk extracellular matrix presents a novel approach. Uterine collagens are primarily interstitial collagens type I, III, and V, stable large molecules that are not completely degraded by endogenous mammalian collagenases, matrix metalloproteinases. However, purified collagenase Clostridium histolyticum (Xiaflex; Endo, Dublin, Ireland) can completely digest interstitial collagens. Collagenase C histolyticum is effective in pathological fibrotic tissues like Dupuytren’s cords in the hand and penile fibrous plaques in Peyronie’s disease.
Currently, collagenase C histolyticum is cleared by the Food and Drug Administration only for these 2 indications. A major advantage of collagenase C histolyticum is that the treatment is administered by local injections, minimizing potential systemic side effects.
Our objective for this study was to investigate whether collagenase C histolyticum can effectively digest the altered collagens in uterine fibroids and reduce their stiffness and thereby evaluate the feasibility that collagenase C histolyticum can be developed into an alternative treatment for fibroids. Proof of principle would provide a basis for the necessary clinical studies. A secondary objective was to describe the collagen content of the fibroid tissue.
Materials and Methods
With institutional review board approval, posthysterectomy samples from 17 fibroids (3.0–8.5 cm) from 7 subjects and myometrial tissue from 4 subjects were cut into 1 cm 3 cubes. Subject-identifying information and fibroid location were not recorded. A total of 154 fibroid cubes and 21 myometrial cubes were treated and analyzed in the experiments. Cubes were injected with 50 μL of diluent control, placebo control, or with reconstituted collagenase C histolyticum at 1, 2, or 4 mg/mL (total dose, 0.05, 0.1, or 0.2 mg collagenase C histolyticum per injection).
Injected tissue cubes, together with noninjected control cubes, were immersed in culture media and incubated in a cell-culture incubator at 37°C for 24, 48, 72, or 96 hours. Thirteen myometrial samples were injected with collagenase C histolyticum (0.05 mg, n = 5; 0.1 mg, n = 1; 0.2 mg, n = 7), and 8 myometrial samples were injected with controls and incubated for 24, 48, or 96 hours. Tissues from 16 fibroids and 3 myometrial samples were available to determine baseline values before treatments were initiated. Before and after the incubation, each tissue cube was photographed and palpated to record qualitative assessments of shape and stiffness.
After incubation, tissue cubes were bisected. Half was formalin fixed and paraffin embedded. Fixed tissues from a fibroid that yielded enough cubes to carry out 19 of the 24 possible dose/time treatment combinations were sectioned (5 μm) and stained with Masson trichrome. Masson trichrome stain does not exclusively stain collagen but is routinely used to evaluate fibrosis. Sixteen untreated fibroids were also stained and analyzed. Microscope slides were scanned (Aperio Scanscope, Leica Biosystems Inc., Buffalo Grove, IL) at ×10 and analyzed using Aperio ImageScope and Adobe Photoshop (Adobe Systems Inc., San Jose, CA) CS6 software to quantify blue-green pixels as a proportion of total pixels.
The second half of the bisected cubes were frozen and stored at –80°C. Two to three 5 mm biopsy cores were cut from each frozen sample to prevent location bias. The cores were thawed immediately before objective measurements of tissue stiffness by rheometry (AR-G2 magnetic bearing rheometer; Rheometric Scientific, Piscataway, NJ).
Preliminary data showed that freezing and thawing of fibroid tissue did not affect stiffness measurements. Each core was placed between 2 plates lined with sandpaper to prevent slippage. The top plate was lowered and the height of the sample was determined at a tare load of 0.5 g. Then the plate was lowered further to apply a 20% compressive strain. Testing was performed in a saline bath at room temperature using dynamic frequency sweeps at increasing angular frequency from 1 to 50 rad/s (oscillatory strain). A complex shear modulus G* (measured in Pascal) was calculated from the recorded torque measurements under the assumption of linear viscoelastic behavior at an angular frequency of 10 rad/s.
Representative fibroid cubes (injected with 0.2 mg collagenase C histolyticum or noninjected) were incubated for 96 hours and then fixed immediately in 4% phosphate-buffered glutaraldehyde, postfixed in 1% osmium-tetraoxide, and embedded in epoxy resin. Sections from 2 different areas of each cube were cut on an ultramicrotome and placed on 200-mesh copper grids. Grids were examined under low and high power by transmission electron microscopy for the presence or absence of the characteristic z-bands of mature collagen fibrils.
Experiments were designed with input from a statistician (M.K.). The experimental unit was the 1 cm 3 fibroid tissue cube. The number of tissue cubes that could be obtained from each fibroid was not sufficient to carry out all 24 treatment combinations on each fibroid. Given the heterogeneity of human uterine fibroid tissues, the study was designed to assure that each dose/time treatment combination was applied to tissue cubes from at least 6 different fibroids originating from at least 3 different subjects. This replication was necessary to assure that the treatments were effective across the biological spectrum.
Appropriateness of the sample size was confirmed by Cohen’s d calculations of effect sizes after the completion of the study. For the outcomes, simple comparisons of diluent-controls vs the 3 doses of collagenase C histolyticum had very large Cohen’s d values between 2.1 and 2.7. Similarly, comparisons of time 0 vs treatment times had Cohen’s d values ranging from 1.9 to 4.1, which translate into 100% power for an alpha of 5%.
Tissue stiffness was determined as the average of the measurements from 2–3 cores from each sample. Stiffness data measured in Pascals is presented in the results as mean ± SEM. Multivariate analysis included analysis of variance analysis of variance. The outcome for the analysis of variance was square root transformed to achieve normality. The model included time, dose, and time-by-dose interaction. Multiple comparisons in analysis of variance were adjusted using Tukey-Kramer. Analysis was conducted using SAS 9.4 (SAS Institute, Inc, Cary, NC). Differences were considered significant at P ≤ .05. Percent fibrosis, determined in 19 tissue cubes by Masson trichrome staining, was compared between controls and collagenase C histolyticum -treated tissues using an unpaired 2-tailed Student’s t test.
Results
Photographs of control fibroid cubes before and after incubation demonstrated that incubation time alone did not affect the tissue shape ( Figure 1 ). Treatment with collagenase C histolyticum at all doses and time points softened the injected fibroid cubes as noted by palpation. No differences were noted in the gross appearance of cubes that were treated with placebo or diluent or untreated at 24, 48, 72, or 96 hours.
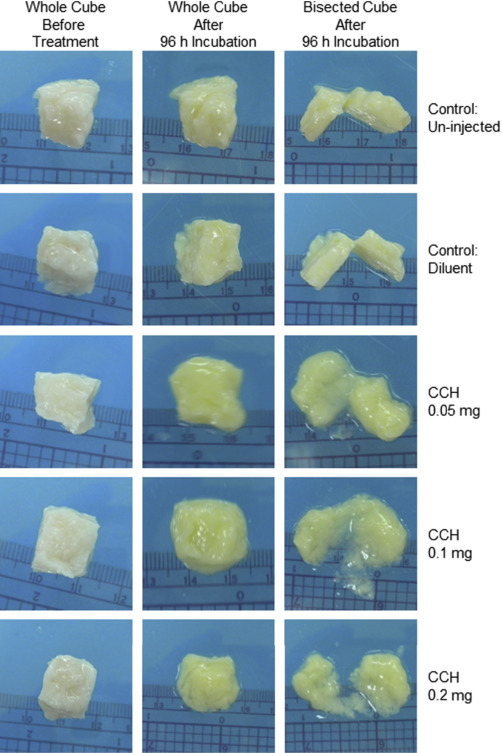
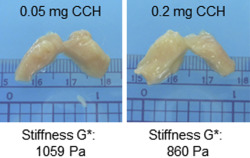
Treatment with collagenase C histolyticum softened the injected fibroid cubes, with the most softening occurring at 72 and 96 hours. These cubes often had rounded edges and a gelatinous consistency. Upon bisection, it was noted that the centers of the cubes were often partially liquefied ( Figure 1 ). Myometrial control cubes before treatments felt softer by palpation than fibroid cubes. Direct injection of myometrial tissues with collagenase C histolyticum softened them further after incubation for 24–96 hours. However, no liquefaction was observed ( Figure 2 ).
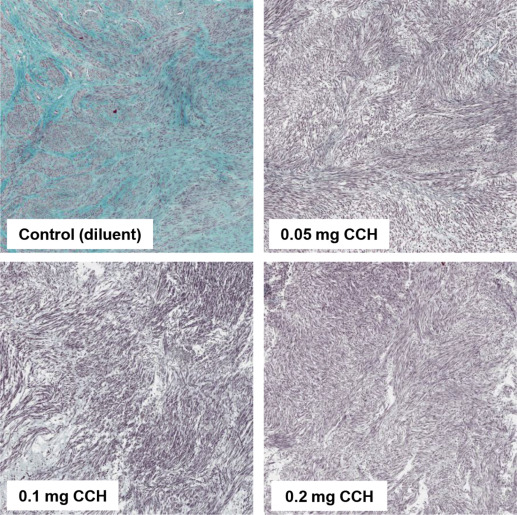
Masson trichrome–stained slides were utilized to evaluate tissue architecture and to determine the amount of fibrosis within the fibroids. The stained sections did not demonstrate evidence of necrosis, and no cellular swelling was observed. We found an abundance of positive Masson trichrome staining in control tissues. The percentage of fibrosis in untreated samples from 16 fibroids ranged from 37% to 77%. Incubated control tissues retained an abundance of collagen across all time points (24, 48, 72, 96 hours) and averaged 67 ± 4% fibrosis. In collagenase C histolyticum –treated tissues average percent fibrosis across all time points was reduced to 20 ± 5% ( P < .001). Percentage fibrosis was inversely related to collagenase C histolyticum dosage and incubation time, and after treatment with collagenase for 96 hours fibrosis ranged from 5.3 to 2.4% ( Figure 3 ).
Electron microscopy revealed that collagen fibrils were visible in the extracellular space of noninjected control samples. In collagenase C histolyticum –treated fibroid tissue, collagen fibrils were not observed in any of the 400 grid squares examined. This total absence of a characteristic collagen band pattern is evidence that collagenase C histolyticum is capable of digesting the altered, disorganized collagen contained in uterine fibroids.
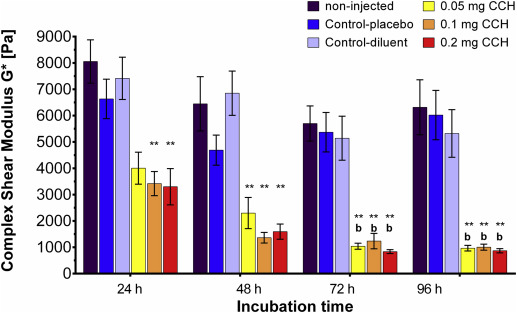
Baseline stiffness in untreated and unincubated fibroid tissue was measured at 8014 ± 798 Pa (mean ± SEM). These tissues were stiffer than myometrial samples (3630 ± 276 Pa). Stiffness in the myometrial control cubes incubated for 24–96 hours did not differ between time points. Direct injection of myometrial tissues with collagenase C histolyticum and incubation for 24–96 hours reduced stiffness by 1492 ± 54 Pa on average, but this reduction was not statistically significant at each time point ( P ≥ .06). Over time, myometrial tissue injected directly with collagenase C histolyticum softened further, but only after 96 hours incubation was tissue stiffness significantly lower than after 24 hours (1071 ± 125 Pa vs 2507 ± 162 Pa; P = .04). Directly injected myometrium was not liquefied and the tissue cubes maintained their shape ( Figure 2 ).
Quantitative data clearly demonstrated reduced stiffness in all collagenase C histolyticum –treated fibroid tissues by 4336 ± 236 Pa on average. Tissue controls did not differ within or between time points ( Figure 4 ). All 3 doses of Collagenase C histolyticum were effective in reducing tissue stiffness, and no difference was detected between collagenase C histolyticum doses at each time point. Longer incubation times caused greater reduction in stiffness ( Figure 4 ).
Results
Photographs of control fibroid cubes before and after incubation demonstrated that incubation time alone did not affect the tissue shape ( Figure 1 ). Treatment with collagenase C histolyticum at all doses and time points softened the injected fibroid cubes as noted by palpation. No differences were noted in the gross appearance of cubes that were treated with placebo or diluent or untreated at 24, 48, 72, or 96 hours.




